Translate this page into:
Exploring the therapeutic potential of GC–MS separated compounds from Dracaena cinnabari against dengue virus and Aedes aegypti using in silico tools
⁎Corresponding author. bomotairi@ksu.edu.sa (Bader O. Almutairi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The paper's main aim was to investigate bioactive molecules in Dracaena cinnabari extract using gas chromatography-mass spectroscopy (GC–MS) and to assess their therapeutic potential using molecular docking algorithm, ProTox II and ADME studies on dengue virus and Aedes aegypti. Molecular docking was carried out using AutoDock Vina, followed by drug-likeness potential and toxicity using in silico tools (ProTox II and ADME). A total of 25 different compounds were detected in the methanol extract, and the major compounds were cis-13-Octadecenoic acid (19.04 %), n-Hexadecanoic acid (16.5 %), beta-Sitosterol (10.5 %), and n-Heptadecanol-1 (9.74 %). Molecular docking revealed that beta-Sitosterol and stigmasterol are the lead compounds and scored the highest docking value among the compounds. The best-docked ligand score for dengue virus was recorded for 4V0Q (stigmasterol, −9.0 kcal/mol), whereas the best-docked ligand score for Ae. agypti was recorded for 1PZ4 (beta-Sitosterol, −9.9 kcal/mol). The toxicity prediction for the beta-Sitosterol and 4,4′-Dihydroxy-2 methoxydihydrochalcone did not violate the Lipinski rules. The values of LD50 predicted using ProTox II revealed that stigmasterol, 4,4′-dihydroxy-2-methoxydihydrochalcone, beta-Sitosterol, and vitamin E ranged from 890 to 5000 mg kg − 1 in a rat model. This study depicts the potential of promising molecules of D. cinnabari. However, in vivo and in vitro investigation is needed to support the results of this study.
Keywords
Gas chromatography
Aedes aegypti
Dengue virus
Dracaena cinnabari
Molecular docking
Drug-likeness
1 Introduction
Dengue fever is an infection caused by the dengue virus (DENV) and transmitted through the bite of infected mosquitoes. The number of dengue cases reported increased over 8-fold from 2000 (505,430 cases) to over 2.4 million in 2010 and 5.2 million in 2019 (WHO, 2022). It is estimated that about 60 % of the world population will be at risk of Dengue fever in 2080 (Messina et al., 2019). In Makkah, Saudi Arabia, dengue cases have increased in recent years. The incidence of dengue cases was 204, 163 and 748 in 2017, 2018 and 2019, respectively. In Makkah, the density of Aedes mosquitoes was higher in 2019 with respect to 2017 and 2018. Ae. albopictus has not been reported to be present in Makkah; therefore, Ae. aegypti is thought to be the principal vector for dengue spread (Sami et al., 2021).
Although all the stages of the DENV replication cycle are prone to inhibition (Magden et al., 2005), no drug is licensed for use in infected patients. Therefore, research, development and assessment in this area are vital. Since vector control is the primary tool for controlling arboviruses, investment in research to combat Ae. aegypti is also growing (Geris et al., 2012). Several methods are already used to combat viruses (Goldenthal et al., 1996) and mosquitoes (Benelli 2015). However, antiviral and insecticidal agents derived from natural products offer a promising source of safer new products for viral and mosquito control due to minimal side effects and residues, therefore minimizing ecosystem disruption (Seo et al., 2012). There is considerable research on antiviral and insecticides of natural origin, especially of botanical origin, due to their innumerable secondary metabolites produced mainly as a defence mechanism against natural predators (Williams et al., 1989).
Dracaena cinnabari (common name: Dragon’s blood) is a 30–60 feet tall tree that belongs to the Agavaceae family. The family Agavaceae comprises over 100 genera distributed in subtropical and tropical regions. The dragon’s blood tree is famous for the red sap that oozes out of it when 3injured (Baumer and Dietemann 2010) (Al-Awthan and Bahattab 2021). It has been used traditionally as an analgesic, abortifacient, astringent, antiseptic, hemostatic, antiulcer; and to treat diarrhoea, fevers, fractures, burns (Al-Awthan et al., 2010) (Xin et al., 2011), skin, eye, and dental diseases (Al-Fatimi 2018). Several pharmacological effects have also been reported, such as wound healing, antidiabetic, antimicrobial, anti-inflammatory, antispasmodic, anticancer, antitumor, hypolipidemic, and analgesic relaxant effects (Al-Awthan and Bahattab 2021).
Considering the need to continue searching for specific larvicidal and antivirals from natural sources, this study aimed to explore in silico interactions of secondary metabolites extracted as well as reported in the literature that may open the door for promising compounds as both larvicidal and antiviral agents. Our results revealed that the compounds extracted and reported from D. cinnabari are promising secondary metabolites for developing larvicidal and anti-DENV agents.
2 Materials and methods
2.1 Plant extraction
Twenty grams of D. cinnabari resin were obtained from Riyadh (Al-Morroj, Riyadh, Saudi Arabia) and pulverized using a commercial blender (SFstardust, Japan). The powder was extracted using methanol (MeOH) in a sonicator (WiseClean, Witeg, China) for 10 mins at 40° C. The methanol (Sigma–Aldrich, MO, USA) extract was filtered using Whatman filter paper number 1 and evaporated using a rotary evaporator (Heidolph, Schwabach, Germany) at 40 °C. The process was repeated twice, and the yield was calculated. The stock solution prepared was kept at 80 °C.
2.2 Analysis of extract by gas chromatography and mass spectrometry (GC–MS)
The chemical constituents of D. cinnabari resin MeOH extract were investigated using GC–MS (Turbomass, PerkinElmer, MA, USA). HP-88 capillary column (100 m, ID: 250 μm) was used for the study. The temperature was adjusted to 40 °C for a 2 min hold, followed by increasing the temperature to 200 °C (5 °C/min) and held for 2 min. The temperature was later raised to 300 °C (5 °C/min) and held for another 2 min. The phytochemical composition of MeOH extract was investigated by comparing the mass spectra of detected compounds with the Wiley GC–MS Library (McLafferty and Stauffer 1989) and the National Institute of Standard and Technology Spectral Library, the Adams Library (Adams 2007).
2.3 Preparation of ligands
The 3D structures of the compounds extracted from the D. cinnabari and reported from the literature (Ying et al., 2011, Al-Awthan and Bahattab 2021) were downloaded from the PubChem (https://pubchem.ncbi.nlm.nih.gov/). The structures of D. cinnabari compounds in table 1 and table 8 (supplementary material) were converted to “PDBQT” files from “SDF” format using Open Babel v.2.4.0 software (https://sourceforge.net/projects/openbabel/files/openbabel/2.4.0/). The energy-minimized ligands were the input for AutoDock Vina to perform docking simulation.
2.4 Preparation of receptor
AutoDock Vina was used to dock the crystal structural protein (Tables 1 and 2) and isolated compounds (25 compounds) into the active site of the selected target proteins. All the three-dimensional (3D) crystal enzyme structures were downloaded from the Protein Data Bank (PDB) (https://www.rcsb.org). All the associated water molecules and heteroatoms were removed from the original structures, and polar hydrogen atoms were added along with the charges using Auto Dock 4.2 (MGL tools1.5.6) [44]. The grid box was set using Autogrid. Molecules were saved in “PDBQT” format so they could be further processed using AutoDock Vina [45].
No.
Protein
PDB ID
1
Sterol Carrier Protein-2
2QZT
2
kynurenine aminotransferase
1YIY
3
sterol carrier protein-2
1PZ4
4
FKBP12 Isomerase
3UQI
No.
Protein
PDB ID
1
DENV1-E111
4FFY
2
NS2B/NS3 Protease
2FOM
3
Dengue 4 Envelope protein domain III
3WE1
4
NS5 RNA dependent RNA polymerase
2J7U
5
RNA helicase
2BMF
6
RNA-directed RNA polymerase (NS5)
4V0Q
7
non-structural protein 1(NS1) chain A
4O6B
2.5 Molecular docking
The algorithm provided with Auto Dock Vina was employed to look for the best-docked conformation between proteins and ligands. The conformations with the lowest free binding energy (ΔG) (best-scored complexes) were selected for the Ligand receptor interaction analysis by PyMOL and the protein–ligand interaction profiler (PLIP).
2.6 In silico pharmacokinetic study
Adsorption, distribution, metabolism, and excretion (ADME) are necessary to analyze the pharmacodynamics attributes of the promising compounds. Chemical notation of the ligands (SMILES) was copied from PubChem (https: //pubchem.ncbi.nlm.nih.gov/compound, accessed on 5 June 2021) and used as an input for SWISS-ADME tool (https://www.swissadme.ch, accessed on 23 June 2022) to predict lipophilicity (Log P0/w, iLOGP, SILICOS-IT, XLOGP3, MLOGP, WLOGP), water solubility-Log S (SILICOS-IT, ESOL, Ali), and drug-likeness rules (Muegge, Veber, Ghose, Lipinski, Ghose, and, Egan,). Toxicology prediction is essential to predict the toxicity of ligands. ProTox-II provides details of various predicted toxicity endpoints such as immunotoxicity, mutagenicity, carcinogenicity, hepatotoxicity, cytotoxicity and acute toxicity. ProTox-II was logged on using SMILES of the compounds, and toxicity mode was assessed.
3 Result
3.1 GC–MS of methanol extract
A total of 25 different compounds were present in the MeOH extract (Table 3). The reported compounds were represented in order of their elution. The major compounds were cis-13-Octadecenoic acid (19.04 %), n-Hexadecanoic acid (16.5 %), beta-Sitosterol (10.5 %), n-Heptadecanol-1 (9.74 %). The remaining compounds are present in small amounts such as Hexadecenoic acid, Z-11- (0.59 %), 1,2-Benzenedicarboxylic acid, diisooctyl ester (0.60 %) and Campesterol (0.71 %).
Sr. No.
Name
Molecular weigh
Molecular formula
Area %
4FFY
2FOM
3WE1
2J7U
2BMF
4V0Q
4O6B
1
Camphene
136.125
C10H16
1.029234
−5.6
−4.9
−5.2
−5.0
−5.2
−5.1
−4.8
2
Ethanone, 1-(2-methylcyclopropyl)-
98.073
C6H10O
0.910577
−4.1
−4.3
−4.0
−3.8
−4.1
−4.2
4.5
3
Tetradecanoic acid
228.209
C14H28O2
0.775554
−3.8
−5.3
−3.3
−4.6
−5.2
−5.2
−4.7
4
Butanoic acid, 3-methyl-, 3,7-dimethyl-6-octenyl ester
240.209
C15H28O2
1.974359
−4.5
−5.5
−4.8
−4.9
−5.6
−5.7
−4.3
5
n-Heptadecanol-1
256.277
C17H36O
9.748447
−3.8
−4.6
−3.4
−4.3
−5.1
−4.5
−4.5
6
Hexadecanoic acid, methyl ester
270.256
C17H34O2
1.378555
−3.9
−4.7
−3.3
−4.5
−5.1
−4.8
−6.0
7
7,9-Di-tert-butyl-1-oxaspiro(4,5)deca-6,9-diene-2,8-dione
276.173
C17H24O3
2.846544
−6.1
−6.9
–
−6.6
−6.1
−7.1
−3.9
8
Hexadecenoic acid, Z-11-
254.225
C16H30O2
0.593962
−4.0
−5.4
−3.9
−5.3
−5.0
−5.2
−4.0
9
n-Hexadecanoic acid
256.24
C16H32O2
16.53734
−3.9
−5.1
−3.9
−5.1
−5.2
−5.4
−4.5
10
1,7-Octadiene, 2,7-dimethyl-3,6-bis(methylene)-
162.141
C12H18
2.489961
−4.5
−5.4
−4.5
−4.7
−5.2
−5.2
−4.5
11
1,5-Heptadiyne
92.063
C7H8
7.026721
−3.8
−3.9
−3.7
−4.2
−3.8
−4.4
−4.0
12
Naphthalene, 1,2,3,4,4a,5,6,8a-octahydro-4a,8-dimethyl-2-(1-methylethenyl)-, [2R-(2.alpha.,4a.alpha.,8a.beta.)]-
204.188
C15H24
4.87512
−5.8
−7.2
−5.8
−6.2
−5.8
−8.0
−5.8
13
cis-13-Octadecenoic acid
282.256
C18H34O2
19.42207
−4.5
−4.9
−3.6
−5.0
−4.4
−4.9
−5.0
14
9,12-Octadecadienoic acid (Z,Z)-
280.24
C36H66O4
1.091124
−3.8
−5.4
−4.3
−5.8
−4.8
−5.4
−5.3
15
1H,5H-Pyrido[3,2,1-ij]quinolin-5-one, 2,3-dihydro-6-ethyl-7-hydroxy-
229.11
C14H15NO2
4.528984
−6.5
−7.3
−6.1
−6.6
−6.6
−7.3
−6.1
16
Pentadeca-1,3,7,12,14-pentaen-7-ol-9-one
232.146
C15H20O2
1.121466
−4.9
−5.7
−4.6
−5.8
−6.0
−6.0
−5.2
17
Vitamin E
430.381
C29H50O2
1.625102
−4.9
−7.5
−5.8
−6.1
−8.2
−6.3
−6.3
18
2-Methyl-Z,Z-3,13-octadecadienol
280.277
C19H36O
0.758699
−4.3
−5.2
−4.0
−4.8
−5.2
−5.5
−4.5
19
cis,cis-7,10,-Hexadecadienal
236.214
C16H28O
2.362345
−3.9
−5.3
−3.9
−5.0
−4.9
−5.3
−4.3
20
1,2-Benzenedicarboxylic acid, diisooctyl ester
390.277
C24H38O4
0.604336
−4.3
−6.3
−4.2
−6.1
−5.7
−5.3
−4.7
21
Campesterol
400.371
C28H48O
0.712619
−7.3
−7.9
−6.4
−8.3
−7.6
−7.8
−8.1
22
1,3,12-Nonadecatriene
262.266
C19H34
2.817368
−3.3
−4.9
−3.9
−4.7
−5.2
−5.0
−4.2
23
Stigmasterol
412.371
C29H48O
1.69131
−7.1
−8.2
−6.8
−8.2
−7.3
−9.0
−8.9
24
beta.-Sitosterol
414.386
C29H50O
10.58025
−6.8
−7.6
−5.6
−8.2
−6.3
−7.6
−7.8
25
2-Ethylacridine
207.105
C15H13N
2.497954
−6.0
−7.0
−6.6
−7.1
−8.0
−7.4
−6.3
3.2 Docking studies
Molecular docking was carried out to predict the complex formation between the dengue virus and Ae. aegypti target proteins and twenty-five ligands detected in the GC–MS. Ligands showed differences in binding affinity values and the numbers of hydrophobic and hydrogen bonds for their interaction with the target proteins. The results have revealed 2 compounds that docked strongly to dengue virus nonstructural protein (DENV-NS1) and RNA-directed RNA polymerase (DENV-NS5). Similarly, 3 compounds also docked strongly to Ae. aegypti Sterol Carrier Protein-2 (1PZ4, 2QZT).
3.3 Screening of inhibitors for 4V0Q
With RNA-directed RNA polymerase (DENV-NS5), ligands stigmasterol, and 4,4′-dihydroxy-2-methoxydihydrochalcone showed binding affinities of − 9.0 and − 8.8 kcal/mol, respectively (Table 3). Further analyses of binding sites between the ligands-DENV-NS5 complexes revealed different binding positions of stigmasterol and 4,4′-dihydroxy-2-methoxydihydrochalcone on the DENV-NS5 protein via hydrophobic and hydrogen bond formation. The hydrogen bonds between the ligand and the DENV-NS5 protein stabilize the ligand within the binding residues. The docking result of stigmasterol to DENV-NS5 protein formed two hydrogen bonds (ASP13, VAL132) and six hydrophobic interactions. Similarly, the docking result of 4,4′-dihydroxy-2-methoxydihydrochalcone to DENV-NS5 protein formed 4 hydrogen bond (LEU94, LYS96, VAL97 and GLN351) and 5 hydrophobic interactions (ILE72, LEU94, LYS95, PRO298, and GLN351). The docking complexes were studied in-depth for the interactions of each ligand with the active residues of the DENV-NS5 target protein (Table 5, Fig. 1).
Sr. No.
Name
Molecular weigh
Molecular formula
Area %
2QZT
3UQI
1YIY
1PZ4
1
Camphene
136.125
C10H16
1.029234
−6.1
−6.3
−5.8
−6.8
2
Ethanone, 1-(2-methylcyclopropyl)-
98.073
C6H10O
0.910577
−4.3
−4.6
−5.0
−4.8
3
Tetradecanoic acid
228.209
C14H28O2
0.775554
−5.7
−5.1
−5.3
−6.6
4
Butanoic acid, 3-methyl-, 3,7-dimethyl-6-octenyl ester
240.209
C15H28O2
1.974359
−6.2
−5.3
−6.2
−7.1
5
n-Heptadecanol-1
256.277
C17H36O
9.748447
−5.8
−4.7
−5.0
−6.4
6
Hexadecanoic acid, methyl ester
270.256
C17H34O2
1.378555
−5.8
−4.2
−5.3
−6.8
7
7,9-Di-tert-butyl-1-oxaspiro(4,5)deca-6,9-diene-2,8-dione
276.173
C17H24O3
2.846544
−7.4
−6.6
−7.6
−5.3
8
Hexadecenoic acid, Z-11-
254.225
C16H30O2
0.593962
−6.6
−4.6
−5.6
−6.9
9
n-Hexadecanoic acid
256.24
C16H32O2
16.53734
−6.0
−4.6
−5.9
−6.5
10
1,7-Octadiene, 2,7-dimethyl-3,6-bis(methylene)-
162.141
C12H18
2.489961
−6.0
−5.2
−5.9
−6.6
11
1,5-Heptadiyne
92.063
C7H8
7.026721
−4.6
−4.5
−4.9
−4.7
12
Naphthalene, 1,2,3,4,4a,5,6,8a-octahydro-4a,8-dimethyl-2-(1-methylethenyl)-, [2R-(2.alpha.,4a.alpha.,8a.beta.)]-
204.188
C15H24
4.87512
−8.1
−6.4
−7.3
−6.8
13
cis-13-Octadecenoic acid
282.256
C18H34O2
19.42207
−6.2
−4.5
−6.2
−7.1
14
9,12-Octadecadienoic acid (Z,Z)-
280.24
C36H66O4
1.091124
−6.8
−4.6
−6.3
−7.6
15
1H,5H-Pyrido[3,2,1-ij]quinolin-5-one, 2,3-dihydro-6-ethyl-7-hydroxy-
229.11
C14H15NO2
4.528984
−7.6
−6.6
−7.4
−5.8
16
Pentadeca-1,3,7,12,14-pentaen-7-ol-9-one
232.146
C15H20O2
1.121466
−6.9
−5.3
−6.6
−7.0
17
Vitamin E
430.381
C29H50O2
1.625102
−9.6
−7.2
−7.1
−5.0
18
2-Methyl-Z,Z-3,13-octadecadienol
280.277
C19H36O
0.758699
−6.5
−5.2
−5.5
−7.1
19
cis,cis-7,10,-Hexadecadienal
236.214
C16H28O
2.362345
−5.9
−5.2
−5.5
−6.9
20
1,2-Benzenedicarboxylic acid, diisooctyl ester
390.277
C24H38O4
0.604336
−7.0
−5.1
−5.9
−4.0
21
Campesterol
400.371
C28H48O
0.712619
−7.2
−6.2
−8.0
−6.3
22
1,3,12-Nonadecatriene
262.266
C19H34
2.817368
−6.4
−5.4
−5.3
−7.1
23
Stigmasterol
412.371
C29H48O
1.69131
−7.1
−7.0
−8.1
−6.7
24
beta.-Sitosterol
414.386
C29H50O
10.58025
−6.5
−6.6
−7.6
−9.9
25
2-Ethylacridine
207.105
C15H13N
2.497954
−8.6
−6.6
−8.3
−9.7
Sr. No.
compounds
Target
No. of H-bond
Interact
residuesNo. of Hydrophobic interaction
Interact
residuesBinding energy (kcal/mol)
23
Stigmasterol
4V0Q
2
ASP13, VAL132
6
TRP87, LYS105, ASP146, ILE147
−9.0
23
Stigmasterol
4O6B
1
ASP1
7
VAL5,LYS14, PHE20, LYS189, ARG192, VAL194
−8.9
5
4,4′-Dihydroxy-2-methoxydihydrochalcone
4V0Q
4
LEU94, LYS96, VAL97, GLN351
5
ILE72,LEU94, LYS95,PRO298, GLN351
−8.8
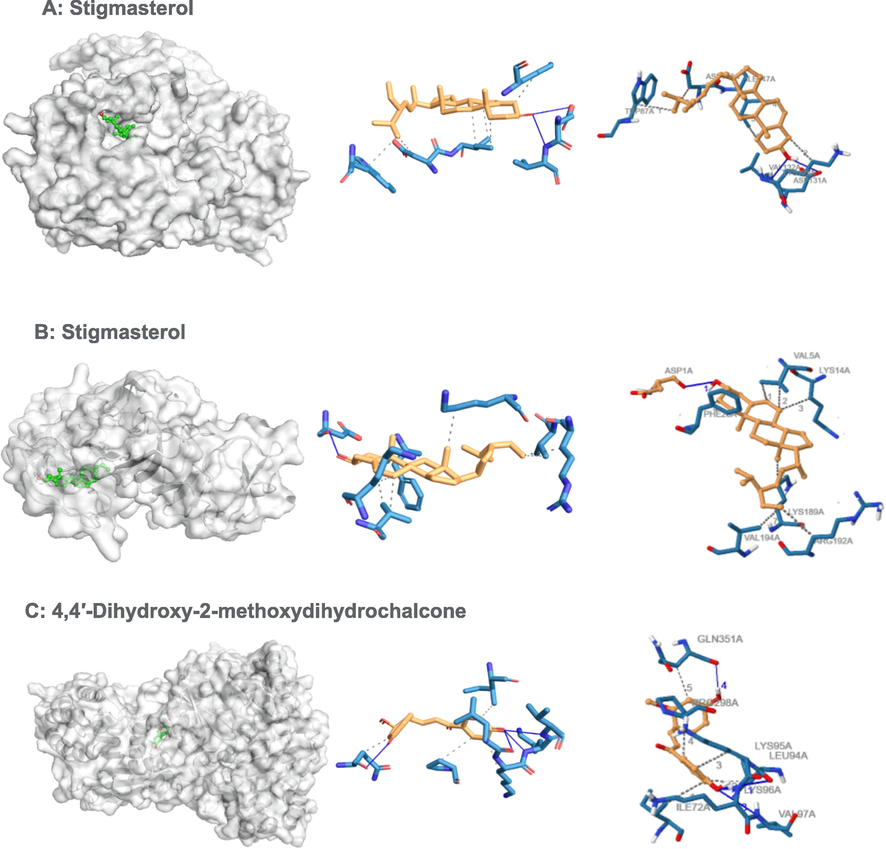
Binding poses of three top-ranked ligands at the binding site of RNA-directed RNA polymerase (PDB ID: 4V0Q), nonstructural protein 1(NS1) (PDB ID: 4O6B) and 3D and 2D interaction diagrams. (A) Stigmasterol − 4V0Q; (B) Stigmasterol 4O6B; and (C) 4,4′-Dihydroxy-2-methoxydihydrochalcone − 4V0Q.
3.4 Screening of inhibitors for 4O6B
Stigmasterol, campesterol and beta.-sitosterol were the 3 ligands with the greatest negative values of binding free energy (Table 3). With DENV-NS1 protein, ligands stigmasterol (−8.9 kcal/mol), campesterol (−8.1 kcal/mol) and beta-sitosterol (−7.8 kcal/mol) showed good to moderate affinity binding. The docking result of Stigmasterol to DENV-NS1 protein formed a hydrogen bond (ASP1) and seven hydrophobic interactions (VAL5, LYS14, PHE20, LYS189, ARG192, VAL194). The docking complexes were studied in-depth with the active residues of the DENV-NS1 target protein (Table 5 and Fig. 1).
3.5 Screening of inhibitors for 1PZ4
The ligands showed different binding free energy values with the target protein of AeSCP2 (PDB code: 1PZ4). Ligands beta-Sitosterol (-9.9), 2-Ethylacridine (-9.7), and (2S)-7, 3′-Dihydroxy- 4′-methoxyflavane (-9.7) were the 3 ligands with the greatest negative binding free energy values for complex formation (Table 4). Further analyses of potential binding sites between the ligand-1PZ4 complexes revealed different binding positions of all 3 peptides on the DENV NS1 protein via hydrogen and hydrophobic bond formation (Table 2). The docking result of beta-Sitosterol to AeSCP2 formed a hydrogen bond (GLY75) and 16 hydrophobic interactions. Similarly, the docking result of 2-Ethylacridine to AeSCP2 formed a hydrogen bond (PHE105) and 9 hydrophobic interactions. Nevertheless, some common binding sites are recognized by ligand at specific amino acid residues on the DENV NS1 structure, including ILE12, LEU102, PHE105, GLN25 and LEU109 (Table 6 and Fig. 2).
Sr. No.
compounds
Target
Interact
residuesNo. of Hydrophobic interaction
No. of Hydrophobic interaction
Interact
residuesBinding energy (kcal/mol)
24
beta.-Sitosterol
1PZ4
1
GLY75A
16
ILE12, ARG15, LEU15, ILE16, ILE19, ARG24, GLN25, VAL36, PHE32,LEU102,PHE105, ILE106, LEU109
−9.9
25
2-Ethylacridine
1PZ4
1
PHE105
9
ILE12, GLN25,VAL26,LEU48, LEU102, PHE105, LEU109
−9.7
17
Vitamin E
2QZT
1
PRO22
17
ILE10, VAL14, VAL 17, ARG23, PHE29, LEU21, VAL44, LEU46, ILE73, VAL80, LEU101, VAL104
−9.6
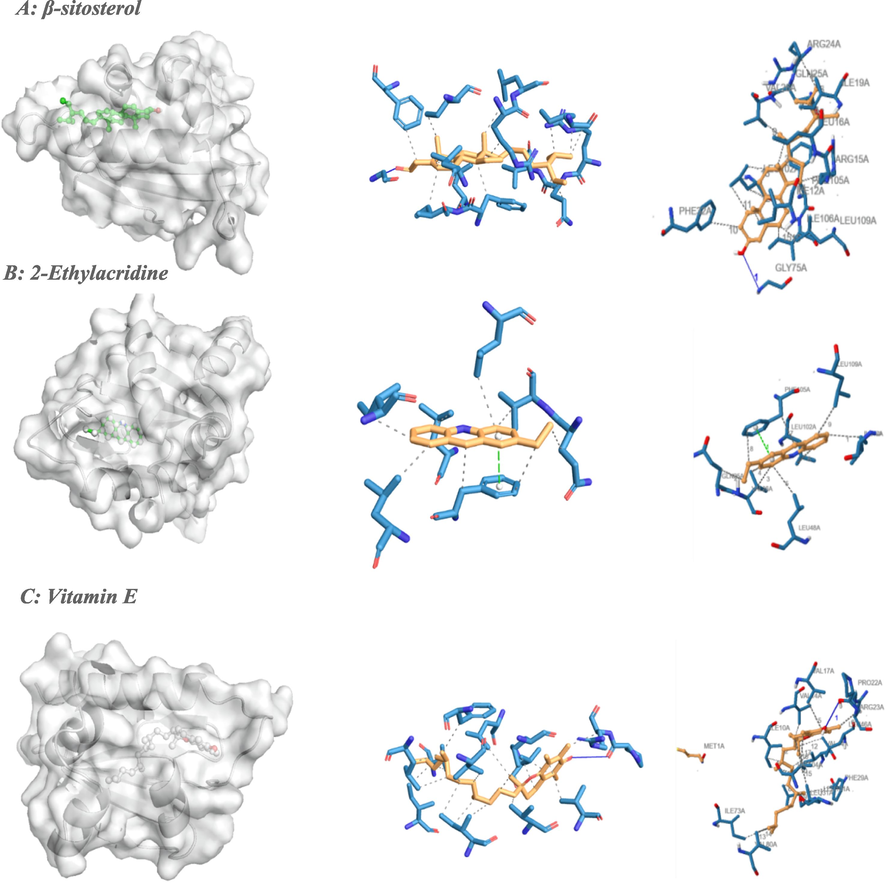
Binding poses of three top-ranked ligands at the binding site of sterol carrier protein-2 (PDB ID: 1PZ4 and PDB ID: 2QZT) and 3D and 2D interaction diagrams. (A) Stevioside-1PZ4; (B) 2-Ethylacridine −1PZ4; and (C) Vitamin E −2QZT.
3.6 Screening of inhibitors for 2QZT
Vitamin E, 2-Ethylacridine, 7,4′-dihydroxy-8-methylflavone and pinoresinol were the 4 ligands with the greatest negative values of binding free energy for complex formation (Table 4). With Sterol Carrier Protein-2, ligands Vitamin E (−9.6 kcal/mol), 2-Ethylacridine (−8.6 kcal/mol), 7,4′-dihydroxy-8-methylflavone (−8.4 kcal/mol) and Pinoresinol (−8.4 kcal/mol) showed good to moderate affinity binding (Table 4). The docking result of vitamin E to Sterol Carrier Protein-2 formed a hydrogen bond (PRO22) and 17 hydrophobic interactions (ILE10, VAL14, VAL17, ARG23, PHE29, LEU21, VAL44, LEU46, ILE73, VAL80, LEU101, VAL104). The docking complexes were visually inspected in-depth for the interactions of each ligand with the active residues of the 2QZT target protein (Table 6 and Fig. 2).
3.7 In silico pharmacokinetic study
Promising candidates should have satisfactory ADME properties and be non-toxic. Therefore, the compounds' toxicity and ADME profile were assessed using ProTox II and Swiss ADME approach. The predicted toxicity of selected compounds is shown in Fig. 3 and Table 7. The toxicity prediction for the beta-Sitosterol, and 4,4′-Dihydroxy-2 methoxydihydrochalcone did not violate the Lipinski rules (Fig. 3). The compounds' cytotoxicity, mutagenicity, carcinogenicity and hepatotoxicity have been assessed(Lounkine et al., 2012). Based on the ProTox II result, compound 2-Ethylacridine is mutagenic. Stigmasterol, 4,4′-dihydroxy-2-methoxydihydrochalcone, and beta-Sitosterol are found to be immunotoxic. However, all compounds are not cytotoxic or hepatotoxic. The values predicted LD50 using ProTox II revealed that stigmasterol, 4,4′-dihydroxy-2-methoxydihydrochalcone, beta-sitosterol, and vitamin E range from 890 to 5000 mg kg − 1 in the rat model (Table 7).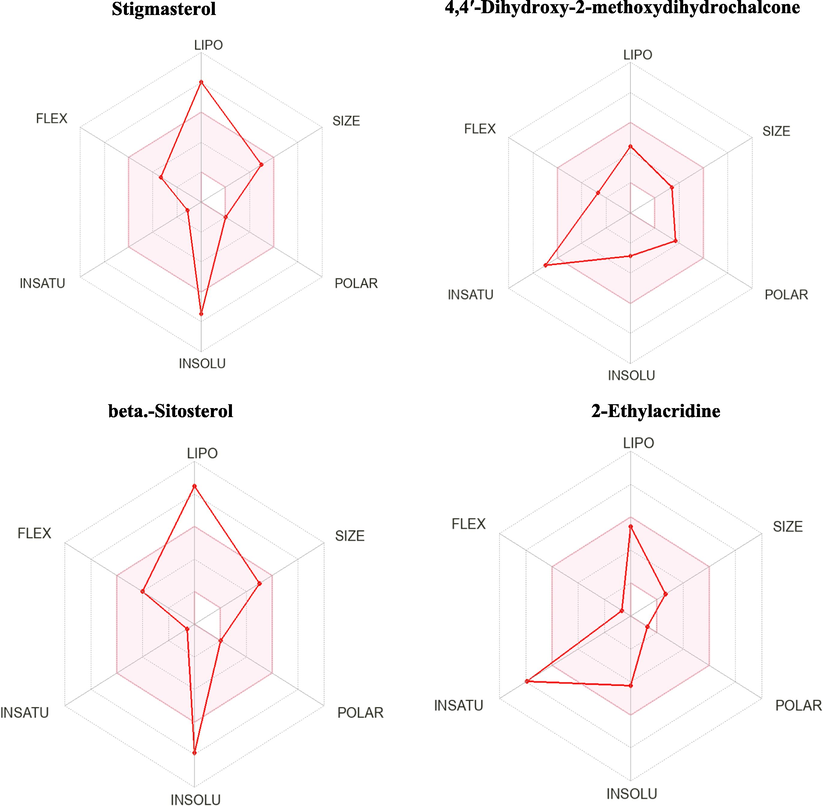
SwissADME bioavailability radar of the 4 promising drug-likeness ligands, where the pink areas signify each property (FLEX: flexibility, INSOLU: insolubility, LIPO: lipophilicity.
Compounds
Predicted:
Hepatotoxicity
Carcinogenicity
Immunotoxicity
Mutagenicity
Cytotoxicity
LD50 (mg/kg)
Toxicity Class
Stigmasterol
Inactive
Inactive
Active
Inactive
Inactive
890
4
4,4′-Dihydroxy-2-methoxydihydrochalcone
Inactive
Inactive
Active
Inactive
Inactive
1000
4
beta-Sitosterol
Inactive
Inactive
Active
Inactive
Inactive
890
4
2-Ethylacridine
Inactive
Inactive
Inactive
Active
Inactive
940
4
Vitamin E
Inactive
Inactive
Inactive
Inactive
Inactive
5000
5
4 Discussion
Computational chemistry plays a substantial role in the drug development process. Virtual screening is widely utilized to reduce the cost and time of drug development. Molecular docking is a technique used to discover novel ligands for target proteins and plays a significant role in structure-based drug design (Kitchen et al., 2004). Due to the merging of new diseases and resistance development to many drugs, plant-based products are the best choice for discovering new promising agents (Khan et al., 2019) (Piscopo et al., 2020).
Interestingly, diverse bioactive secondary metabolites support the traditional use of D. cinnabari for treating many diseases. This is the first study to report molecular docking of D. cinnabari secondary metabolites with different targets protein from the dengue virus and Ae. agypti. Our study revealed that the selected bioactive molecules could efficiently bind to the targeted receptors, and molecular docking can be effectively used in finding promising inhibitors from D. cinnabari extract. The higher negative docking score signified a high binding affinity between the receptor (target protein) and ligand, indicating the higher efficacy of bioactive molecules. In the present investigation, beta-Sitosterol and stigmasterol are the lead compounds showing the highest docking score among the bioactive compounds. The best-docked ligand scores for dengue virus was 4V0Q (stigmasterol, −9.0), whereas the best-docked ligand scores for Ae. agypti was recorded for 1PZ4 (beta-Sitosterol, −9.9).
An effective drug for DENV infection is required to reduce the DENV infection. It is a challenge to find a good drug candidate (Marnolia et al., 2018). The Dengue NS5 methyltransferase is an important nonstructural protein (104 kDa), a component of viral replication complex, that has enzymatic activities, and the important drug target for antiviral discovery (Yin et al., 2009). It contains both RNA-dependent RNA polymerase and methyltransferase (Marnolia et al., 2018).
AeSCP-2 is an essential gene for the development and survival of mosquitoes(Spates et al., 1988). Searching for Ae. agypti AeSCP-2 inhibitors is a way to find a compound that could be employed in mosquito control. Therefore, If the carrier protein AeSCP-2 is blocked, it would disrupt the cholesterol uptake and cause mosquito larval death (Kim et al., 2005). Targeting cholesterol metabolism to control the mosquito population is one of the aims of diseases causing vector management.
Different compounds can be used as inhibitors for treating DENV infection and as a larvidical agent. One of them is phytosterols. Phytosterols are a large group of compounds with various biological activities. Among phytosterols, β-sitosterol, campesterol, and stigmasterol are the major compounds found with a high percentage in plants. β-sitosterol and stigmasterol are a nutritional complement with a long history of use as a pharmaceutical products (Paniagua-Pérez et al., 2005). Many scientific reports recognized that they possess anxiolytic, antinociceptive and sedative effects, immunomodulatory, anti-inflammatory, antimicrobial, anticancer, hepatoprotective, lipid-lowering effect, wound healing effect, antidiabetic, and antioxidant, larvicidal, neuroprotective, antibaceterial activities (Fraile et al., 2012, Dighe et al., 2016, Sharmila and Sindhu 2017) (Ododo et al., 2016) (Sharmila and Sindhu 2017) (Abdou et al., 2019), (Yuan et al., 2019) (Park et al., 2019) (Park et al., 2019) (Ponnulakshmi et al., 2019) (Ghosh 2013) (Sultana and Khalid 2010) (Alawode et al., 2021).
Computational chemistry results positively correlated with the previous in vitro studies where stigmasterol (Gade et al., 2017) and β-sitosterol (Ghosh 2013) compounds were very effective against Ae. agypti. For instance, the β-sitosterol isolated from Abutilon indicum extract exhibited an LC50 value of 11.5 mg/L against Ae. egypti and LC50 value of 26.7 mg/L against Anopheles stephensi (Abdul Rahuman et al., 2008). Similarly, Cestrum diurnum extract was reported for its toxicity against Culex quinquefasciatus (Ghosh et al., 2008). Our result also correlated with the reported studies in which the ethyl acetate (EtOAc) extract of Melochia umbellata (stem bark) inhibited the dengue virus (DENV-2) with an IC50 value of 2.81 μg/mL (Soekamto et al., 2018). In the same way, stigmasterol isolated from the EtOAc extract of M. umbellata inhibited DENV-2 with IC50 values of 9.11 μg/mL (Soekamto et al., 2019).
The present investigation focused on identifying different secondary metabolites from D. cinnabari using GC–MS analysis. The bioactive compounds identified are responsible for various pharmacological activities. The compounds stigmasterol and β-sitosterol showed promising binding affinity against the selected target proteins in the molecular docking studies. From our investigations, D. cinnabari may enable us to develop promising drugs against various infections. Despite the effectiveness of computational chemistry (in silico studies), the main limitation is the lack of confidence on the ability of scoring functions to give precise binding energies Therefore, in vivo and in vitro investigations are needed to support the results of silico studies.
Acknowledgement
Researchers Supporting Project number (RSP2022R414), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Assessment of the hepatoprotective effect of developed lipid-polymer hybrid nanoparticles (LPHNPs) encapsulating naturally extracted β-Sitosterol against CCl4 induced hepatotoxicity in rats. Sci. Rep.. 2019;9(1):1-14.
- [Google Scholar]
- Isolation and identification of mosquito larvicidal compound from Abutilon indicum (Linn.) Sweet. Parasitol. Res.. 2008;102(5):981-988.
- [Google Scholar]
- Adams, R. P., 2007. Identification of essential oil components by gas chromatography/mass spectrometry, Allured publishing corporation Carol Stream.
- Stigmasterol and β-Sitosterol: Antimicrobial Compounds in the Leaves of Icacina trichantha identified by GC–MS. Beni-Suef University Journal of Basic and Applied Sciences.. 2021;10(1):1-8.
- [Google Scholar]
- Al-Awthan, Y. S. and O. S. Bahattab, 2021. Phytochemistry and Pharmacological Activities of Dracaena cinnabari Resin. BioMed Research International. Volume 2021 | Article ID 8561696 | https://doi.org/10.1155/2021/8561696.
- Flavonoids content of Dracaena cinnabari resin and effects of the aqueous extract on isolated smooth muscle preparations, Perfused heart, blood pressure and Diuresis in the rat. Jordan J Pharm Sci.. 2010;3(1):8-16.
- [Google Scholar]
- Ethnobotanical survey of Dracaena cinnabari and investigation of the pharmacognostical properties, antifungal and antioxidant activity of its resin. Plants.. 2018;7(4):91.
- [Google Scholar]
- Identification and differentiation of dragon's blood in works of art using gas chromatography/mass spectrometry. Anal. Bioanal. Chem.. 2010;397(3):1363-1376.
- [Google Scholar]
- Research in mosquito control: current challenges for a brighter future. Parasitol. Res.. 2015;114(8):2801-2805.
- [Google Scholar]
- Analgesic and anti-inflammatory activity of β-sitosterol isolated from leaves of Oxalis corniculata. Int J of Pharmc Res.. 2016;6(3):109-113.
- [Google Scholar]
- Immunomodulatory properties of beta-sitosterol in pig immune responses. Int. Immunopharmacol.. 2012;13(3):316-321.
- [Google Scholar]
- Acetylcholinesterase inhibitory activity of stigmasterol & hexacosanol is responsible for larvicidal and repellent properties of Chromolaena odorata. Biochimica et Biophysica Acta (BBA)-General Subjects.. 2017;1861(3):541-550.
- [Google Scholar]
- Bioactive natural products as potential candidates to control Aedes aegypti, the vector of dengue. Stud. Nat. Prod. Chem.. 2012;37:277-376.
- [Google Scholar]
- Efficacy of phytosterol as mosquito larvicide. Asian Pacific Journal of Tropical Disease.. 2013;3(3):252.
- [Google Scholar]
- Laboratory evaluation of a phytosteroid compound of mature leaves of Day Jasmine (Solanaceae: Solanales) against larvae of Culex quinquefasciatus (Diptera: Culicidae) and nontarget organisms. Parasitol. Res.. 2008;103(2):271-277.
- [Google Scholar]
- Goldenthal, K., K. Midthun and K. Zoon, 1996. Chapter 51: Control of viral infections and diseases. Medical Microbiology. 4th ed. Galveston, TX: University of Texas Medical Branch; 1996. Available from: https://www.ncbi.nlm.nih.gov/books/NBK8492/. [Last accessed on 2022 May 5].
- Anticancer plants: A review of the active phytochemicals, applications in animal models, and regulatory aspects. Biomolecules. 2019;10(1):47.
- [Google Scholar]
- Identification of mosquito sterol carrier protein-2 inhibitors. J. Lipid Res.. 2005;46(4):650-657.
- [Google Scholar]
- Docking and scoring in virtual screening for drug discovery: methods and applications. Nat. Rev. Drug Discov.. 2004;3(11):935-949.
- [Google Scholar]
- Large-scale prediction and testing of drug activity on side-effect targets. Nature. 2012;486(7403):361-367.
- [Google Scholar]
- Inhibitors of virus replication: recent developments and prospects. Appl. Microbiol. Biotechnol.. 2005;66(6):612-621.
- [Google Scholar]
- Marnolia, A., E. Toepak, S. Siregar, et al., 2018. Computational screening of flavonoid based inhibitor targeting DENV NS5 methyltransferase. AIP Conference Proceedings, AIP Publishing LLC. (Vol. 2023, No. 1, p. 020070).
- The Wiley/NBS registry of mass spectral data. New York: Wiley; 1989.
- The current and future global distribution and population at risk of dengue. Nat. Microbiol.. 2019;4(9):1508-1515.
- [Google Scholar]
- Structure elucidation of β-sitosterol with antibacterial activity from the root bark of Malva parviflora. Springerplus. 2016;5(1):1-11.
- [Google Scholar]
- Genotoxic and cytotoxic studies of beta-sitosterol and pteropodine in mouse. J. Biomed. Biotechnol.. 2005;2005(3):242-247.
- [Google Scholar]
- Effects of β-Sitosterol from corn silk on TGF-β1-induced epithelial–mesenchymal transition in lung alveolar epithelial cells. J. Agric. Food Chem.. 2019;67(35):9789-9795.
- [Google Scholar]
- Antimicrobial and antioxidant activity of proteins from Feijoa sellowiana Berg. fruit before and after in vitro gastrointestinal digestion. Nat. Prod. Res.. 2020;34(18):2607-2611.
- [Google Scholar]
- In silico and in vivo analysis to identify the antidiabetic activity of beta sitosterol in adipose tissue of high fat diet and sucrose induced type-2 diabetic experimental rats. Toxicol. Mech. Methods. 2019;29(4):276-290.
- [Google Scholar]
- The epidemiology and incidence of dengue in Makkah, Saudi Arabia, during 2017–2019. Saudi Med. J.. 2021;42(11):1173-1179.
- [Google Scholar]
- Larvicidal activity of ajowan (Trachyspermum ammi) and Peru balsam (Myroxylon pereira) oils and blends of their constituents against mosquito, Aedes aegypti, acute toxicity on water flea, Daphnia magna, and aqueous residue. J. Agric. Food Chem.. 2012;60(23):5909-5914.
- [Google Scholar]
- Evaluate the Antigenotoxicity and anticancer role of β-Sitosterol by determining oxidative DNA damage and the expression of phosphorylated mitogen-activated protein Kinases’, C-fos, C-Jun, and endothelial growth factor receptor. Pharmacogn. Mag.. 2017;13(49):95.
- [Google Scholar]
- Soekamto, N. H., S. Liong, S. Fauziah, et al., 2018. Dengue antiviral activity of polar extract from Melochia umbellata (Houtt) Stapf var. Visenia. Journal of Physics: Conference Series, IOP Publishing. (Vol. 979, No. 1, p. 012017).
- Soekamto, N., F. Ahmad and F. Appa, 2019. Potential of stigmasterol from EtOAc extract Melochia umbellata (Houtt) Stapf var. Visenia as Dengue Antivirus. Journal of Physics: Conference Series, IOP Publishing. (Vol. 1341, No. 3, p. 032044).
- Ingestion, utilization and excretion of blood meal sterols by the stable fly, Stomoxys calcitrans. J. Insect Physiol.. 1988;34(11):1055-1061.
- [Google Scholar]
- Phytochemical and enzyme inhibitory studies on indigenous medicinal plant Rhazya stricta. Nat. Prod. Res.. 2010;24(4):305-314.
- [Google Scholar]
- WHO, (10 January 2022). “Dengue and severe dengue”. https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed 20/9/20222).
- Why are secondary metabolites (natural products) biosynthesized. J. Nat. Prod.. 1989;52(6):1189-1208.
- [Google Scholar]
- Dragon's Blood extract has antithrombotic properties, affecting platelet aggregation functions and anticoagulation activities. J. Ethnopharmacol.. 2011;135(2):510-514.
- [Google Scholar]
- An adenosine nucleoside inhibitor of dengue virus. Proc. Natl. Acad. Sci.. 2009;106(48):20435-20439.
- [Google Scholar]
- Chemical constituents from dragon's blood of Dracaena cambodiana. Chin. J. Nat. Med.. 2011;9(2):112-114.
- [Google Scholar]
- Effect of β-sitosterol self-microemulsion and β-sitosterol ester with linoleic acid on lipid-lowering in hyperlipidemic mice. Lipids Health Dis.. 2019;18(1):1-11.
- [Google Scholar]







