Translate this page into:
Exploring the therapeutic potential of edible Pleurotus mushroom species for oxidative stress and diabetes management
⁎Corresponding author. balaji_paulraj@yahoo.com (Paulraj Balaji)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
In this study, extracts from eight Pleurotus mushroom species were examined for their antioxidant properties.
Methods
The pure cultures of eight Pleurotus were grown, harvested, air-dried, pulverized, and for various biological activities.
Results
The methanolic extract of Pleurotus florida showed the highest concentrations of total phenolics, total tannins, and flavonoids. It also displayed the greatest antioxidant activity in the FRAP, ABTS, metal chelating, superoxide anion radicals, hydrogen peroxide, nitric oxide and DPPH assay. In the hydroxyl radical scavenging assay, Pleurotus florida and Pleurotus flabellatus showed notable effects in methanolic and acetone extracts, respectively. Moreover, Pleurotus florida demonstrated a strong antioxidant effect on β-carotene in methanolic extracts. The study also explored the potential of mushrooms as natural anti-diabetic treatments by targeting a key enzyme in biopharmaceuticals. Pleurotus florida's methanolic extract at a concentration of 1000 µg/ml exhibited significant α-amylase inhibition (99.02%), surpassing acarbose's efficiency at the same concentration (96.54%).
Conclusion
Overall, the bioactive components from Pleurotus florida showed effective antioxidant and antidiabetic effects under in vitro conditions.
Keywords
Pleurotus
Bioactive compounds
Phenolics
Antioxidants
α-amylase
1 Introduction
For ages, mushrooms have been integrated into diets for vital nutrients like dietary fiber, copper, selenium, beta-glucans, riboflavin, and potassium. Recently, mushroom extracts have gained popularity for their health benefits. Serving as both food preservatives and supplements, mushrooms aid in addressing diverse ailments. As part of a balanced diet, mushrooms hold significant nutritional and medicinal value (Deveci et al., 2021).
These Pleurotus mushrooms possess culinary appeal due to their low fat, high fiber, and protein content. Unlike cholesterol found in high-protein sources like meat, they contain ergosterol, which serves various physiological functions and can be converted into vitamin D for dietary supplements or food production. Beyond their nutritional significance, Pleurotus mushrooms have attracted researchers for potential medicinal uses. Over the past decade, numerous academic papers and patents have emerged about these mushrooms. Studies indicate their potential for anti-diabetic, anti-hypercholesterolemic, anti-hypertensive, anti-aging, antimicrobial, antioxidant, anti-obesity, and hepatoprotective effects (Madhanraj et al., 2019). As a source of nutraceuticals, Pleurotus mushrooms are getting more and more attention for their possible health benefits (Das et al., 2021). According to a recent study, the steroids, fatty acids, and other compounds found in different Pleurotus species mushrooms could be employed as effective nutraceuticals (Illuri et al., 2022). It has been found that in obese human subjects, Pleurotus eryngii regulates postprandial glycaemia by regulating the absorption of glucose (Kleftaki et al., 2022).
In addition to hyperglycemia (also known as high blood sugar), hypertension (also called high blood pressure) is a serious condition that can lead to more severe diseases like diabetes and cardiovascular disease (Balaji et al., 2020). α-Amylase and α-glucosidase enzymes break down carbs during digestion. Inhibiting these enzymes, as done by miglitol and acarbose, slows monosaccharide release from carbohydrates, delaying glucose absorption. This helps control blood sugar, but adverse effects like gastrointestinal problems are notable in current diabetes treatments (Maya et al., 2021). Searching for natural antidiabetic agents has become increasingly important, as society seeks multifaceted solutions to health problems that minimize reliance on synthetic solutions with potential harm (Maya et al., 2022). As a result, there is an increase in research into the anti-diabetic effects of mushrooms and the development of novel medications with a natural basis.
This study had two main objectives: evaluating safe-to-consume Pleurotus mushrooms' antioxidant properties and assessing their potential in vitro anti-diabetic effects, possibly linked to their antioxidants. The researchers conducted in vitro experiments on the mushrooms' ability to hinder α-amylase enzyme activity, aiding carbohydrate digestion and blood sugar control. This research contributes to the growing body of knowledge on health benefits from edible mushrooms, especially Pleurotus species. The primary objective of this study is to explore the link between the antioxidant capabilities and antidiabetic effects of various Pleurotus mushroom species. This correlation may offer valuable information on the possible utilization of these mushrooms as natural and effective treatments for diabetes.
2 Materials and Methods
2.1 Basidiomycetes mushroom fungi
In this current study, various strains of edible mushrooms from the Pleurotus genus were used, including Pleurotus eous (Berk.) Sacc., Pleurotus ostreatus (Jacq. ex Fr.) Kumm., Pleurotus flabellatus (Berk and Br.) Sacc., Pleurotus eryngii (DC.) Quel., Pleurotus florida (Mont.) Singer, Pleurotus citrinopileatus Singer, Pleurotus cystidiosus (OK) Miller, and Pleurotus pulmonarius (Fr.) Quelet.
2.1.1 Preparation of mushroom fruiting body
Different mushroom spawns were prepared using unfilled paddy grains, and the fruit bodies of Pleurotus species were cultivated in polybags using paddy straw as the substrate. The seed spawn for the mushroom cultures was maintained in a controlled environment room at a temperature of 25 ± 2 °C. Subsequently, the spawn run for the mushroom species was conducted in a dedicated mushroom house where the temperature was maintained at 28 ± 2 °C, with a relative humidity of 80%.
To initiate the growth of fruiting bodies, the paddy straw substrate was thoroughly moistened by immersing it in water overnight. Afterward, the substrates underwent pasteurization by exposing them to hot water at 80 °C for 2 h. Following pasteurization, the substrate was allowed to cool and excess water was drained overnight (approximately 15–18 h). The next step involved carefully blending the substrate with the spawn, and the resulting mixture was manually packed into clear polyethylene bags measuring 20 × 40 cm and with an average thickness of 0.2 mm. Each bag contained 1 kg of moistened substrate, and the spawn was introduced into the mixture at a rate of 5% based on the wet mass of the substrate.
For fruiting initiation, the temperature in the mushroom house's crop run section was maintained at 25 ± 2 °C, along with a relative humidity of 95%. These distinct cultures were cultivated in a controlled environment at MGR College, Hosur, where the conditions were carefully regulated at a temperature of 25 ± 2 °C and a relative humidity of 95% for a duration of 3–4 weeks, allowing for the formation of basidiocarps. Following this period, the polyethylene bags were removed, and the cultures were relocated to an environment with a temperature of 22 ± 1 °C and exposed to light from cool white fluorescent tubes for 12 h a day. During the incubation phase, electric fans operated for 4 h a day to ensure uniform ventilation within the incubation room. The bags' moisture levels were maintained by periodically spraying them with tap water twice daily throughout the cropping period.
Mushroom fruiting bodies were harvested approximately a week after the formation of pinheads, as soon as the gills were fully developed and while the caps' edges remained curled under. Following the harvest of the fruiting bodies, they were dried in a shaded area within the laboratory and subsequently ground into a fine powder using an electric pulverizer. The resulting powder derived from the mushroom fruiting bodies was carefully stored in an airtight container until it was ready for future utilization.
2.2 Extraction of active compounds
To extract the mushroom fruiting bodies, powdered samples of about 10 g were combined with 100 ml each of methanol and acetone and left to extract for 48 h. A rotary evaporator was used to concentrate the resultant extracts (Rotavapor® R-300 Buchi, India), and until further analysis, the extracts were stored at 4 °C. At a concentration of 1 mg/ml, the concentrated extracts dissolved in the right solvents, and their antioxidant activity was tested in a number of ways.
2.3 Quantification of total phenolics, tannins and flavonoid contents
The total phenolic and tannin amount was estimated by adding one milliliter of distilled water to every 100 µl of mushroom fruiting body extract, then adding a 20% sodium carbonate solution of about 2.5 ml and Folin-Ciocalteau phenol reagent (1:1 with water) of 0.5 ml. Tubes were left in the dark for 40 min before absorbance at 725 nm was estimated in comparison to a reagent blank (Siddhuraju and Becker 2003). After polyvinyl polypyrrolidone treatment, the same extract was used to measure the tannin concentration. The tannin concentration was determined as the fraction of total phenolics and non-tannin polyphenolic compounds (Siddhuraju and Manian 2007). The flavonoid concentration was evaluated by diluting the extracted samples with distilled water and adding with 5% NaNO2 solution, 10% AlCl3 solution, and 4% NaOH solution successively. The absorbance of the mixture at 510 nm was measured after it had rested for 15 min. A standard rutin chemical was used to measure total flavonoids. The phenolic content was presented as gallic acid equivalents (GAE), and the flavonoid content was presented as rutin equivalents (RE) (Zhishen et al., 1999). These experiments were conducted in triplicates.
2.4 Antioxidant activity estimations
2.4.1 Ferric reducing antioxidant power assay
The ferric-reducing antioxidant power assay (FRAP) was estimated by adaptation of a standard procedure that was slightly modified (Sayah et al., 2017). In 40 mmol/L HCl, the acetate buffer (300 mmol/L, pH 3.6), and TPTZ (10 mmol/L) were dissolved and in a ratio of 10/1/1 (v/v/v), 20 mmol/L of ferric chloride were combined to form the FRAP reagent. Following that, in the dark at room temperature, the mixture of reagent (3 ml) and 100 µl of mushroom fruiting body extracts was left after mixing. After 30 min, the absorbance was measured at 700 nm in order to determine the FRAP scavenging and expressed as mmol Fe (II) equivalent/mg extract.
2.4.2 ABTS•+ scavenging activity
ABTS radical scavenging activity was determined following the established protocol (Gan et al., 2010) with some modifications. In order to make the ABTS solution, 88 µl of potassium persulfate (140 mmol/L) and 5 ml of ABTS (7 mmol/L) were combined, and amber glass bottles were used to store the mixture for 16 h at room temperature in complete darkness. The absorbance of the ABTS solution was measured and adjusted to 0.70 ± 0.05 at 734 nm using a spectrophotometer. Then, 100 L of extracts from mushroom fruiting bodies were treated with 3 ml of ABTS solution and left at room temperature in the dark for 2 h. At 734 nm, the absorbance of each extract's ABTS concentration was calculated, and the results were given in trolox equivalents (TE) per gram of extract.
2.4.3 Metal chelating activity
Chelating activity was evaluated using a modified version of the standard method described in (Mohan et al., 2012). 2 mmol/L FeCl2 (0.05 ml) mixed with fruiting body extracts at different concentrations (100 µl) and left for 5 min. As a positive control, EDTA (2 mg/mL) was used, and without any extract, the reaction mixture was a negative control. After adding 0.1 ml of 5 mmol/L ferrozine to the solutions, the final volume was reached by mixing 0.05 ml of 100% ethyl alcohol in the mixtures. The absorbance at 562 nm was measured after the mixtures were incubated at 25 °C for 10 min. The results were reported as mean values, presented as mg of EDTA equivalents.
2.4.4 Phosphomolybdenum assay
Activity in the samples was determined by measuring the production of green phosphomolybdenum complexes (Jan et al., 2013). In a 4 ml vial, 100 µl of the extract of fruiting body (in 1 mM dimethyl sulphoxide) were combined with one ml of the reagent solution (28 mM sodium phosphate, 0.6 M sulphuric acid and 4 mM ammonium molybdate). After being sealed, the vials were placed in a water bath for incubation at 95 °C for 90 min. Once the mixture cooled to room temperature, the absorbance was measured at 695 nm using a blank. The results were reported as a mean value in mg of ascorbic acid equivalents.
2.4.5 Assay of superoxide anion radical activity
This experiment was used based on a previously described procedure to assess formazan formation inhibition by superoxide radical scavenging, which is produced in the riboflavin-light-NBT system (Beauchamp and Fridovich 1971) with slight modifications. Each 3 ml of the reaction mixture included 20 μg riboflavin, 50 mM sodium phosphate buffer (pH 7.6), 12 mM EDTA, 0.1 mg NBT, and 100 μl of the sample solution. For 90 s, the reaction mixture was illuminated by extracts from the fruiting bodies, and the reaction was started. The absorbance was evaluated after illumination at 590 nm. The reaction component was stored inside a container covered with aluminium foil to protect it from outside light. Similar reaction mixtures were stored in the dark in identical test tubes to serve as blanks. The mean values of the formazan formation inhibition, represented as a percentage inhibition of superoxide radicals, were used to represent the results.
Following were the calculations for the % inhibition of superoxide anion generation: where A0 – the absorbance of the control and A1 – the absorbance of the sample extract or standard.
2.4.6 DPPH scavenging activity
Mushroom extracts' ability to scavenge the stable radical DPPH was measured (Blois 1958). Samples were diluted with methanol to obtain 100 µl volume, and different amounts were used for each extract. The sample extracts were combined with 5 ml of a 0.1 mM DPPH solution in methanol, which was then vigorously mixed. The reaction mixture was left at 27 °C for 20 min. The absorbance of the resulting solution was measured at 517 nm to determine how effective the extracts were as antioxidants. The percentage of DPPH radical scavenging activity was determined by using the following formula:
2.4.7 Hydrogen peroxide scavenging activity
The capacity of fruiting body extracts to remove hydrogen peroxide was evaluated (Ruch et al., 1989). In phosphate buffer (0.2 M, pH 7.4), a hydrogen peroxide solution (2 mmol/l) was prepared. Spectrophotometric analysis was used to measure the concentration of hydrogen peroxide by evaluating its absorbance at 230 nm with a molar absorbtivity of 81 (mol/l)-1/cm. One hundred µL of fruiting body extract were combined with 0.6 ml of hydrogen peroxide solution and 3.4 ml of phosphate buffer. At 230 nm, hydrogen peroxide absorbance was measured after 10 min, and the result was compared with that of a blank solution, which was made entirely of phosphate buffer (without H2O2).
where A1 denotes the extract or standard absorbance and A0 denotes the control absorbance; this control is the reaction mixture without the extract.
2.4.8 Hydroxyl radical scavenging activity
To assess ability of mushroom to scavenge hydroxyl radicals, the solution made up of ferrous ammonium sulphate (0.13%) and EDTA (0.26%) known as iron-EDTA (one ml), 0.5 ml of 0.018% EDTA solution, and 1.0 ml of dimethyl sulphoxide (DMSO) (0.85% v/v) in a phosphate buffer (0.1 M) at pH 7.4, and 100 µl of the acetone and methanolic extracts were mixed. The reaction was started by adding 0.22% ascorbic acid (5 ml), and then in a water bath, the mixture was incubated for fifteen minutes at 80–90 °C. One ml of ice-cold trichloroacetic acid (17.5% w/v) was added to stop the reaction. Nash reagent (2 ml acetyl acetone, 3 ml glacial acetic acid, and 75 g ammonium acetate dissolved in distilled water) was added and at fifteen minutes, the mixtures were incubated at room temperature. For controls, the reaction mixtures (to which the sample has not been added) were used. A spectrophotometer was used to detect the colour intensity at 412 nm and compare it to a reagent blank (Klein et al., 1981). The percentage of hydroxyl radical scavenging activity (HRSA) is estimated by applying the following formula: where A1-extract or standard absorbance and A0-control absorbance.
2.4.9 Assay of nitric oxide scavenging activity
Nitric oxide scavenging activity was measured using the Griess reaction (Balakrishnan et al., 2009). With 100 µl of the extract, 500 µl of 10 millimolar sodium nitroprusside made in phosphate-buffered saline at pH 7.4 were mixed. After 150 min at 25 °C, Griess reagent in the amount of about 1.5 ml made up of naphthylethylenediamine dihydrochloride and 1% sulfanilamide in phosphoric acid (2.5%) and the mixture were mixed. An identical amount of buffer was used in the control sample following the same procedure. The absorbance at 546 nm was determined after 30-minutes incubation at room temperature. The potential to scavenge nitrous oxide was calculated using an equation: Where, A-control absorbance and B-extract or standard absorbance.
2.4.10 β-carotene/linoleic acid antioxidant activity
The beta-carotene and linoleic acid peroxidation protocols were used to measure the antioxidant properties of extracts derived from mushroom fruiting bodies (Wong et al., 2014). First, 12.5 g linoleic acid solution and 500 µl of beta-carotene (500 µg/mL, prepared in chloroform) were dissolved, followed by the addition of Tween-40 of about 100 mg. A speed vacuum concentrator was then used to evaporate the chloroform at a temperature of 43 °C Distilled water (25 ml) was used to dilute the resulting mixture, and in order to result in emulsion formation, the mixture was shaken vigorously for two to three minutes. Incubate the mixture for 2 h at 50 °C. The mixture contains an emulsion (150 µl) and mushroom extract (100 µl). Every 30 min, the absorbance is measured at 470 nm, for a total of 120 min. Equation used to calculate the antioxidant potential of β-carotene/linoleic acid: Where As120 represents the sample absorbance after 120 min, Ac120 represents the control absorbance after 120 min, and Ac0 represents the control absorbance after 0 min.
2.5 In vitro α-amylase inhibitory activity
Assessment of β-amylase action of inhibition by following the starch-iodine approach, where it is modified (Quan et al., 2019). In addition to mushroom fruiting body extracts, porcine pancreatic β-amylase solution (2 mg/mL) and pH 6.9 phosphate buffered saline in 0.2 M concentration were dissolved. Deionized water was used to make a 0.5% soluble starch and 0.25 mM iodine solution. A total volume of 100 µl was made up of various doses of β-amylase with various concentrations of fruiting body extracts. Each test sample was then mixed with a starch solution of about 30 uL, and the mixture kept for eight minutes. 1 M hydrochloric acid (20 µl) and iodine solution (100 µl) were added to stop the reaction. The resulting solution was analysed in a microplate reader at 565 nm. Acarbose was used as the positive control. Using the method, the inhibition percentage and IC50 values were calculated as follows: Where, A represents reaction's absorbance in the sample where the fruiting body extracts were present, B represents the reaction's absorbance in the absence of enzymes, and C represents the reaction's absorbance in the absence of fruiting body extracts.
2.6 Statistical analysis
Five individual replicates were carried out independently (n = 5) in each experiment and the standard error of mean (Mean ± SEM) for each group was computed. IC50 values were determined by dose–response curve generated using Graphpad Prism software.
3 Results
The total phenolic, flavonoid, and tannin content of mushroom extracts from the fruiting bodies of Basidiomycetes species is shown in Table 1. In comparison to acetone extracts (84.10 mg GAE/g extract), P. florida methanolic extracts had a higher total phenol concentration (91.29 mg GAE/g extract). P. florida methanolic extract also had the highest amount of flavonoids and tannins, at about 70.71 mg RE/g extract and 90.91 mg GAE/g extract, respectively. Table 2 indicates the results of the FRAP assay conducted on acetone and methanolic extracts of fruiting bodies from different basidiomycetes mushroom fungi. P. florida methanolic and acetone extracts showed the highest ferric reducing power (654.12 and 440.95, respectively) among all other mushrooms tested, including BHT and Rutin standards. Similar to that of BHT and rutin, the methanolic extract of P. florida had a strong scavenging effect on ABTS radicals. For P. florida methanolic extracts, their inhibition of ABTS radicals was found to be 998.56 µM TEAC/g extract, followed by P. pulmonarius (983.37 µM TEAC/g extract). In addition, P. eryngii acetone extracts demonstrated an ABTS scavenging activity of 916.87 M TEAC/g extract. Values are expressed as mean ± SEM (n = 3 for each concentration). GAE-Gallic acid equivalents. RE-Rutin equivalent. AE- Acetone extract. ME- Methanol extract. FRAP assay: BHT: 91.81 ± 2.15; Rutin: 216.59 ± 2.59. Fe (II) E: Fe (II) equivalent. Values are means of three independent analysis ± SEM (n = 3) / ABTS activity: BHT: 1245.2 ± 126.7; Rutin: 1109.8 ± 162.1. TEAC: Trolox equivalent antioxidant capacity. Values are means of three independent analysis ± SEM (n = 3).
S. No.
Mushroom fungi
Total Phenolic Content (TPC)
Total Tannin Content (TTC)
Total Flavonoid Content (TFC)
AE (mg GAE / g extract)
ME
(mg GAE / g extract)AE (mg GAE / g extract)
ME (mg GAE / g extract)
AE (mg RE / 1 g)
ME (mg RE / 1 g)
1.
Pleurotus citrinopileatus
21.23 ± 0.13
26.19 ± 0.10
79.12 ± 0.12
69.79 ± 0.05
21.25 ± 0.14
22.75 ± 0.36
2.
Pleurotus cystidiosus
37.32 ± 0.12
47.58 ± 0.13
18.33 ± 0.10
72.14 ± 0.17
19.06 ± 0.63
30.45 ± 0.39
3.
Pleurotus eous
46.59 ± 0.11
66.49 ± 0.07
59.43 ± 0.08
69.13 ± 0.11
51.35 ± 0.25
62.45 ± 0.12
4.
Pleurotus eryngii
37.91 ± 0.19
55.32 ± 0.21
59.41 ± 0.14
78.12 ± 0.61
21.69 ± 0.21
32.75 ± 0.40
5.
Pleurotus flabellatus
44.92 ± 0.16
56.25 ± 0.21
48.32 ± 0.11
57.30 ± 0.19
14.15 ± 0.32
25.91 ± 0.39
6.
Pleurotus florida
84.10 ± 0.17
91.29 ± 0.46
41.31 ± 0.15
90.91 ± 0.13
49.65 ± 0.23
70.71 ± 0.35
7.
Pleurotus ostreatus
25.92 ± 0.11
48.91 ± 0.16
40.32 ± 0.18
62.27 ± 0.54
22.36 ± 0.26
32.64 ± 0.55
8.
Pleurotus pulmonarius
68.12 ± 0.21
86.61 ± 0.31
61.38 ± 0.19
73.40 ± 0.12
29.25 ± 0.21
40.11 ± 0.01
S. No.
Mushroom fungi Concentration
1000 µg/mlFerric Reducing Antioxidant Power
ABTS Scavenging activity
AE (mmol Fe (II) E / mg extract)
ME (mmol Fe (II) E / mg extract)
AE (µM TEAC/g extract)
ME
(µM TEAC/g extract)
1.
Pleurotus citrinopileatus
298.14 ± 1.00
501.36 ± 1.12
388.12 ± 1.76
560.23 ± 1.72
2.
Pleurotus cystidiosus
368.79 ± 1.98
485.81 ± 1.56
548.92 ± 1.13
619.09 ± 1.00
3.
Pleurotus eous
359.12 ± 1.13
579.56 ± 1.56
762.12 ± 1.01
902.11 ± 1.57
4.
Pleurotus eryngii
218.89 ± 1.65
340.25 ± 1.08
916.87 ± 2.01
976.31 ± 1.22
5.
Pleurotus flabellatus
344.00 ± 1.75
544.80 ± 1.02
417.27 ± 1.20
517.28 ± 1.82
6.
Pleurotus florida
440.95 ± 1.01
654.12 ± 1.20
763.97 ± 1.25
998.56 ± 1.42
7.
Pleurotus ostreatus
200.18 ± 1.21
550.95 ± 1.25
459.58 ± 1.52
659.96 ± 1.13
8.
Pleurotus pulmonarius
390.82 ± 2.32
410.81 ± 1.21
773.11 ± 1.13
983.37 ± 1.22
The eight Pleurotus mushroom fruiting bodies and their acetone and methanolic extracts exhibited metal chelating activity, which is averse to the ferrous ion, as shown in Table 3. As a reference standard, EDTA was used. P. florida methanolic extracts had the highest metal chelating effect against ferrous ions (7.74 mg EDTAE/g extract), while P. flabellatus acetone extracts also had the highest metal chelating effect (6.72 mg EDTAE/g extract). On ferrous ions, several basidiomycetes’ mushrooms showed a significantly higher chelating action. EDTAE: Ethylene diamine tetra acetic acid equivalent. Values are means of three independent analysis ± SEM (n = 3) / AAE: Ascorbic acid equivalent. BHT: 90.37 ± 1.15; Rutin: 61.18 ± 1.90 (For Phosphomolybdenum assay).
S. No.
Mushroom fungi Concentration
1000 µg/mlMetal Chelating Activity
Phosphomolybdenum Assay
AE (mg EDTAE / g extract)
ME
(mg EDTAE / g extract)AE (mg AAE / mg extract)
ME
(mg AAE / mg extract)
1.
Pleurotus citrinopileatus
5.52 ± 0.001
7.68 ± 0.003
6.48 ± 0.001
11.76 ± 0.005
2.
Pleurotus cystidiosus
5.12 ± 0.003
6.21 ± 0.003
3.46 ± 0.002
9.96 ± 0.001
3.
Pleurotus eous
3.18 ± 0.002
6.87 ± 0.001
9.13 ± 0.003
11.48 ± 0.004
4.
Pleurotus eryngii
5.45 ± 0.002
6.50 ± 0.004
5.11 ± 0.004
9.16 ± 0.003
5.
Pleurotus flabellatus
6.72 ± 0.01
7.12 ± 0.005
4.72 ± 0.001
15.34 ± 0.002
6.
Pleurotus florida
4.54 ± 0.004
7.74 ± 0.002
5.30 ± 0.001
14.99 ± 0.005
7.
Pleurotus ostreatus
5.46 ± 0.001
6.00 ± 0.002
5.12 ± 0.002
6.08 ± 0.003
8.
Pleurotus pulmonarius
5.80 ± 0.003
6.60 ± 0.01
6.48 ± 0.004
8.80 ± 0.002
The phosphomolybdenum assay was used in this current investigation to assess the total antioxidant capacity of various basidiomycetes mushroom fungi. By this technique, Mo (IV) is reduced to Mo (V), forming Mo (V) green phosphate compounds with absorbance maxima at 695 nm. P. flabellatus methanolic extracts had the highest activity (14.99 mg AAE/mg extract), and the P. eous acetone extract had the highest potential (9.13 mg AAE/mg extract). (Table 3). The study also found that, even at low concentrations, fruiting body extracts of different mushrooms may scavenge superoxide anion radicals. When tested against rutin and BHT, the methanolic extract of P. florida showed excellent scavenging activity, inhibiting free radicals by 98.4 % at a concentration of 1000 µg/mL (Table 4). BHT: 42.18 ± 1.41; Rutin: 39.65 ± 2.08. Values are means of three independent analysis ± SEM (n = 3).
S. No.
Mushroom fungi Concentration
1000 µg/mlAssay of Superoxide Radical
DPPH scavenging activity
AE (%)
ME (%)
AE (%)
ME (%)
1.
Pleurotus citrinopileatus
49.48 ± 0.28
77.70 ± 0.40
52.62 ± 0.04
68.76 ± 0.18
2.
Pleurotus cystidiosus
69.14 ± 0.32
81.58 ± 0.27
29.36 ± 0.08
67.46 ± 0.04
3.
Pleurotus eous
27.46 ± 0.05
38.26 ± 0.20
60.94 ± 0.01
81.64 ± 0.01
4.
Pleurotus eryngii
37.12 ± 0.17
52.54 ± 0.28
66.48 ± 0.13
87.46 ± 0.16
5.
Pleurotus flabellatus
37.24 ± 0.15
42.24 ± 0.23
27.64 ± 0.01
59.48 ± 0.01
6.
Pleurotus florida
48.84 ± 0.23
98.40 ± 0.40
55.39 ± 0.16
92.60 ± 0.26
7.
Pleurotus ostreatus
42.65 ± 0.05
45.29 ± 0.22
39.00 ± 0.17
65.46 ± 0.13
8.
Pleurotus pulmonarius
72.08 ± 0.35
95.12 ± 0.43
41.06 ± 0.11
63.98 ± 0.05
Moreover, Table 4 showed that P. florida methanolic extracts had the maximum DPPH radical scavenging ability, with a scavenging effect of 92.6% at 1000 μg/ml concentration, while P. eryngii showed an 87.46% scavenging effect. In contrast, the maximum scavenging effect among acetone extracts was observed in P. eryngii with 66.48% scavenging effect. These results suggest that methanolic extracts are more effective in enhancing the scavenging abilities on reactive radicals.
Table 5 displays the scavenging activities of fruiting body extracts on hydrogen peroxide and hydroxyl radicals. The maximum rate of hydrogen peroxide scavenging on P. florida methanolic extracts was 93.60% at 1000 µg/ml, followed by P. pulmonarius with 73.98%. However, P. eous and P. eryngii had lower effects on hydrogen peroxide scavenging, with 69.94% and 66.48%, respectively, in their acetone extracts. The scavenging effect of hydroxyl radical was less effective for all strains tested, with P. florida showing the highest scavenging rate of 13.94% in its methanolic extract, while acetone extracts of P. flabellatus had 13.72% scavenging. Values are means of three independent analysis ± SEM (n = 3).
S. No.
Mushroom fungi Concentration
1000 µg/mlHydrogen Peroxide Scavenging Activity
Hydroxyl radical scavenging activity
AE (%)
ME (%)
AE (%)
ME (%)
1.
Pleurotus citrinopileatus
32.81 ± 0.04
58.76 ± 0.18
11.36 ± 0.001
12.36 ± 0.001
2.
Pleurotus cystidiosus
49.72 ± 0.08
57.46 ± 0.04
10.24 ± 0.002
13.84 ± 0.001
3.
Pleurotus eous
69.94 ± 0.01
71.64 ± 0.11
12.62 ± 0.002
11.42 ± 0.002
4.
Pleurotus eryngii
66.48 ± 0.13
47.46 ± 0.16
11.30 ± 0.002
10.50 ± 0.003
5.
Pleurotus flabellatus
47.64 ± 0.01
69.48 ± 0.01
13.72 ± 0.001
13.12 ± 0.001
6.
Pleurotus florida
35.78 ± 0.16
93.60 ± 0.26
11.44 ± 0.003
13.94 ± 0.001
7.
Pleurotus ostreatus
39.10 ± 0.17
55.46 ± 0.14
11.46 ± 0.001
12.06 ± 0.003
8.
Pleurotus pulmonarius
61.06 ± 0.11
73.98 ± 0.05
13.09 ± 0.001
12.07 ± 0.001
Table 6 presents the results of nitric oxide scavenging by fruiting body extracts. P. florida demonstrated the highest scavenging effect in both acetone and methanolic extracts, with 59.96% and 81.34%, respectively, among the strains tested. Conversely, P. cystidiosus had the lowest activity of 31.24% in its acetone extract. In addition, the methanolic extracts of P. florida exhibited a strong β-carotene antioxidant effect (98.14%), whereas the acetone extracts of P. pulmonarius showed the highest scavenging rate of 82.08%. Values are means of three independent analysis ± SEM (n = 3).
S. No.
Mushroom fungi Concentration
1000 µg/mlNitric oxide scavenging activity
β-carotene/linoleic acid antioxidant activity
AE (%)
ME (%)
AE (%)
ME (%)
1.
Pleurotus citrinopileatus
74.10 ± 0.14
45.12 ± 0.28
59.48 ± 0.22
87.70 ± 0.46
2.
Pleurotus cystidiosus
31.24 ± 0.76
52.46 ± 0.41
79.14 ± 0.32
91.92 ± 0.33
3.
Pleurotus eous
43.44 ± 0.35
74.92 ± 0.01
47.46 ± 0.05
58.26 ± 0.20
4.
Pleurotus eryngii
33.60 ± 0.22
64.18 ± 0.65
47.12 ± 0.17
72.58 ± 0.28
5.
Pleurotus flabellatus
55.56 ± 0.62
37.18 ± 0.25
37.24 ± 0.15
62.24 ± 0.23
6.
Pleurotus florida
59.96 ± 0.45
81.34 ± 0.15
68.84 ± 0.23
98.14 ± 0.40
7.
Pleurotus ostreatus
44.46 ± 0.12
54.34 ± 0.60
53.30 ± 0.05
75.58 ± 0.22
8.
Pleurotus pulmonarius
51.24 ± 0.28
32.46 ± 0.01
82.08 ± 0.35
81.18 ± 0.43
The percentage of α-amylase inhibition and their IC50 values are depicted in Table 7 and Fig. 1 respectively. According to Table 7, as sample concentrations increased, the percentage of all fruiting body extracts with α-amylase inhibitory actions increased. The highest level of inhibition, 99.02%, was achieved by P. florida methanolic extracts at 1000 g/ml concentration, followed by P. citrinopileatus methanolic extracts with 98.74%. At the same concentration, Acarbose exhibited 96.54% inhibition. These findings suggest that the methanolic extracts of P. florida may contain bioactive compounds with potential as efficient antioxidant and antidiabetic agents in in vitro conditions, as shown by the fruiting body extracts used in the current study. (Results are mean ± SE of three replicates).
Species
Samples
Invitro Porcine pancreatic amylase activity
(% Inhibition)
Concentration (µg/ml)
50
250
500
1000
P. citrinopileatus
AE
79.52 ± 1.43
83.28 ± 1.12
85.17 ± 1.12
90.16 ± 1.22
ME
89.12 ± 1.07
93.96 ± 0.62
95.15 ± 0.76
98.74 ± 0.82
P. cystidiosus
AE
90.48 ± 1.25
87.78 ± 1.16
95.96 ± 1.26
97.42 ± 0.56
ME
75.49 ± 0.33
77.19 ± 1.20
85.74 ± 1.41
87.22 ± 1.11
P. eous
AE
87.48 ± 0318
91.16 ± 1.02
93.28 ± 1.07
96.13 ± 1.09
ME
85.54 ± 1.06
88.70 ± 1.70
93.46 ± 1.09
97.46 ± 1.90
P. eryngii
AE
73.84 ± 0.67
76.46 ± 0.78
80.17 ± 0.55
82.13 ± 1.48
ME
83.48 ± 1.43
86.70 ± 1.09
90.87 ± 1.06
93.48 ± 1.19
P. flabellatus
AE
78.36 ± 2.19
79.14 ± 2.14
91.85 ± 2.14
93.56 ± 2.11
ME
83.16 ± 1.05
84.12 ± 1.14
88.30 ± 1.10
93.96 ± 1.11
P. florida
AE
88.20 ± 1.23
89.90 ± 2.14
90.26 ± 2.89
94.62 ± 1.38
ME
85.16 ± 1.21
89.08 ± 0.81
95.24 ± 0.65
99.02 ± 0.70
P. ostreatus
AE
75.30 ± 2.08
79.52 ± 2.10
76.34 ± 2.10
77.43 ± 2.13
ME
80.10 ± 0.53
83.50 ± 0.29
86.72 ± 0.84
91.00 ± 1.16
P. pulmonarius
AE
90.08 ± 1.23
92.10 ± 2.32
92.09 ± 2.12
95.74 ± 2.11
ME
83.16 ± 1.06
89.01 ± 1.06
92.16 ± 1.11
97.87 ± 1.12
Acarbose
32.73 ± 1.48
63.16 ± 1.82
73.32 ± 2.64
96.54 ± 1.73
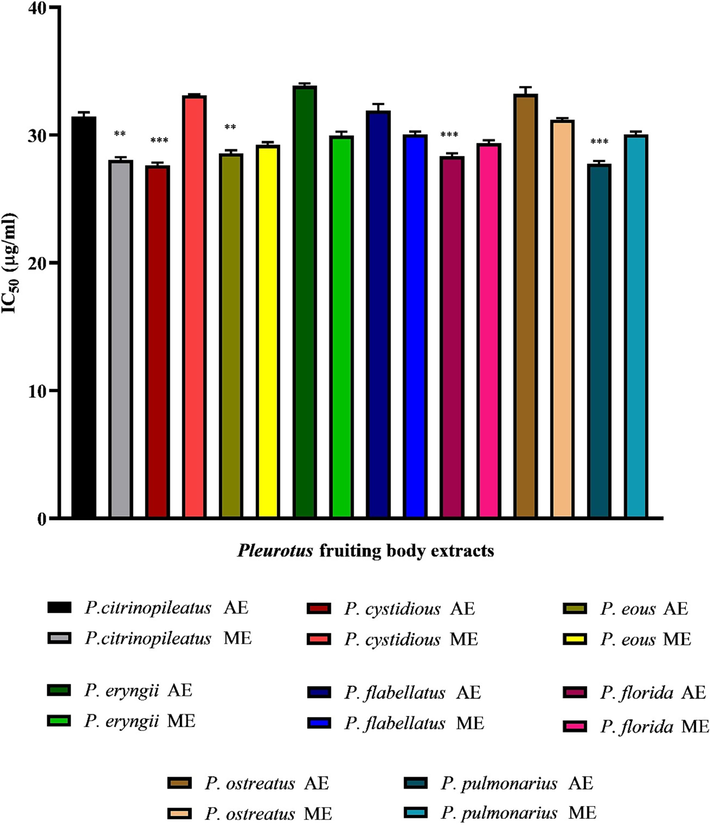
IC50 values of different fruiting body extracts of basidiomycetes mushroom fungi on invitro α-amylase inhibitory activity.
4 Discussion
For thousands of years, humans have consumed mushrooms as part of their diet; but, in recent years, their consumption has significantly risen, with a wide variety of mushrooms being consumed. They are highly important, beneficial foods that are excellent sources of vitamins, minerals, act as health foods, have less calories and fat, and have more vegetable protein. Mushrooms are a significant source of vitamin D for vegetarians because they are the only non-animal source of vitamin D. Several medical benefits of mushrooms have been reported, including lowering blood cholesterol, preventing or treating heart disease, lowering blood sugar, and preventing or treating illnesses brought on by different pathogens. In addition, they have demonstrated anti-tumor, cholesterol-lowering, antiviral, antithrombotic, and immune-modulating activities (Borchers et al., 1999).
Despite being neither an animal nor a plant, mushrooms are classified as a vegetable by the US Department of Agriculture in their categories of food because they share many of the same nutritional characteristics as plant-based meals. Instead, they belong to the Fungi kingdom. Phytoestrogens, phytosterols, glucosinolates, limonoids, isoflavonoids, polyphenols, flavonoids, carotenoids, anthocyanidins, and terpenoids are a few examples of phytochemicals, which are bioactive substances found in numerous foods, particularly mushrooms (Illuri et al., 2021). These substances play critical roles in overall health promotion and maintenance, as well as in the prevention and treatment of certain physiological conditions. Free radicals such as reactive nitrogen species and reactive oxygen species can impair proteins, lipids, and DNA, all of which have been linked to early ageing and a variety of diseases. They are produced either by the body's own internal mechanisms or by the response of tissues to various pathophysiological conditions or physicochemical situations (Prema et al., 2022).
Among the eight mushrooms investigated in this research, the TPC ranged between 21.23 and 86.61 mg GAE/g extract. Compared to acetone extracts, which had 84.10 mg GAE/g extract, the methanolic extract of P.florida had 91.29 mg GAE/g extract, which was a high TPC level. Important mycochemical constituents such as tannins and flavonoids are present in mushrooms and function as antioxidants by neutralizing free radicals, preventing disease, and encouraging the production of antibodies. The P. florida methanolic extract in this study had the highest concentrations of flavonoids (70.71 mg RE/g extract) and tannins (90.91 mg GAE/g extract).
Normally, the cellular metabolism of the human body produces ROS and free radicals continuously. Endogenous enzymatic and non-enzymatic defense mechanisms typically remove these radicals and species. During some conditions, such as smoking, air pollution, drug use, inflammation, and irradiation, endogenous antioxidant systems can become overactive, which leads to oxidative stress and a variety of conditions, including atherosclerosis, ageing, and also cancer (Khanday et al., 2019). By offering protons and/or electrons to free radicals, antioxidants can counteract them directly. They can also inhibit radicals indirectly by restricting the endogenous oxidases activity, promoting antioxidant enzyme activity (for example, by activating the Keap1-Nrf2 signalling pathway), and chelating metal ions that involve formation of radicals (Tonolo et al., 2020). The consumption of dietary antioxidants is inversely connected to the risk of developing oxidative stress-related diseases. The discovery of antioxidants in different food-based sources, such as mushrooms, is gaining interest.
Vitamins C, E, phenols, peptides, ergosterol, polysaccharides, proteins, carotenoids, and many others are considered antioxidant varieties and can all be found in mushrooms. Mushrooms can be grown faster than plants, they are a good source of naturally occurring compounds with biological activity that are used in industry (Chandra et al., 2020).
Phenolic compounds are a key type of phytochemicals found in mushrooms that act as powerful antioxidants. More studies have investigated the TPC and the antioxidant potential of mushrooms. Several mushrooms, including Pleurotus, Armillaria, Agaricus, Auricularia, Lentinula, and Fistulina, have significant amounts of phenolic acids that were evaluated. The benefits of these mushrooms were found to be largely connected to their antioxidant activities, since phenolic compounds can take part in redox reactions as well as operate as hydrogen atom donors or reductants. To maintain their physiological functions, mushrooms produce and store a variety of secondary metabolites, which carry out metabolic processes. Phenolic chemicals are a marker of a great source of antioxidants and synergists that are non-mutagenic. Antioxidant substances protect against oxidative harm brought on by ageing and illnesses like cancer, diabetes, atherosclerosis, and cirrhosis (Margret et al., 2022).
The compound's potential to decrease may be a useful indicator of its significant antioxidant activities. Ascorbic acid is a strong reducing agent that is known to act as an effective antioxidant by acting on free radicals and breaking the chain by moving an atom of hydrogen. This is an example of a substance that has reducing properties.
P. florida had the highest levels of ferric reduction of acetone and methanolic fruiting body extracts, with 440.95 and 654.12 mmol Fe (II) E / mg extract, respectively, and was comparatively more prominent than BHT (91.81) and rutin (216.59). Researchers have found that as the concentration of P. ostreatus extract increases, its reducing power increases (Jayakumar et al., 2009). At 10 mg/ml, the reducing power of Pleurotus citrinopileatus mushroom ethanolic extract was determined to be 1.05.
When ABTS salt reacts with a potent oxidising agent like potassium persulfate, blue-green ABTS•+ is produced. Yet, the chromophore can quickly return to its colourless neutral form when hydrogen-donating antioxidant substances are present. Hence, monitoring the decline in absorbance can be used to assess the effectiveness of an antioxidative molecule that functions as a radical-scavenger. According to the findings, P. florida's methanolic extract had a considerable radical quenching potential, which continuously increased with concentration. According to findings from several authors, ethanol and aqueous extracts have a significant capacity to scavenge ABTS radicals. Working with methanol extracts as well as many other extracts prepared from various mushroom species, (Gargano et al., 2017). It was shown that the concentration of antioxidants used may influence their effectiveness. However, less antioxidants were needed to completely get rid of the ABTS•+ radicals.
It is thought that transition metals stimulate the production of the first radicals. Chelating substances can reduce oxidative damage from free radicals, stabilize transition metals in living things, and stop free radical generation. It was discovered that the polysaccharides of Auricularia auricula had greater Fe-chelating activity than the usual butylated hydroxytoluene activity, depending on the dose. Pleurotus florida methanol extract has the chelating ability that was previously reported. As reported, the extract chelated at a rate of 64% when it was mixed with 500 g/mL. Although the chelating potential varied with concentration, the EC50 was calculated to be 355 g/mL for a similar mushroom species. Yet, at a comparable dose, EDTA is a synthetic metal chelator that showed an exceptional capacity of 81%. In this research, methanolic and acetone extracts of Pleurotus flabellatus and Pleurotus florida showed high chelating potential, with 7.74 and 6.72 mg EDTAE/g of extract, respectively. Similar to this, it has been observed that edible wild mushroom extracts showed more potent ferric ion chelating abilities than those from cultivated mushrooms. Several authors say that the bioactive compounds and good nutrients in both wild and cultivated mushrooms can help prevent and treat a wide range of health problems and diseases. (Alispahić et al., 2015).
The phosphomolybdenum assay is frequently used to evaluate how effective different compounds are as antioxidants. The phosphomolybdenum assay was used in this study to find out how well acetone extract and methanol extract of unknown compounds worked as antioxidants. The results of this study are in line with those of previous studies: as antioxidants, methanol extracts have stronger capabilities than acetone extracts (Sudha et al., 2016).
Active free radical precursors, known as superoxide anions, have the capability to interact with macromolecules and cause damage to the soft tissue. It has been noted that superoxides can occasionally start lipid peroxidation directly. When other reactive oxygen species (ROS) are generated, like hydroxyl radicals, hydrogen peroxide, or singlet oxygen, the superoxide anion plays a crucial role in ROS production. These species have the potential to oxidatively damage lipids, proteins, and DNA (Sánchez 2017). In this study, it was observed that the scavenging effect against superoxide radicals was highest for Pleurotus florida methanolic extracts (98.4%), followed by Pleurotus pulmonarius (95.12%). Acetone extracts of Pleurotus pulmonarius demonstrated a scavenging effect of 72.08% that was more effective than those previously reported (Sánchez 2017).
By preventing lipid oxidation, the DPPH radical test is helpful in estimating antioxidant activity. The amount of free radical scavenging is proportional to the amount of DPPH radical scavenging. The DPPH radical scavenging method is frequently employed since the analysis requires less time. Pleurotus florida acetone extract has a 98.4% activity level compared to the methanolic extract, which has a 92.60% scavenging effect. It has been reported that, at a dose of 2.5 mg/mL, methanolic extracts of Antrodia camphorata have been shown to scavenge between 96.3% and 99.1% of free radicals. 6.4 mg/ml of an extract of the fungus Dictyophora indusiata made with methanol was able to scavenge 92.1% of the DPPH radicals, while other mushroom extracts were able to scavenge 63.3–67.8% of the DPPH radicals. Pleurotus ostreatus methanolic extract (6.4 mg/mL) scavenged 81.8% DPPH radicals, whereas extracts of several commercial mushrooms produced scavenging activities ranging from 42.9 to 69.9% (Mocan et al., 2018). Verpa conica observed 75.7% activity, Agaricus bisporus 77.5% activity, Pleurotus ostreatus 81.3% activity, and Boletus badius 68.7% scavenging activity. Verpa conica showed a notable increase in activity.
One common metabolite formed when superoxide is reduced is hydrogen peroxide (H2O2). Since hydrogen peroxide can quickly be transformed into a dangerous hydroxyl radical, excess H2O2 is cytotoxic. It is found polyphenols, which are found naturally in mushrooms, are responsible for their antioxidant effects. In the current investigation, methanolic extracts of Pleurotus florida and Pleurotus pulmonarius were discovered to have the most effective H2O2 scavenging activity, which is consistent with the previous findings (Vamanu 2012).
Important oxidizing species, hydroxyl free radicals (OH), can target and oxidize molecules in their immediate environment in an endeavor to balance the arrangement of the electrons that are unpaired in their molecule. The reaction between hydroxyl radicals and oxidative systems is violent due to their strong oxidizing power (Herraiz and Galisteo 2015). Since it has the potential to inflict serious harm to nearby biomolecules, the most reactive ROS is the hydroxyl radical. Our result showed the methanolic extracts of Pleurotus florida had the highest hydroxyl radical scavenging effect with 13.94%, which was consistent with previous findings that methanol and ethyl acetate extracts of Pleurotus florida demonstrated robust hydroxyl radical scavenging activities.
Negative health effects from exposure to nitrite/nitrate radicals include headaches, nausea, rapid heartbeat, and stomach pain. Methanolic extract of Pleurotus florida showed the maximum scavenging activity against NO (81.34%), whereas the lowest activity was found in Pleurotus cystidiosus (31.24%). The study reported that the methanol-prepared Pleurotus florida extract successfully prevented sodium nitroprusside from producing nitric oxide confirmed our findings. The mushroom extract's high affinity for oxygen prevented nitrite from forming. As a result, for direct nitric oxide reactions, it competed with oxygen and prevented their formation. The production of nitric oxide has a strong association with inflammatory diseases like atherosclerosis and vascular disease and their immunopathogenesis. The common mushrooms, which are all edible, and their methanolic extracts inhibit COX-2 and induce the expression of nitric oxide synthase (iNOS) to restrict nitric oxide synthesis (Baskaran et al., 2017).
Carotenoids have antioxidant potential because of the radical adducts they form when linoleic acid free radicals react with them. Highly unsaturated β -carotene models are subject to free radical damage from linoleic acid. Mushrooms contain more antioxidants, and some of them get rid of the linoleate free radical and other free radicals in the body, which makes β -carotene less likely to bleach. The results supported the previous findings that the presence of linoleic acid and β-carotene in the examined basidiomycetes mushrooms was responsible for their enhanced antioxidant action. Carotenoids presence demonstrates a reduction in the free radical concentration present as well as the Fe3+ to Fe2+ conversion by carotenoids. It is likely that the anti-oxidative components in the mushroom extract lessen the degree to which β -carotene is degraded during the process, where linoleate free radicals and other scavenged free radicals are generated (Yoon et al., 2011).
Starch digestion is done by a number of enzymes, but β-glucosidase and α-amylase are being looked into as possible treatments for diabetes (Deveci et al., 2021). One approach to treating type 2 diabetes involves restricting the enzymes α-glucosidase and α-amylase to delay glucose absorption and reduce postprandial hyperglycemia. In this study, eight edible basidiomycetes mushrooms were tested for their inhibitory properties on α-amylase enzymes. The study found that this inhibitory effect is based on dose dependence, with the maximum inhibitory activity for α-amylase at 99.02 percent shown by P. florida's methanolic extracts, while the acetone extract of Pleurotus cystidiosus had excellent inhibitory activity for α-amylase. The β-amylase inhibitory activity of methanol extracts from seven different mushroom species was much higher than that of acetone extracts. Bioactive compounds such as polysaccharides, phenolics, triterpenes, and proteins extracted from mushrooms are used as anti-diabetic therapies (Wińska et al., 2019). As a result, the various bioactive chemicals found in methanol extracts may be responsible for their high α-amylase inhibitory action.
5 Conclusions
Mushrooms encompass an array of bioactive components such as polysaccharides, glycosides, phenols, flavonoids, phenolics, tocopherols, ascorbic acid, and organic acids, each carrying immunological and inhibitory attributes. These compounds' antioxidant characteristics are integral for neutralizing free radicals within the body. This study aimed to assess the antioxidant activity and α-amylase inhibition effects present in acetone and methanolic extracts of eight different edible basidiomycetes mushroom fungi. The outcomes highlighted that, in comparison to acarbose, the most elevated antioxidant and α-amylase inhibitory traits were exhibited by the methanolic extracts of Pleurotus florida. It became evident that the choice of extraction solvent and the specific mushroom type exert substantial influence over the antioxidative potentials and α-amylase inhibitory functions. These findings contribute noteworthy insights into the pharmacological prowess of the mushrooms under scrutiny. Moreover, they underscore the potential utility of methanolic extracts, particularly those derived from Pleurotus florida, as an organic source for antioxidants, as well as dietary agents to counter diabetes, and even as potential compounds for the pharmaceutical industry via targeted enzyme inhibition.
Acknowledgments
The authors wish to express their gratitude to the management of MGR College in Hosur, Tamilnadu, India, for their assistance in conducting the research. The authors extend their appreciation to Researchers Supporting Project number (RSPD2023R1095), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Phenolic content and antioxidant activity of mushroom extracts from Bosnian market. Bull. Chem. Technol. Bosnia Herzegovina. 2015;44(2):5-8.
- [Google Scholar]
- Evaluation of antidiabetic activity of Pleurotus pulmonarius against streptozotocin-nicotinamide induced diabetic wistar albino rats. Saudi J. Biol. Sci.. 2020;27(3):913-924.
- [Google Scholar]
- The evaluation of nitric oxide scavenging activity of Acalypha indica Linn root. Asian J. Res. Chem.. 2009;2(2):148-150.
- [Google Scholar]
- Pleurotus giganteus (Berk. Karun & Hyde), the giant oyster mushroom inhibits NO production in LPS/H2O2 stimulated RAW 264.7 cells via STAT 3 and COX-2 pathways. BMC Complement. Altern. Med.. 2017;17(1):1-10.
- [Google Scholar]
- Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal. Biochem.. 1971;44(1):276-287.
- [Google Scholar]
- Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199-1200.
- [Google Scholar]
- Antioxidant compounds from microbial sources: a review. Food Res. Int.. 2020;129:108849
- [Google Scholar]
- Edible mushrooms as functional ingredients for development of healthier and more sustainable muscle foods: a flexitarian approach. Molecules. 2021;26(9):2463.
- [Google Scholar]
- Inhibitory activities of medicinal mushrooms on α-amylase and α-glucosidase-enzymes related to type 2 diabetes. S. Afr. J. Bot.. 2021;137:19-23.
- [Google Scholar]
- Antioxidant activity and total phenolic content of medicinal plants associated with prevention and treatment of cardiovascular and cerebrovascular diseases. J. Med. Plants Res.. 2010;4(22):2438-2444.
- [Google Scholar]
- Medicinal mushrooms: valuable biological resources of high exploitation potential. Plant Biosyst. – Int. J. Deal. Aspects Plant Biol.. 2017;151(3):548-565.
- [Google Scholar]
- Hydroxyl radical reactions and the radical scavenging activity of β-carboline alkaloids. Food Chem.. 2015;172:640-649.
- [Google Scholar]
- Production, partial purification and characterization of ligninolytic enzymes from selected basidiomycetes mushroom fungi. Saudi J. Biol. Sci.. 2021;28(12):7207-7218.
- [Google Scholar]
- Bio-prospective potential of Pleurotus djamor and Pleurotus florida mycelial extracts towards Gram positive and Gram negative microbial pathogens causing infectious disease. J. Infect. Public Health. 2022;15(2):297-306.
- [Google Scholar]
- Assessment of antioxidant potential, total phenolics and flavonoids of different solvent fractions of Monotheca buxifolia fruit. Osong Public Health Res. Perspect.. 2013;4(5):246-254.
- [Google Scholar]
- In-vitro antioxidant activities of an ethanolic extract of the oyster mushroom, Pleurotus ostreatus. Innov. Food Sci. Emerg. Technol.. 2009;10(2):228-234.
- [Google Scholar]
- Antioxidant and cytotoxic potential of leaf extracts of Costus igneus. J. Nat. Sci. Biol. Med.. 2019;10(2):157-166.
- [Google Scholar]
- Pleurotus eryngii improves postprandial glycaemia, hunger and fullness perception, and enhances ghrelin suppression in people with metabolically unhealthy obesity. Pharmacol. Res.. 2022;175:105979
- [Google Scholar]
- Production of formaldehyde during metabolism of dimethyl sulfoxide by hydroxyl radical-generating systems. Biochemistry. 1981;20(21):6006-6012.
- [Google Scholar]
- Evaluation of anti-microbial and anti-haemolytic activity of edible basidiomycetes mushroom fungi. J. Drug Deliv. Therapeut.. 2019;9(1):132-135.
- [Google Scholar]
- Extrapolating mycochemical profile of selected wild mushrooms (Sirumalai hills) with the commercial cultivates and strategizing their antidepressant efficacy. Acta Ecol. Sin.. 2022;42(1):46-55.
- [Google Scholar]
- Protective efficacy of Capsicum frutescens fruits in pancreatic, hepatic and renal cell injury and their attenuation of oxidative stress in diabetic Wistar rats. J. Taibah Univ. Sci.. 2021;15(1):1232-1243.
- [Google Scholar]
- Evaluation of antioxidant, anti-inflammatory, and anti-hyperglycemic effects of Wattakaka volubilis Linn. f. Process Biochem.. 2022;112:183-191.
- [Google Scholar]
- Chemical composition and bioactive properties of the wild mushroom Polyporus squamosus (Huds.) Fr: a study with samples from Romania. Food Funct.. 2018;9(1):160-170.
- [Google Scholar]
- Metal ion chelating activity and hydrogen peroxide scavenging activity of medicinal plant Kalanchoe pinnata. J. Chem. Pharm. Res.. 2012;4(1):197-202.
- [Google Scholar]
- Microbial synthesis of silver nanoparticles using Lactobacillus plantarum for antioxidant, antibacterial activities. Inorg. Chem. Commun.. 2022;136:109139
- [Google Scholar]
- Momilactones A and B are α-amylase and α-glucosidase inhibitors. Molecules. 2019;24(3):482.
- [Google Scholar]
- Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis. 1989;10(6):1003-1008.
- [Google Scholar]
- Reactive oxygen species and antioxidant properties from mushrooms. Synth. Syst. Biotechnol.. 2017;2(1):13-22.
- [Google Scholar]
- Antioxidant activity and inhibitory potential of Cistus salviifolius (L.) and Cistus monspeliensis (L.) aerial parts extracts against key enzymes linked to hyperglycemia. BioMed Res. Int. 2017
- [Google Scholar]
- Studies on antioxidant activities of mucuna seed (Mucuna pruriens var utilis) extract and various non-protein amino/imino acids through in vitro models. J. Sci. Food Agric.. 2003;83(14):1517-1524.
- [Google Scholar]
- The antioxidant activity and free radical-scavenging capacity of dietary phenolic extracts from horse gram (Macrotyloma uniflorum (Lam.) Verdc.) seeds. Food Chem.. 2007;105(3):950-958.
- [Google Scholar]
- Comparative study on the antioxidant activity of methanolic and aqueous extracts from the fruiting bodies of an edible mushroom Pleurotus djamor. Food Sci. Biotechnol.. 2016;25:371-377.
- [Google Scholar]
- Milk-derived bioactive peptides exhibit antioxidant activity through the Keap1-Nrf2 signaling pathway. J. Funct. Foods. 2020;64:103696
- [Google Scholar]
- Determination of antioxidant and antimicrobial properties of Agaricus bisporus from Romanian markets. Ovidius Univ. Ann. Chem.. 2012;23(1):47-52.
- [Google Scholar]
- Mushrooms of the genus Ganoderma used to treat diabetes and insulin resistance. Molecules. 2019;24(22):4075.
- [Google Scholar]
- β-Sitosterol protects against carbon tetrachloride hepatotoxicity but not gentamicin nephrotoxicity in rats via the induction of mitochondrial glutathione redox cycling. Molecules. 2014;19(11):17649-17662.
- [Google Scholar]
- Antioxidant and antityrosinase activities of various extracts from the fruiting bodies of Lentinus lepideus. Molecules. 2011;16(3):2334-2347.
- [Google Scholar]
- The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem.. 1999;64(4):555-559.
- [Google Scholar]







