Exploring the potential: Inhibiting quorum sensing through marine red seaweed extracts – A study on Amphiroa fragilissima
⁎Corresponding authors. prakash@andavancollege.ac.in (Prakash Piruthiviraj), parthasangi@yahoo.com (Rengasamy Parthasarathi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives
A great setback in concern with public health is the development of emerging bacterial resistance towards conventional antibiotics making it a huge issue in the medical sector. To combat bacterial resistance, innovative strategies have been developed. One of the significant strategies is to target the bacterial pathogenicity through inhibition of quorum sensing (QS) mechanism among bacteria. QS is cell density dependent molecular communication prevailing in bacteria trends to express several genes conferring for its virulence, biofilm formation, antibiotic resistance, motility and plant-bacterial interactions etc. Methods: The current study investigates the anti-QS activity of red seaweed, against the targeted test pathogens Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter sp. and E. coli. Results and conclusion: At the concentration of 100 mg/mL of red seaweed extract, the biofilm was effectively reduced in Acinetobacter sp. and E. coli with 36 % and 40 % respectively. The exo polymeric substance (EPS) quantification from test pathogens in the presence of algal extract has declined to 40.8 % in Acinetobacter sp. and 14.8 % in E. coli reduction compared to S. aureus and K. pneumonia. Furthermore, the QS dependent motility activities are also reduced with the effect of algal extract. The efflux pump expression, being the noteworthy factor for antibiotic resistance has been inhibited by algal extract. The bioactive compounds from the algal extract A. fragilissima such as Melamine, Silicic acid, diethyl bis(trimethylsilyl) ester, Trimethyl [4-(1-methyl-1-methoxyethyl) phenoxy] silane, Benzo[h]quinoline, 2,4-dimethyl, Pyrazol-3(2H)-one, 4-nitro and 1,2-Bis(trimethylsilyl)benzene etc. Among them, Melamine showed high peak area and may responsible for the anti-QS activity and helps to overcome antibiotic resistance of the opportunistic pathogens. The QS dependent phenotypic expression among the test pathogens has been interrupted by the bioactive components of A. fragilissima.
Keywords
Quorum sensing
Anti-QS
Antibiofilm activity
Amphiroa fragilissima
Staphylococcus aureus
Klebsiella pneumoniae
Acinetobacter sp.
1 Introduction
The discovery of antibiotics at the turn of 20th century freed all people from a variety of life-threatening illnesses. However, after the mid – twentieth century, overuse of antibiotics have elevated a wide number of issues, especially emergence of antibiotic resistant bacterial strains. The whole medical sector has been intense on resolving the great set back owing to the emerging antibiotic resistant strains. Further, antibiotic resistance has observed to be higher in bacterial biofilm than in their planktonic counterparts. Therefore, it is important to investigate the biofilm formation bacterial populations that are associated to at least 65 % of all major infections. Quorum sensing (QS) is the communication/ signalling system prevailing among the bacterial population which pathogenecity. The expression of signalling molecules varies with gram positive and gram negative bacteria to extinguish their QS system that regulates bacterial behaviour in biofilms along with other virulence production in a cell-dependent manner (Sarkar and Das, 2019). The QS signal concentration rises along with the population, binds to the it’s appropriate receptor and ensure the QS response that takes its upper hand upon motility, biofilm formation, EPS (exopolysaccharide) production (physical barrier of bio film), plant-bacterial interaction and sporulation etc., (Tran and Hadinoto, 2021). Acyl homoserine lactone (AHL) is one among the QS signal molecule that has exhibited by the majority of Gram-negative bacteria. In response to population density, bacteria can express particular genes by using these signalling molecules. The investigation of Kumar et al. (2016) has revealed 2-hydroxy-4-((methylamino)(phenyl)methyl) cyclopentanone as the QS signal molecules in Ralstonia solanacearum enhancing the biofilm formation that has been confirmed by the Confocal laser scanning microscopic analysis.
The test microorganism employed in the present study include Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter sp. and Escherichia coli are antibiotic resistant species and are involved in nosocomial infections. Indeed, microbes causing nosocomial infections with antibiotic resistant characteristic makes the treatment extremely challenging. In fact, the QS mechanism facilitates various gene expressions among the population in a niche resulting in the antibiotic resistance, biofilm formation, sporulation etc. The QS machinery in S. aureus has been denoted by the accessory gene regulator (agr) system constituting of agrACDB cassette (Kannapan et al. 2023). The agr system in S. aureus has found to be active in the presence of a certain threshold extracellular AIP (autoinducing peptide) concentration. Further, the AIP molecules bind with AgrC of AgrC-AgrA complex to phosphorylate AgrA, which in turn induces the promoters (P2 and P3) of agrACDB cassette. Therefore, the expression of the structural genes such as AgrB, AgrD, AgrC, AgrA have accomplished along with the aid of RNAII and RNAIII to promote P2 and P3 respectively (Arunachalam et al., 2023; Vasquez et al., 2017). LuxR/LuxI homologs that are encoded in several gram-negative bacteria exhibit to regulate a variety of biological activities (Srinivasan et al., 2021). But only the LuxR homolog SdiA is encoded in most of the gram negative bacteria including Klebsiella pneumoniae, Escherichia coli, and Salmonella (Ahmed et al., 2021). However, the earlier study of Sun et al. (2021) has insisted on abaI/abaR QS system in Acinetobacter sp. such as A. baumannii and A. nosocomialis regulating its virulence, biofilm formation, antibiotic resistance, energy metabolism, and lipid metabolism. AbaI is the synthase of AHL signal while AbaR encodes for the receptor to bind with the auto-inducers during the activation of abaI/abaR based QS system (Choe et al., 2022). On the other hand, finding innovative approache to treat infections and targeting virulence through QS mechanism has become crucial to resolving the issues (Seleem et al., 2020). However, the inhibition of QS mechanism has accomplished by receptor protein degradation, AIs synthase inhibition and signal degradation (Paluch et al., 2020).
QS inhibitors have been categorized under natural and synthetic inhibitors. Certain natural inhibitors include the secondary metabolites of marine environmental organisms especially microalgae /seaweed, plant leaves, bark, fruits, fungal and bacterial enzymes. Furthermore, in recent years ample of bioactive substances from marine algae has been identified to be a good solution in the hunt for alternative medication to manage the pathogens with QS and multidrug resistance. Interestingly, identification of distinct seaweeds excerting certain biological chemicals with anti-QS property has become the pioneer among the researchers and therefore, bioactive component from macroalgae have been the current trend by numerous researchers to explore anti-QS molecules (Boominathan et al., 2022; Muthukrishnan et al., 2023; Rima et al., 2022; Borges and Simões, 2019).
In the present research study, the marine red algae, Amphiroa fragilissima has employed to reveal the anti-QS activity among the test pathogens. In addition, the expression of efflux pumps in bacteria has also been investigated as it has been a significant factor to confer antibiotic resistance and bacterial biofilm formation. Therefore, the current study has undertaken in the aim to explore the anti-QS molecules in marine red algae, Amphiroa fragiliss inhibiting the virulence and QS dependent biofilm formation.
2 Material and methods
2.1 Collection and authentication of marine red seaweed
The marine red seaweed, Amphiroa fragilissima (100 g) obtained from R.K. Algae project centre, Mandapam, Ramanathapuram, Tamil Nadu, India (shown in Fig. 1) and authenticated in Botanical Survey of India, Coimbatore, India.
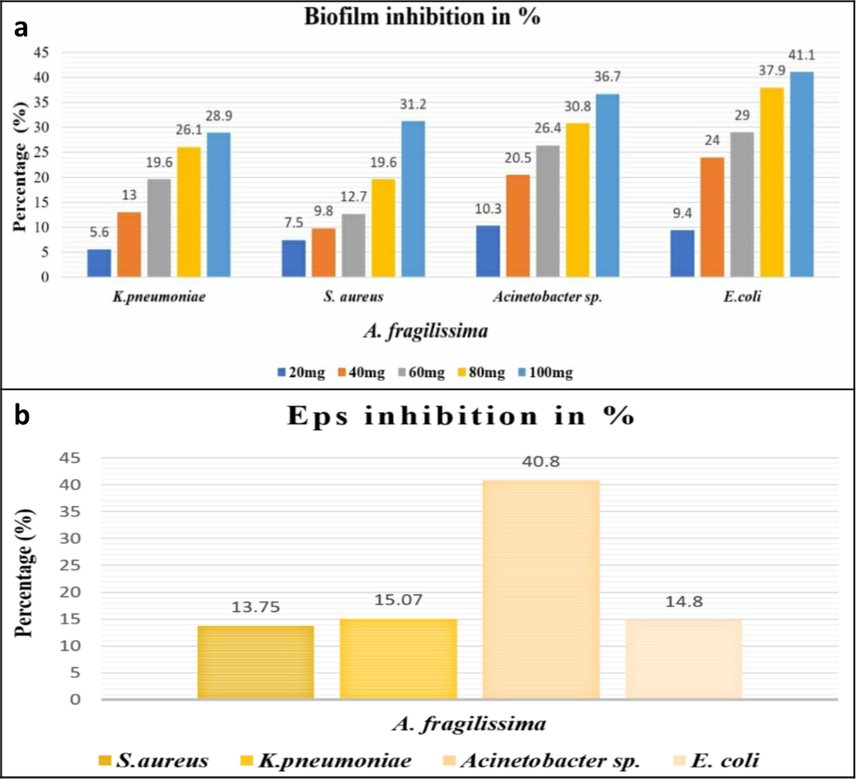
- (a) Percentage of Biofilm inhibition (b) Percentage of EPS inhibition by A. Fragilissima.
2.2 Extraction of red seaweed
The obtained marine seaweed was shade dried and powdered using a sterile mechanical blender aseptically. To 100 mL of sterile distilled water, 20 g of algal powder was added and kept at room temperature with 140 rpm shaking for two days. The mixture was then freeze-dried in a Lyophilizer after being filtered via Whatman no.1 filter paper (Kulshreshtha et al., 2016).
2.3 Bacterial strains and culture conditions
The test pathogens employed in the present study were Staphylococcus aureus strain ATCC 1690, Klebsiella pneumoniae strain ATCC 13883, Acinetobacter sp. strain ATCC 49139, and Escherichia coli strain ATCC 15224 respectively. All bacterial culture work was done from Bishop Heber College in Trichy, Tamil Nadu, India.
2.4 Antibacterial activity
To evaluate the antibacterial efficacy of the algal extract among the test pathogens such as S. aureus, K. pneumoniae, Acinetobacter sp. and E. coli, the overnight test cultures were swabbed on Muller–Hinton agar (MHA) following Mc.Farland standard. The sterile disc was then placed over the medium and to which 20 mg of algal extract was added individually on each plate aspectically. The positive control antibiotics ampicillin was loaded separately. The plates were then incubated for 24 h at 30 °C (Packiavathy et al., 2012) respectively.
2.5 Anti-biofilm activity
The anti-biofilm activity among of algal extracts the test pathogens were investigated using the 96 well containing micro-titre plates at various concentrations (20–100 mg/mL). In the sterile, fresh nutrient broth medium containing algal extract, 2 % of test organisms are inoculated. The Uninnoculated culture broth without algal extract was kept as the control. After 24 h incubation at 37 °C, the media were taken out from the micro-titre plates and rinsed with phosphate-buffered saline (PBS) to eliminate planktonic cells. The wells were then treated with 100 μl of 0.1 % crystal violet for 10 min. The wells were then rinsed with sterile distilled water to remove the excess stain. In order to solubilize the biofilm, 100 μl of 95 % ethanol were added to each well. The biofilm was then quantified using a microplate reader at a wavelength of 570 nm (Al-kafaween et al., 2019). The percentage of biofilm inhibition was calculated by the formula,
2.6 Cover glass based microscopic visualization of biofilm
In the 3 mL of fresh nutrient broth medium in test tubes nutrient broth medium with cover glass (12X24 mm), algal extract (50 mg/mL) was added. To which, 1 % of overnight cultures of the test pathogens were inoculated individually. The mixture was then incubated for 24 h. After incubation, planktonic cells were removed from the cover glasses by washing it with sterile distilled water and finally treated the cover glasses with 0.2 % CV. The stained cover glasses were then air dried and visualized the biofilms under light microscope (Packiavathy et al., 2012).
2.7 Biofilm visualization using tube method
The test pathogens were inoculated into the test tube containing 25 mg of algal extract and 5 mL of nutrient broth. The tubes were then incubated after the addition of 1 % glucose. The control tubes were maintained by inoculating overnight cultures without extract. The tubes were stained by adding 0.1 % crystal violet after being incubated at 37 °C for 24 h. To get rid of excess stains the tubes were rinsed with sterile distilled water, air dried and then manually visualized for biofilms (Freeman et al., 1989).
2.8 EPS quantification
The test pathogens were allowed to develop biofilms on a cover glass (size?) in a 6-well microtiter plate in the presence and absence of algal extract (100 mg/mL). It was incubated at 37 °C for 18 h. Following incubation, the cover glass was treated with 0.9 % NaCl (0.5 mL), 5 % phenol (0.5 mL) along with 5 volumes of concentrated H2SO4 and left in the dark for an hour. Then the absorbance was recorded using UV–VIS spectrophotometer at 490 nm (Favre-Bonté et al., 2003). The percentage of EPS inhibition was calculated using the formula,
2.9 Swimming assay
Solid agar medium containing 25 mg of concentrated algal extracts along with 1 % tryptone and 0.5 % NaCl was prepared. The plate devoid of algal extract was kept as the control. The test pathogens were then point-inoculated at the centre of medium. The plates were incubated for 18 h at 37 °C in an upright position and after incubation the result was recorded (Musthafa et al., 2012).
2.10 Ethidium bromide–agar cartwheel assay
The effect of algal extract upon the efflux pump of the test pathogens were assessed by employing Ethidium bromide (EtBr) agar cartwheel assay. The presence of efflux pump was detected through the accumulation of EtBr inside the cells resulting in the elevation of fluorescence. For which, 2 mg/mL of EtBr was added to MH agar plates along with 25 mg of algal extract. The test was performed both in the presence and absence of algal extract. Further, the test bacterial cultures were swabbed onto agar plates like a cartwheel. The plates were evaluated with a UV transilluminator after 16 h of incubation at 37 °C to detect EtBr concentration within the test pathogens as an indication of efflux pump (Eleftheriadou et al., 2021).
2.11 Identification of anti-QS compounds by GC − MS and FTIR analysis
GC–MS (gas chromatography-mass spectrometry) analysis for aqueous extract of A. fragilissima was performed at TUV SUD South Asia Pvt. Ltd, Tirupur, Tamil Nadu to identify the bio- active compounds present in the algal extracts. Along with GC–MS, Fourier transform infrared spectroscopy (FTIR) was also used to identify the structure and functional groups of those compounds at St. Joseph College, Trichy.
3 Results
3.1 Authentication of marine red seaweed
The red seaweed has been identified as Amphiroa fragilissima (L.) J.V. Lamour-LITHOPHYLLACEAE (BSI/SRC/5/23/2022/Tech/468) by Dr. S.S. Hameed, The Scientist- E & Head of Office, Botanical Survey of India, TNAU Campus, Coimbatore- 641003.
3.2 Extraction yield
The Amphiroa fragilissima has yielded of 8 % of aqueous extract which has been quantified in terms of percentage by using the following formula adapting Bhuyar et al. (2020).
3.3 Anti-bacterial activity
While examining the algal extract for its antibacterial activity against test pathogens, none of the four test strains of S. aureus, K. pneumoniae, Acinetobacter sp., and E. coli showed any activity. Since then, microbes including S. aureus and K. pneumoniae have become resistant to the antibiotics like ampicillin. At a concentration of 20 mg/mL of algal extract, the zone of inhibition for ampicillin and algal extract has shown as 10 mm in S. aureus, 16 mm in Acinetobacter sp., and 14 mm in E. coli respectively.
3.4 Anti-biofilm activity
The marine seaweed A. fragilissima has exhibited a well profound anti-biofilm activity at distinct concentration ranging from 20 to 100 mg/mL. The biofilm formation among the test pathogens have been much reduced when treated with A. fragilissima extract. The percentage of biofilm inhibition by the algal extract has been visualized in Fig. 1.a.
3.5 Quantification of EPS
The EPS production of the test pathogens have declined to an appreciable level owing to the effect of aqueous extract of A. fragilissima comparing with the control. The algal extract at 100 mg/mL has reduced the EPS synthesis upto 40.8 %, 14.8 %, 15 %, and 13 % in Acinetobacter sp., E. coli, K. pneumoniae, and S. aureus, respectively (Fig. 1.b).
3.6 Biofilm visualization using tube method
Biofilm formation has been visualized from the naked CV stained thick film lining appearance at the test tube wall. The algal extract has remarkable effect to express the anti-biofilm activity by inhibit the biofilm formation among all the test pathogens while comparing with the control.
3.7 Light microscopic analysis of biofilm
Though the biofilm formation is reduced in all test pathogens by the effect of algal extract, Acinetobacter sp. biofilm formation has been greatly declined (depicted in Fig. 2) comparatively and it has been visualized with the aid of light microscope.
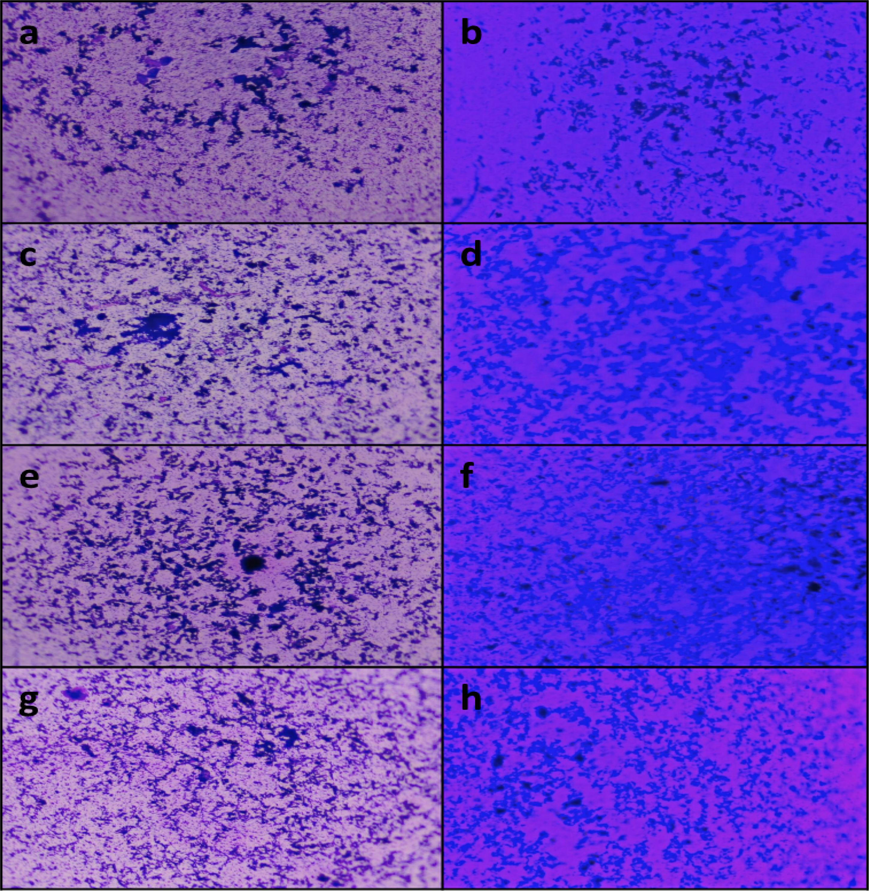
- Biofilm visualization under light microscope – (a, c, e, g) control of Acinetobacter sp., E. coli, K. pneumoniae and S. aureus respectively; (b, d, f, h) treated with A. fragilissima extract.
3.8 Swimming assay
In the presence of algal extract at a concentration of 25 mg/mL, the swimming capacity of the test pathogens has been reduced promisingly except K. pneumonia. As because, the control plate has exhibited the non-motile nature of the strain K. pneumonia (Fig. 3.a and b.). Other strains such as Acinetobacter sp., S. aureus, and E. coli treated with the algal extracts has exhibited the greatest suppression in QS dependent swimming migration (Fig. 3.c and d.).
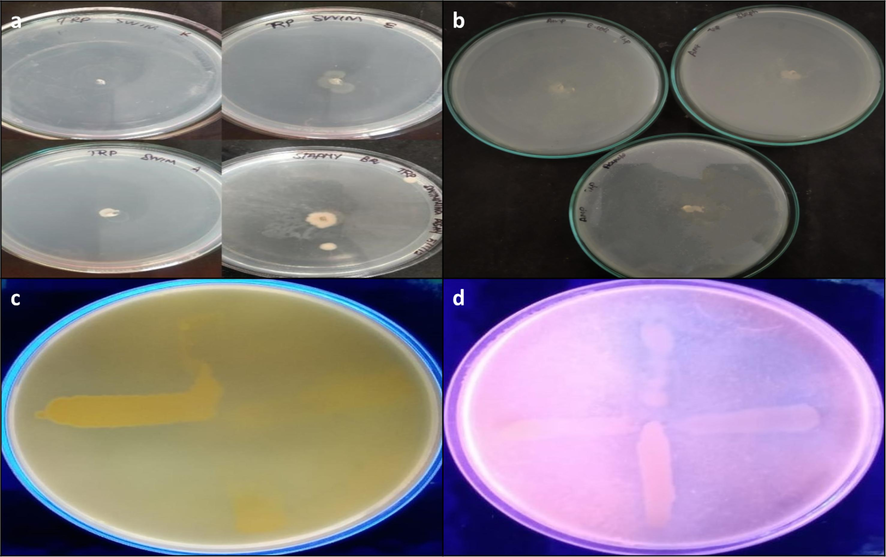
- Swimming assay (a) control (b) treated with A. fragilissima; Efflux pump inhibition assay (c) control (d) Treated with A. fragilissima.
3.9 Ethidium bromide-agar cartwheel assay
The efflux pump inhibitory activity of seaweed extracts (25 mg/mL) against the test pathogens has been identified from the cart wheel test in MHA agar plates containing 2 mg/mL of EtBr. Interestingly, the A. fragilissima extract has disrupted the active efflux pumps in E. coli, K. pneumoniae, and S. aureus.
3.10 Identification of bioactive compounds
The aqueous extract of A. fragilissima has been investigated employing FTIR (Fig. 4) and GC–MS analysis (Fig. 5). The FTIR has shown the presence of amides, alkanes, phosphines, alkyls, carboxylic compounds, ether, alcohols, aromatic compounds and alkyl halides functional groups. The presence of functional groups is of great importance in the prepared compounds. One such functional group is the carboxyl group, which is indicated by a peak at 3778 and 3424 cm-1.FTIR analysis confirmed the presence of amine and amide as functional group the peak at 2918 and 2854 cm−1. The peak at 1610, 1549, and 1425 cm−1 indicated the presence of amino acid, nitro compound, and aromatic functional group, respectively. The peak at 1385, 1319, and 1222 cm−1 confirms the presence of alkane, aldehyde, fluoride, nitro, and alkyl halide group, respectively. The remaining peak at 1093, 861, and 601 cm−1 indicated the presence of alcohol, aliphatic amines, aromatic compound, primary or secondary amines, and halogen group, respectively. The GC–MS interpretation was tabulated in Table 1.
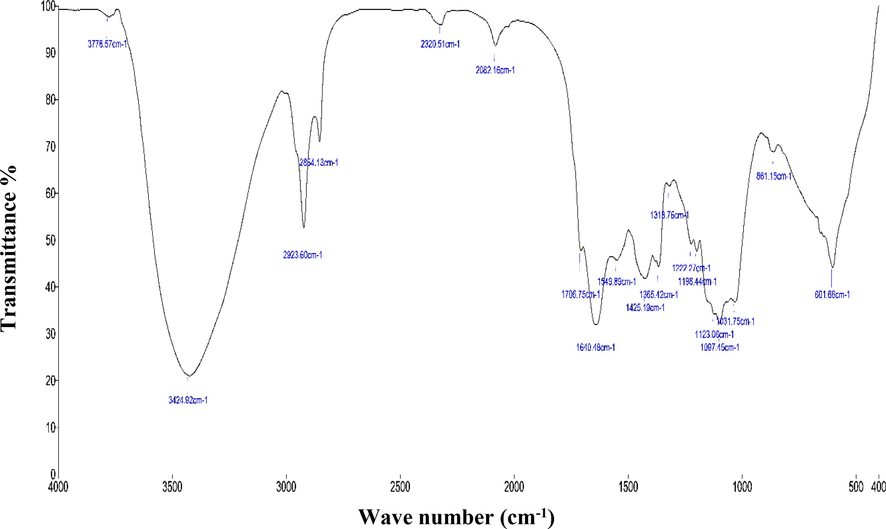
- FTIR analysis - Aqueous extract of A. fragilissima.
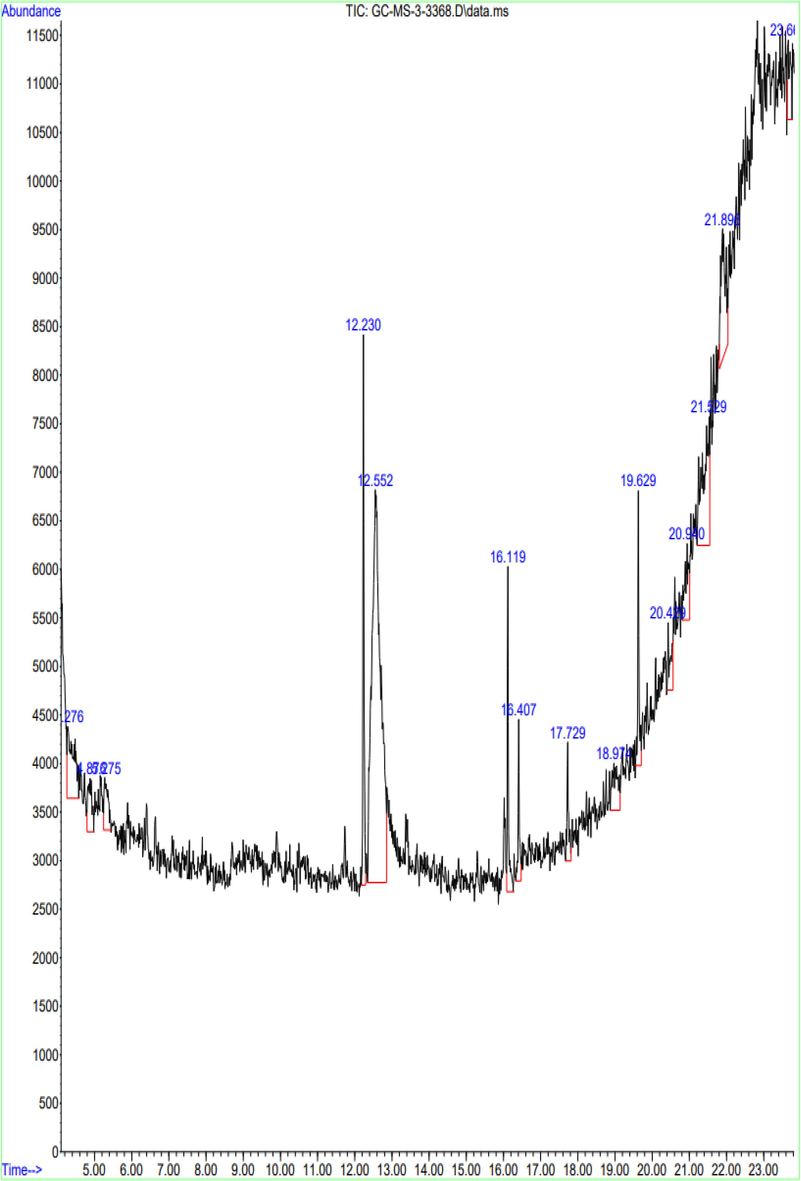
- GC MS analysis of A. Fragilissima.
| Peak no | Retention time | Peak area | Peak % | Compounds |
|---|---|---|---|---|
| 1 | 4.276 | 8876 | 5.47 | Benzo[h]quinoline, 2,4-dimethyl |
| 2 | 4.876 | 4021 | 2.48 | 6,7-Dimethoxy-3-(4-trifluoromethoxy-phenylamino)-3H-isobenzofuran-1 |
| 3 | 5.275 | 3840 | 2.36 | 1,2-Benzenediol, 3,5-bis(1,1-dimethylethyl) |
| 4 | 12.230 | 11,475 | 7.07 | Pentadecanoic acid, 14-methyl-, methyl ester |
| 5 | 12.552 | 66,997 | 41.26 | 1,3,5-Triazine-2,4,6-triamine |
| 6 | 16.119 | 7115 | 4.38 | Pyrazol-3(2H)-one, 4-nitro- |
| 7 | 16.407 | 3810 | 2.35 | 4-Quinolinecarboxylic acid, 2-chloro |
| 8 | 17.729 | 3611 | 2.22 | 1,2-Bis(trimethylsilyl)benzene |
| 9 | 18.974 | 5192 | 3.20 | Silicic acid, diethyl bis(trimethylsilyl) ester |
| 10 | 19.629 | 6300 | 3.88 | Trimethyl[4-(1-methyl-1-methoxyethyl)phenoxy]silane |
| 11 | 20.429 | 3637 | 2.24 | Silane, trimethyl[5-methyl-2-(1-methylethyl)phenoxy]- |
| 12 | 20.940 | 5382 | 3.31 | 1,2-Bis(trimethylsilyl)benzene |
| 13 | 21.529 | 15,730 | 9.69 | Silicic acid, diethyl bis(trimethylsilyl) ester |
| 14 | 21.896 | 12,014 | 7.40 | Trimethyl[4-(1-methyl-1-methoxyethyl)phenoxy]silane |
| 15 | 23.662 | 4387 | 2.70 | Silane, trimethyl[5-methyl-2-(1-methylethyl)phenoxy]- |
4 Discussion
The QSI potential of the marine red seaweed, Amphiroa fragilissima has publicized in the present investigation for the first time against the QS dependent phenotypic expressions in the test pathogens such as E. coli, Acinetobacter sp., K. pneumoniae, and S. aureus. Though initially, the seaweed extract has employed for detecting its antibacterial activity, based on the result with no appreciable effect over the growth of the test pathogens, the alternative strategy has been pursued in the present study. In fact, even before checking out the tactics of combined action of algal extract with the antibiotic ampicillin, precise action of ampicillin towards the individual test pathogens has been evaluated. Though the test pathogens such as K. pneumoniae, and S. aureus has shown resistance towards ampicillin, while combining with algal extract with the antibiotic ampicillin has interestingly exhibited a tremendous activity of anti-biofilm and anti-QS effect. It may be due to the reason that certain bioactive compounds or phytochemicals from various sources exerts its anti-QS activity against the microbial population in a niche by affecting its cellular communication system (QS system) rather directly killing the pathogens. This view has been in acceptance with the earlier reports of Truchado et al., (2009); Vattem et al., (2007) who has insisted that those bioactive components does not involve in the evolution of resistant strains and has suggested that those bioactive components can express its anti-QS effect without even exhibiting its lethal effect upon pathogens respectively. In a similar way, the finding of Packiavathy et al. (2012) has revealed that Cuminum cyminum did not exhibit anti-bacterial activity but has suppressed QS mechanism.
However, there has been the evidence (Tang et al., 2020) of certain components like phlorotannins from the marine algae like Hizikia fusiforme unveiling its efficacy to inhibit QS system to combat pathogenic bacterial species. In which, the antibacterial action of phlorotannins against specific gram-positive and gram-negative bacteria has been demonstrated by insisting that its mode of action would not be specific like that of antibiotics. Interestingly, the seaweed compounds utilised in this investigation interferes with cell–cell communication and is ineffective against bacterial growth when examined individually. Therefore, this present study has concentrated more on anti-QS action of the seaweed Amphiroa fragilissima. Moreover, the QS mechanism highly influences the biofilm formation, EPS production and efflux pumps in bacteria. On this note, the present study has undertaken afore said QS related phenotypic expressions, which has also been conferring for its virulence. The antibiofilm action of the marine red algae Amphiroa fragilissima extract has been well established in the current investigation through the reduction of biofilm formation among the test pathogens E. coli, Acinetobacter sp. Indeed, Sagar et al. (2022) has revealed different quorum sensing regulated virulence parameters, such as swarming motility, pyocyanin pigment, exopolysaccharide (EPS), and biofilms of P. aeruginosa, were reduced by Eucalyptus globulus methanol extract.
Further, the motility is a significant parameter among the bacterial species that produces biofilm (Tang et al., 2020). The motility of E. coli, Acinetobacter sp. and S. aureus have been much reduced by Amphiroa fragilissima extract. While, in the previous study of Tran and Hadinoto (2021), a plant-derived flavonoid Quercetin has complexed with chitosan nanoparticles and has reduced the swimming activity of P. aeruginosa. The study also highlights the EPS production in the presence and absence of red algae Amphiroa fragilissima extract that has effectively reduced the EPS in E. coli, Acinetobacter sp. when comparing with K. pneumoniae, and S. aureus. In yet another study of Karuppiah et al. (2021), a similar report has been submitted insisting on the seaweed Musa paradisiaca having a significant effect on the EPS activity with the influence of the compound 1,8-cineole. In fact, the biofilm, motility and EPS are the pioneer among the virulent factors regulated by the QS machinery, which may add up to its higher level of pathogenicity towards the offering bacterial pathogens. Certain study of Abdul Malik et al. (2020) has insisted on the anti-QS activity by studying the surface attached bacteria from Mexican red algae, Halymeniafloresii. Furthermore, in comparison to certain components like quercetin, nimbolide, nimbin, and azardirachtin, phytocompounds like catechin from Azadirachta indica has demonstrated the maximum biofilm eradication along with the degradation of EPS structural components like carbohydrates and proteins. Catechin is a significant compound which has the ability to reduce QS in the dental biofilm-forming bacteria Alcaligenes faecalis and Pseudomonas gingivalis (Lahiri et al., 2021).
The efflux pump has a direct effect on the antimicrobial resistance of pathogens and has been identified to be QS mediated mechanism. Therefore, efflux pump suppression lowers the level of antibiotic resistance (Rasamiravaka and El Jaziri, 2016). The current study on efflux pump inhibition has employed EtBr as an indicator to fluoresce when accumulating EtBr inside the bacterial cell as a result of efflux pump and has found that red seaweed extract has efficiently suppressed efflux pump in S. aureus, K. pneumoniae, and E. coli. Though the QS machinery varies with the regulation of distinct gene expression in gram positive and negative bacteria, as a consequence has a major impact in the virulence and pathogenicity. The expression of the QS genes such as lasI, lasR, rhlI, rhlR, pqsA and pqsR have decreased and confirmed by qRT-PCR (Abbas et al., 2020). The organic AHL antagonist from Curcumin plant has been demonstrated in an In-silico investigation. Due to specific hydrogen bonding and hydrophobic interactions, the P. aeruginosa genes for LasR and LuxR can be rendered inactive (Shukla et al., 2020). Indeed, the researchers are concentrating on the QS inhibitors to manage the antimicrobial resistant pathogens. The QS inhibitors extracted from Plumula nelumbini iseffective against P. aeruginosaand exhibits biofilm inhibition activity of 44.63 % at 100 mM without impeding bacterial growth (Chen et al., 2022). P. aeruginosa infections can be treated with sitagliptin, a new anti-quorum sensing drug. In the present study, through the identification FTIR and GCMS analysis, A. fragilissima extract has found to constitute the major bioactive compounds such as 1,3,5-Triazine-2,4,6-triamine, Silicic acid, diethyl bis(trimethylsilyl) ester, Trimethyl [4-(1-methyl-1-methoxyethyl) phenoxy] silane, Benzo[h]quinoline, 2,4-dimethyl, Pyrazol-3(2H)-one, 4-nitro and 1,2-Bis(trimethylsilyl)benzene respectively exhibiting anti-QS activity. In accordance, Haddadin et al. (2019) has suggested an effective QS inhibitory action against E. coli by a red alga Gracilaria arcouata. Additionally, the discovery of bioactive molecules by Karnjana et al. (2020) from the red seaweed, Gracilariafisheri namely N-benzyl cinnamamide molecule has reduced the QS virulence and bioluminescence of Vibrio harveyi. However, A. fragilissima extract shows a high peak of 1,3,5-triazine-2,4,6-triamine (Melamine), which is also found in desert medicinal plants and exhibits anti-QS activity (Singh et al., 2022). These bioactive compounds may target the receptor and synthase of QS system to actively reduce the expression of virulence factors.
5 Conclusion
Targeting bacterial virulence is one of the novel strategies that can be used to combat bacterial resistance. The present study has detail assessment upon the QS mediated phenotypic expressions such as biofilm formation, swarming/ swimming motility, exopolysaccharide (EPS) quantification and efflux pump both in the presence and absence of red algae Amphiroa fragilissima extract. Promisingly, the seaweed extracts has effectively reduced the expression of these virulence factors. The bioactive compounds from the red algae Amphiroa fragilissima along with their functional groups are identified using FTIR and GCMS analysis. These bioactive compounds have targetted the QS mechanism of pathogenic bacteria. The bioactive components interrups the varying QS machinery and controls the regulation of distinct gene expression to influence the virulence and pathogenicity among the target pathogens. Yet, still more research is required in the view of pharmacological aspect to combat quorum sensing approach in pathogens and to explore its commercialization.
CRediT authorship contribution statement
Prakash Piruthivraj: Conceptualization, Writing – original draft, Formal analysis, Methodology, Supervision, Project administration, Software. B.R. MahaSwetha: Data curation, Formal analysis, Methodology. Chitra Balasubramanian: Visualization. Rajapandiyan Krishnamoorthy: Funding acquisition, Writing – review & editing. Mansour K.Gatasheh: Funding acquisition. Anis Ahmad: Funding acquisition. Rengasamy Parthasarathi: Investigation, Validation. Poonguzhali Pandurangan: Investigation, Validation. V.K. Bhuvaneshwari: Resources. Natesan Vijayakumar: Writing – review & editing.
Acknowledgment
The work was supported by the Researchers Supporting Project number (RSP2024R393), King Saud University, Riyadh, Saudi Arabia
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Curtailing quorum sensing in Pseudomonas aeruginosa by sitagliptin. Curr. Microbiol.. 2020;77(6):1051-1060.
- [Google Scholar]
- Screening of surface-associated bacteria from the Mexican red alga Halymeniafloresii for quorum sensing activity. Microbiology. 2020;89(6):778-788.
- [Google Scholar]
- Identifying novel inhibitor of quorum sensing transcriptional regulator (SdiA) of Klebsiella pneumoniae through modelling, docking and molecular dynamics simulation. J. Biomol. Struct. Dyn.. 2021;39(10):3594-3604.
- [Google Scholar]
- Determination of optimum incubation time for formation of Pseudomonas aeruginosa and Streptococcus pyogenes biofilms in microtiter plate. Bull. Natl. Res Centre. 2019;43(1):1-5.
- [Google Scholar]
- Regulation of Staphylococcus aureus Virulence and Application of Nanotherapeutics to Eradicate S. aureus Infection. Pharmaceutics. 2023;15(310)
- [Google Scholar]
- Antioxidant and antibacterial activity of red seaweed Kappaphycusalvarezii against pathogenic bacteria. Global J. Environ. Sci. Manage.. 2020;6(1):47-58.
- [Google Scholar]
- Quorum quenching action of marine red alga Halemeniadurvillei on biofilm forming Gram negative bacterial isolates from contact lens. Algal Res.. 2022;64:102693
- [Google Scholar]
- Inhibitory Effects of Compounds from Plumula nelumbinis on Biofilm and Quorum Sensing Against P. aeruginosa. Curr. Microbiol.. 2022;79(8):1.
- [Google Scholar]
- Acinetobacter quorum sensing contributes to inflammation-induced inhibition of orthopaedic implant osseointegration. Eur. Cell. Mater.. 2022;43:267-276.
- [Google Scholar]
- Cocktail of CuO, ZnO, or CuZn nanoparticles and antibiotics for combating multidrug-resistant Pseudomonas aeruginosa via efflux pump inhibition. ACS Appl. Nano Mater.. 2021;4(9):9799-9810.
- [Google Scholar]
- Biofilm formation by Pseudomonas aeruginosa: role of the C4-HSL cell-to-cell signal and inhibition by azithromycin. J. Antimicrob. Chemother.. 2003;52(4):598-604.
- [Google Scholar]
- Freeman, DJ., Falkiner, FR., Keane, CT., 1989. New method for detecting slime production by coagulase negative staphylococci. J. Clin. Pathol. 42(8),872-4.
- Haddadin, RN., Al-Zibdah, M., Al-Bakri, AG., 2019. Investigating Potential Quorum SENSING Inhibition and Antimicrobial Activity Of Some Algae Collected From Gulf Of Aqaba, The Red Sea. Feb-Fresenius Environmental Bulletin. 4542.
- Purification and evaluation of N-benzyl cinnamamide from red seaweed Gracilariafisheri as an inhibitor of Vibrio harveyi AI-2 quorum sensing. Mar. Drugs. 2020;18(2):80.
- [Google Scholar]
- Anti-quorum sensing and antibiofilm potential of 1, 8-cineole derived from Musa paradisiaca against Pseudomonas aeruginosa strain PAO1. World J. Microbiol. Biotechnol.. 2021;37(4):1-2.
- [Google Scholar]
- Red seaweeds Sarcodiothecagaudichaudii and Chondrus crispus down regulate virulence factors of Salmonella enteritidis and induce immune responses in Caenorhabditis elegans. Front. Microbiol.. 2016;7:421.
- [Google Scholar]
- Detection of quorum sensing molecules and biofilm formation in Ralstonia solanacearum. Curr. Microbiol.. 2016;72(3):297-305.
- [Google Scholar]
- Catechin as the most efficient bioactive compound from Azadirachta indica with antibiofilm and anti-quorum sensing activities against dental biofilm: An in vitro and in silico study. Appl. Biochem. Biotechnol.. 2021;193(6):1617-1630.
- [Google Scholar]
- 5-Piperazinedione inhibits quorum sensing-dependent factor production in Pseudomonas aeruginosa PAO1. J. Basic Microbiol.. 2012;52(6):679-686.
- [Google Scholar]
- Anti-biofilm and Anti-quorum Sensing Activities of the Red Seaweed, Gracilaria changii and its Associated Bacteria. J. Appl. Phycol. 2023:1-12.
- [Google Scholar]
- Antibiofilm and quorum sensing inhibitory potential of Cuminum cyminum and its secondary metabolite methyl eugenol against Gram negative bacterial pathogens. Food Res. Int.. 2012;45(1):85-92.
- [Google Scholar]
- Prevention of biofilm formation by quorum quenching. Appl. Microbiol. Biotechnol.. 2020;104(5):1871-1881.
- [Google Scholar]
- Quorum-sensing mechanisms and bacterial response to antibiotics in P. aeruginosa. Curr. Microbiol.. 2016;73(5):747-753.
- [Google Scholar]
- Seaweed extracts: A promising source of antibiofilm agents with distinct mechanisms of action against Pseudomonas aeruginosa. Mar. Drugs. 2022;20(2):92.
- [Google Scholar]
- Inhibition of quorum sensing regulated virulence factors and biofilm formation by Eucalyptus globulus against multidrug-resistant Pseudomonas aeruginosa. J. Pharmacopuncture.. 2022;25(1):37.
- [Google Scholar]
- Drugs with new lease of life as quorum sensing inhibitors: For combating MDR Acinetobacter baumannii infections. Eur. J. Clin. Microbiol. Infect. Dis.. 2020;39(9):1687-1702.
- [Google Scholar]
- Twin peaks: presenting the antagonistic molecular interplay of curcumin with LasR and LuxR quorum sensing pathways. Curr. Microbiol.. 2020;77(8):1800-1810.
- [Google Scholar]
- Potential of desert medicinal plants for combating resistant biofilms in urinary tract infections. Appl. Biochem. Biotechnol. 2022:1-5.
- [Google Scholar]
- Bacterial Biofilm Inhibition: A focused review on recent therapeutic strategies for combating the biofilm mediated infections. Front. Microbiol.. 2021;12:676458.
- [Google Scholar]
- The abaI/abaR quorum sensing system effects on pathogenicity in Acinetobacter baumannii. Front. Microbiol. 2021:1791.
- [Google Scholar]
- Antimicrobial and anti-quorum sensing activities of phlorotannins from seaweed (Hizikiafusiforme) Front. Cell. Infect. Microbiol.. 2020;10:586750
- [Google Scholar]
- A Potential Quorum-sensing inhibitor for bronchiectasis therapy: Quercetin–chitosan nanoparticle complex exhibiting superior inhibition of biofilm formation and swimming motility of Pseudomonas aeruginosa to the native quercetin. Int. J. Mol. Sci.. 2021;22(4):1541.
- [Google Scholar]
- Quorum sensing inhibitory and antimicrobial activities of honeys and the relationship with individual phenolics. Food chemistry. 2009;115(4):1337-1344.
- [Google Scholar]
- Simplified AIP-II peptidomimetics are potent inhibitors of Staphylococcus aureus AgrC quorum sensing receptors. Chembiochem. 2017;18(4):413-423.
- [Google Scholar]
- Dietary phytochemicals as quorum sensing inhibitors. Fitoterapia. 2007;78(4):302-310.
- [Google Scholar]







