Exploring the influence of saline-alkaline stress on germination and seedling growth traits of Arta (Calligonum comosum L. Herit) as influenced by saline-alkaline stresses
⁎Corresponding author. malwhibi@ksu.edu.sa (Mona S. Alwahibi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
This research was conducted to study the effects of saline stress (9:1 M ratio of NaCl: Na2SO4, pH 6.27–6.49), alkaline stress (9:1 M ratio of NaHCO3: Na2CO3, pH 9.11–9.25) and saline/alkaline combined stress (NaCl: Na2SO4:NaHCO3: Na2CO3 9:1:9:1, pH 8.94–9.17) using concentrations 25, 50, 75 and 100 mmol/L on seed germination and seedling growth traits of Calligonum comosum L’Her. The experiments were carried out in the laboratories of the Botany and Microbiology Department in the female university dormitory of the Science College, King Saud University. The results of seed germination included the percentage of seed germination, the lengths of both radicle and plumule, and both fresh and dry weights of the seedlings after 14 days of growth under stress treatments. Germination results indicated a significant gradual decrease in seed germination rates with an increase in the concentration of stress treatments, and seed germination was inhibited at concentrations higher than 100 mmol/L in all stress treatments. Also, there was a differential decrease in the parameters of seedling growth represented by the radicle and plumule lengths and both fresh and dry seedling weights with the increase in the concentrations and the greatest effect was the alkaline stress.
Keywords
Germination
Seedling growth
Arta
Saline-alkaline
1 Introduction
Calligonum comosum L. (Arta) is a wild plant that grows in the sandy deserts of the Kingdom of Saudi Arabia (Chaudhary and Al-Jowaid, 1999). The plant has an important role in stabilizing sand due to the presence of a root system that is very branching and fast growing, as it has been proven that its roots reach great depths in the soil 30 m (Slama et al., 2015). In addition to its ability to produce antioxidants and free radicals (Okla et al., 2014). The plant has medicinal benefits as an anti-inflammatory (Liu et al., 2001). It is also used in folk medicine as an ointment to treat skin diseases (Phondani et al., 2016). However, plants that make up the vegetation cover in desert environments are more susceptible to deterioration when exposed to environmental factors that stress the plant, such as an increase in soil salinity or alkalinity where, which seriously affects lands globally (Roa et al., 2008).
Recent studies have indicated the importance of preserving plant diversity by studying the appropriate environments for the growth of plants (Aref et al., 2009). This damage may not be limited to the same area, but may spread to other neighboring areas (Al-Saadi et al., 2013). Thus, the loss of a local plant species will cause an imbalance in the ecosystem and accelerate its collapse.
Alkaline soils are common in dry lands where the percentage of sodium bicarbonate salts NaHCO3 and sodium carbonate Na2CO3 is higher, and soil can be considered saline-alkaline when the electrical conductivity exceeds four Siemens (Vu et al., 2015). As for the stages of seed germination and seedling growth, they are considered among the critical stages in the life of the plant because they are more sensitive to environmental stresses than other stages, so the ability of the plant to grow in saline or alkaline environments is considered a turning point for benefiting from saline and alkaline soils. In view of the scarcity of studies concerned with the impact of both alkaline, saline and joint stress on plant species in the region, and the fact that carrying out such studies will serve sustainable environmental and economic development plans (Dhief et al., 2012), this research aims to study the impact of salt and alkaline stress individually and their combined effects on the germination of seeds and related growth traits of plants exposed to stress.
2 Materials and methods
C. comosum seeds were collected from the northwestern part of Jubail city in the eastern region of Saudi Arabia according to the coordinates https://t.co/1xkkqV9kjp?ssr = true. Two neutral salts were mixed in a 9:1 M ratio (NaCl: Na2SO4) and applied to the saline stress group. Two alkaline salts were mixed in a 9:1 M ratio (NaHCO3: Na2CO3) and applied to the alkaline stress group. Similar concentrations of saline and alkaline solutions were mixed in equal proportion to obtain the combined solution (saline and alkali) group. Within each group, five concentrations were used: 25, 50, 75 and 100 mmol/L. The pH range in the saline stress group was 6.27–6.49, alkaline stress group was 9.11–9.25 and combined group was 8.94–9.17. Distilled water was used as a control. Before the germination assay, seeds were soaked in concentrated sulfuric acid for 30 min, removed and rinsed with sterile deionized water. The germination experiment and related growth traits were conducted in the laboratories of the Department of Botany and Microbiology in the university dormitory for female students of the College of Science, King Saud University during the period (1440–1441 AH / 2019–2020 CE).
Germination experiment and related growth traits were conducted in the laboratories of the Department of Botany and Microbiology in the university dormitory for female students of the College of Science, King Saud University during the period (1440–1441 AH / 2019–2020 CE) under salt stress, where healthy seeds of equal sizes were selected after rubbing them by hand to remove the thorns first, then they were placed in a glass container containing (96 %) sulfuric acid for 30 min (Dhief et al., 2012). To break the dormancy with continuous stirring by a glass stem, then pour the acid and wash it with distilled water for 15 min, then sterilize it by soaking it with 5 % sodium hypochlorite solution for 5 min, then wash it with distilled water several times, then put it on filter paper with a diameter of 9 cm inside Petri dishes (each dish contains 10 seeds) and the seed germination experiment was started using 2.5 ml of solutions of stress treatments for irrigation. It was irrigated daily according to the treatment for 14 days, and it was exposed to light daily for 4 h after germination. It was distributed to the first control group, which contains 5 replicates of dishes that were irrigated with 2.5 ml of distilled water, and to the second group, salt stress, which includes four treatments of saline solution (25, 50, 75, 100 mmol/L). Each treatment has five replicates. Germination and seedling growth traits were measured after seeds germinated daily for 14 days, and the seed was considered germinated at root emergence from the seed. After 14 days, the germinated seeds were sorted and measurements of the selected growth parameters were started by taking fresh weights and measuring the lengths of both the radicle and plumule using a ruler, then drying using the drying oven at a temperature of 50° C until the weight was stable to take the dry weight of those organs for all the germinated seedlings for all coefficients and concentrations used. The following equation has been used to calculate germination percentage:
Statistical analysis was carried out using the SPSS program, where the replicates were subjected to analysis of variance (ANOVA) to calculate the means and standard error (SE). Means were analyzed and grouped using Duncan’s multiple range test (P=0.05) by following the standard procedures described by (Gomez et al., 2015).
3 Results
3.1 Saline stress
The germination behavior of C. comosum revealed that there is a significant difference between control and all saline exposure, i.e., 25, 50, 75 and 100 mmol/L treatments (Fig. 1).
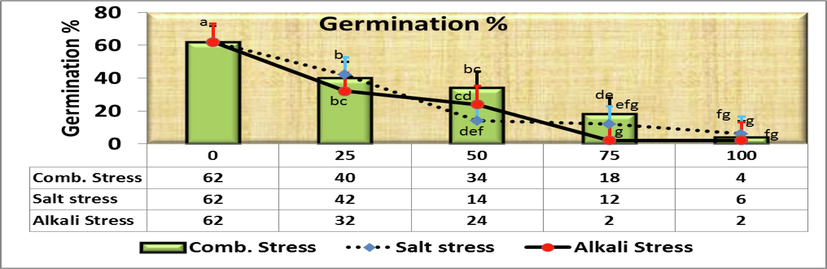
- Effect of irrigation with five different concentrations of three stress factor solutions (saline, alkaline, and combined) on germination percentage of C. comosum seeds (columns followed by standard error values).
However, the final germination percentage observed at saline stress below a concentration of 50 mmol/L salt solution reached 14 % and showed no significant decrease at 75 and 100 mmol/L [e.g.,no significant differences between 50 (14def ± 5.1), 75 (12efg ± 2.0) and 100 (6fg ± 4.0) mmol/L]. Comparing the germination percentage in the control treatment (as being 100 %) with the rest of the salt concentrations, it was found that the decreasing percentage was 32.3, 77.4, 80.7 and 90.3 % at the concentrations of 25, 50, 75 and 100 mmol/L, respectively over the control (Fig. 2). These results indicate gradual decrease in seed germination with the increase in salt concentrations, and germination stopped after concentration 100 mmol/L for all salt treatments.
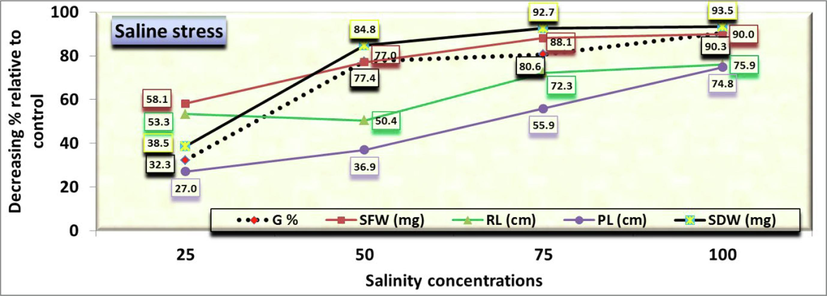
- Final germination percentage (G), both radicle (RL) and plumule (PL) lengths as well as both fresh (SFW) and dry (SDW) seedling weight of C. comosum as affected by salinity. Data were expressed as percentage of the control (under distilled water) at each salinity level.
The growth measurements of radicle and plumule lengths were calculated for all crop seedlings in response to different concentrations (control, 25, 50, 75, 100 mmol/L). After 14 days of germination, radicle length was 2.74 cm in control (Fig. 3). It decreased to 1.28b ± 0.086 cm, 1.36b ± 0.163 cm, 0.76c ± 0.06 cm and 0.66c ± 0.18 cm at 25, 50, 75, 100 mmol/L, respectively. However, no significant differences between both 25 and 50 mmol/L and between both 75 and 100 mmol/L were observed. On the other hand, Comparing the radicle length in the control treatment (as being 100 %) with the rest of the salt concentrations, it was found that the decreasing length was 53.3, 50.4, 72.3 and 75.9 % at the concentrations of 25, 50, 75 and 100 mmol/L, respectively over the control (Fig. 2).
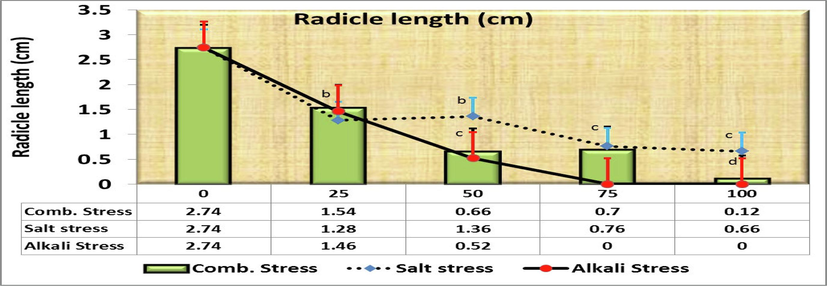
- Effect of irrigation with five different concentrations of three stress factor solutions (saline, alkaline, and combined) on the radicle length of C. comosum seedlings (columns followed by standard error values).
Moreover, the length of plumule was 2.22 cm in control, but decreased gradually significant to 0.56 cm for increasing saline concentrations with no significant differences between both 25 and 50 mmol/L (Fig. 4). As for comparing the plumule length in the control treatment (as being 100 %) with the rest of the salt concentrations, it was found that the decreasing length was 27.0, 36.9, 55.9 and 74.8 % at the concentrations of 25, 50, 75 and 100 mmol/L, respectively over the control (Fig. 2). Fig. 5&6 show a significantly reduction in the fresh (SFW) and dry (SDW) weight of the C. comosum seedling under salinity stress treatments compared to the control one in which the weights were reached 54 and 10.4 mg, respectively. Seeds exposure to different concentrations of saline stress treatments exhibited 22.6, 12.4, 6.4, 5.4 mg seedlings fresh weight (Fig. 5) and 6.4, 1.58, 0.76, 0.68 mg seedlings dry weight (Fig. 6) at 25, 50, 75, 100 mmol/L, respectively. However, no significant differences were obtained among the effects of 50, 75 and 100 mmol/L saline solutions in both traits. On the other hand, Comparing the SFW in the control treatment (as being 100 %) with the rest of the salt concentrations, it was found that the decreasing fresh weight was 58.2, 77.0, 88.2 and 90.0 % at the concentrations of 25, 50, 75 and 100 mmol/L, respectively over the control (Fig. 2), while the decreasing dry weight was the highest overall studied traits at the concentrations 50, 75 and 100 mmol/L, i.e., 84.8, 92.7 and 93.5 % of control.
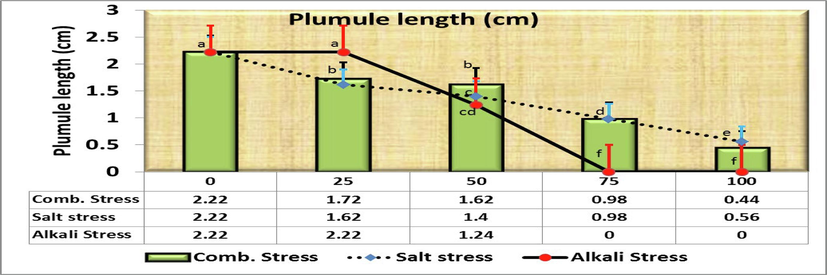
- Effect of irrigation with five different concentrations of three stress factor solutions (saline, alkaline, and combined) on the plumule length of C. comosum seedlings (columns followed by standard error values).
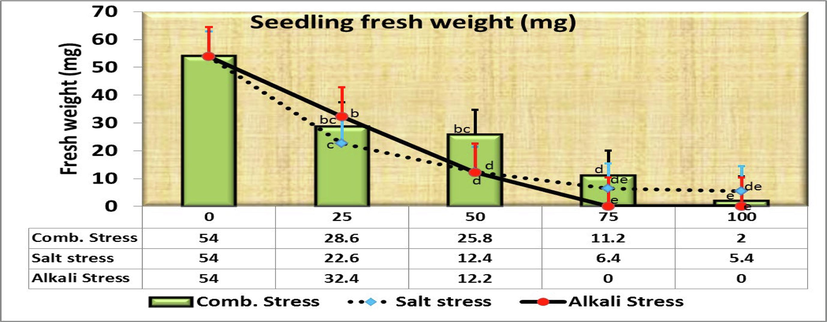
- Effect of irrigation with five different concentrations of three stress factor solutions (saline, alkaline, and combined) on the fresh weight of C. comosum seedlings (columns followed by standard error values).
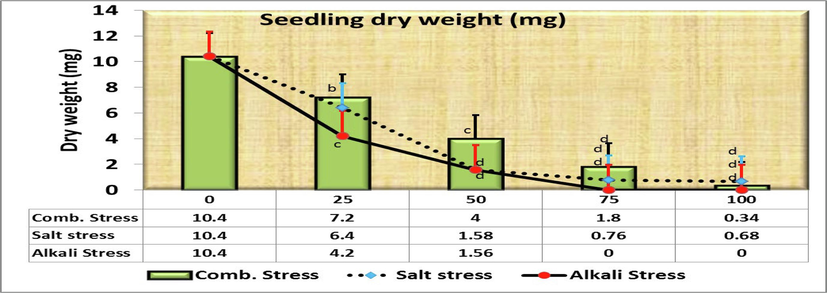
- Effect of irrigation with five different concentrations of three stress factor solutions (saline, alkaline, and combined) on the dry weight of C. comosum seedlings (columns followed by standard error values).
3.2 Alkali stress
The studies conducted on the germination behavior of C. comosum seeds revealed that there is a significant difference between control and all alkaline stress (NaHCO3:Na2CO2 9:1) treatments, i.e., 25, 50, 75 and 100 mmol/L (Fig. 1). However, the germination percentage observed at alkaline stress below a concentration of 50 mmol/L solution reached 24 % with no significant differences between the effect of 25 and 50 mmol/L. However, the germination behavior of seed differed significantly (Fig. 1&7), i.e., 25 mmol alkaline treatment had highest germination percentage (32 %) followed by 50 mmol/L (24 %) with no significant differences between them while the 75 and 100 mmol/L had the lowest equal germination percentage (2 %). Moreover, it was observed that seed germination showed a sensitive effect for the two types of salt and alkaline stress apparently without noticing any significant differences for the same concentration in both types, which was further analyzed in the other traits. Respecting to comparing the germination percentage in the control treatment (as being 100 %) with the rest of the alkaline solution concentrations, it was found that the decreasing percentage was 48.4, 61.3, 96.8 and 96.8 % at the alkali concentrations of 25, 50, 75 and 100 mmol/L, respectively over the control (Fig. 7) which were 32.3, 77.4, 80.7 and 90.3 % at the corresponding saline concentrations, respectively over the control (Fig. 2).
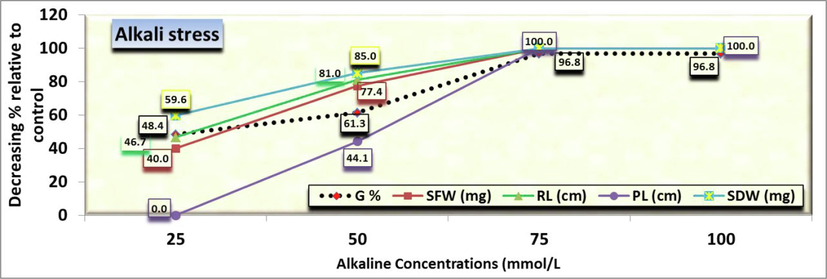
- Seed germination percentage (G), Radicle (RL) and Plumule (PL) lengths as well as both fresh (SFW) and dry (SDW) seedling weight of Arta (Calligonum comosum) plants as affected by alkalinity. Data were expressed as decreasing percentage of the control (under distilled water) at each level.
Results showed that both saline and alkaline could significantly decrease germination percentage of seeds (Figs. 1, 2 and 7), and the latter applied more negative effect (Fig. 7). After 14 days of germination, radicle length was 2.74 cm in control (Fig. 3). It decreased to 1.46b ± 0.143 cm, 0.52c ± 0.048 cm, 0.0d ± 0.0 cm and 0.0d ± 0.0 cm at 25, 50, 75, 100 mmol/L, respectively. However, both 75 and 100 mmol/L treatments showed negligible values. On the other hand, Comparing the radicle length in the control treatment (as being 100 %) with the rest of the salt concentrations, it was found that the length decreasing under alkaline effects was 46.7, 81.0, 100 and 100 % of control (Fig. 7) by corresponding to 53.3, 50.4, 72.3 and 75.9 % influenced by saline solution at the concentrations of 25, 50, 75 and 100 mmol/L, respectively over the control (Fig. 2).
Significant differences were also detected for other parameters at alkaline stress by Duncan significant differences procedure. Both SFW and SDW (mg) were 54.0a ± 4.615 and 10.4a ± 1.939 mg, respectively in control treatments where for seedling fresh weight (Fig. 5) decreased to 32.400b ± 3.880 mg and 12.2d ± 1.854 mg at 25 and 50 mmol/L, respectively whereas the two other alkaline levels (75 and 100 mmol/L) did not record any values. Similarly, seedling dry weight (SDW, Fig. 6) decreased gradually to 4.2c ± 0.583 mg and 1.56d ± 0.426 mg at the same levels of 25 and 50 mmol/L, respectively.
Comparing the SFW and SDW in the control treatment (as being 100 %) with the rest of the salt concentrations, it was found that the decreasing fresh weight was 40.0, 77.4, 100 and 100 % and dry weight was 59.6, 85.0, 100 and 100 % at 25, 50, 75 and 100 mmol/L, respectively over the control (Fig. 7).
3.3 Combined stress of salinity and alkalinity on germination parameters
Saline-alkali combined stress presented a highly important effect on the germination of C. comosum seeds. At low salinity (25 %), the level of seed germination was not substantially reduced, but decreased with higher salinity and alkalinity. The highest final germination percentage occurred under the corresponding same salinity concentration; however, the final germination percentages observed under combined saline-alkaline salt solutions were not significantly different than alkaline or saline solutions at 50 mmol/L or 75 mmol/L, respectively (Fig. 8).
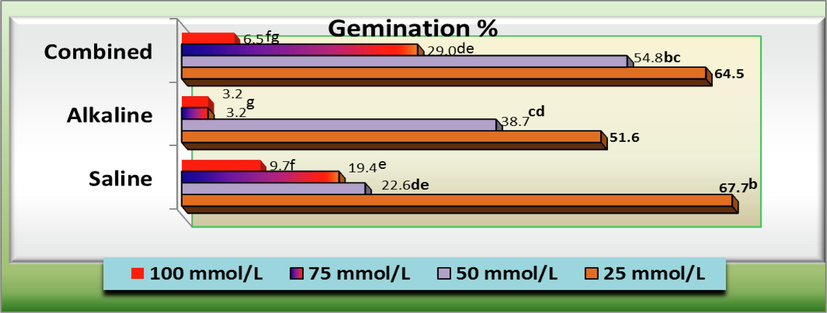
- Seed germination percentage (G) of Arta (Calligonum comosum) plants as affected by different concentration of salinity, alkalinity and their combined stresses. Data were expressed as increasing percentage relative to the control (under distilled water) at each level.
Similarly, no significant difference (P>0.05) was detected at 25 mmol/L of both saline and combined of salt solutions and 50 mmol/L of salinity stress (un-alkaline stress) in both radicle (RL) and plumule (PL) lengths as well as at 25 mmol/L of alkaline and 50 of combined stresses for radicle and PL, respectively. Also, no significant difference (P>0.05) was detected at 50 mmol/L alkali with 75 mmol/L of both saline and combined of salt solutions in both radicle and plumule lengths as well as 50 mmol/L of combined and 100 mmol/L of salinity stresses in radicle length (Fig. 9). Moreover, the effects of 25 mmol/L on plumule length (Fig. 10) was significantly equal with the control treatment (2.22 ± 0.166 cm) and 100 mmol/L salinity (0.560 ± 0.112 cm) with combined stress (0.440 cm).
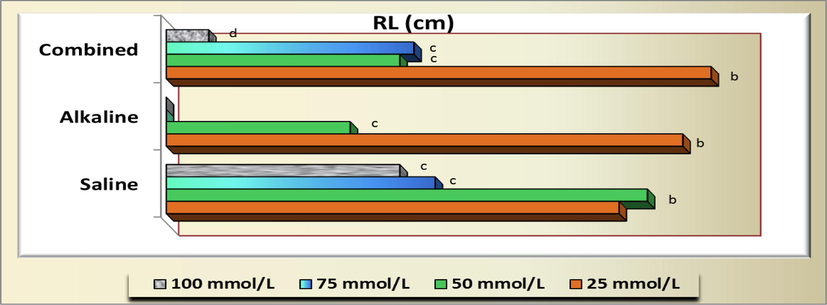
- Radicle length (RL) of Arta (Calligonum comosum) plants as affected by different concentration of salinity, alkalinity and their combined stresses. Data were expressed as increasing percentage relative to the control (under distilled water) at each level.
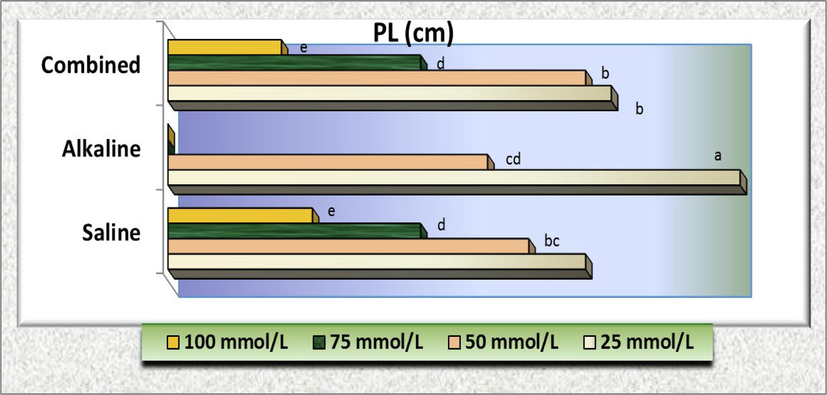
- Plumule length (PL) of C. comosum) plants as affected by different concentration of salinity, alkalinity and their combined stresses. Data were expressed as increasing percentage relative to the control (under distilled water) at each level.
As for seedling fresh (Fig. 11) and dry weight (Fig. 12), no significant difference (P>0.05) was detected at 25 mmol/L of both saline and combined of salt solutions and among 50 mmol/L combined stress and each of 25 mmol/L alkaline stress, 50, 75 and 100 salinity in both traits as well as between 25 and 50 mmol/L of combined stresses for seedling fresh weight (FW). However, other treatments in both traits exhibited neglected values (see Fig. 13).
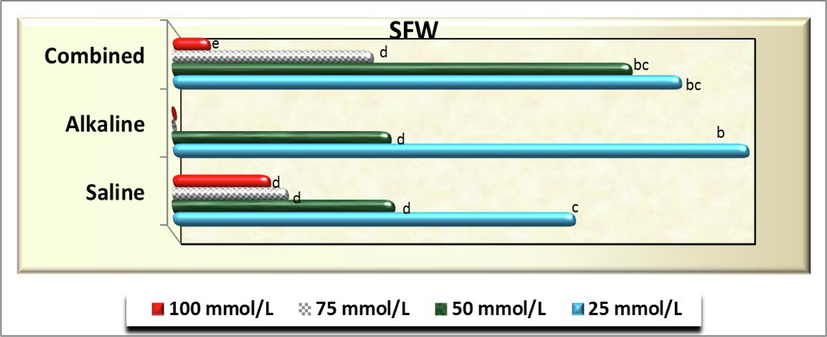
- Seedling fresh weight (SFW) of C. comosum) plants as affected by different concentration of salinity, alkalinity and their combined stresses. Data were expressed as increasing percentage relative to the control (under distilled water) at each level.
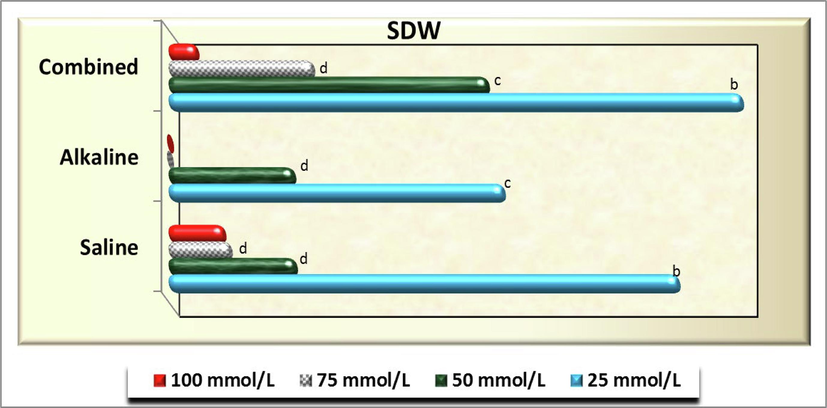
- Seedling dry weight (SDW) of C. comosum) plants as affected by different concentration of salinity, alkalinity and their combined stresses. Data were expressed as increasing percentage relative to the control (under distilled water) at each level.
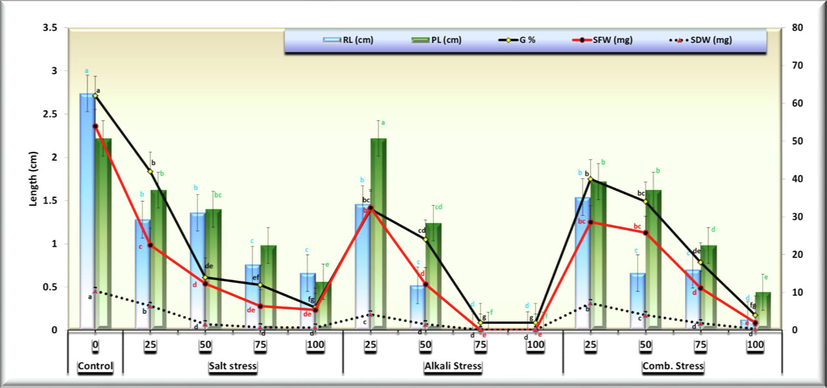
- Comparative Effects of irrigation with five different concentrations of three stress factor solutions (saline, alkaline, and combined) on C. comosum studied traits (columns followed by standard error values).
These results indicate that there is a significant decrease in seedling growth parameters, i.e., radicle length, plumule length, seedling fresh and dry weight with the increase in the concentrations and the greater effect of alkaline stress. Generally, the results indicate the inhibition effect of all the treatments on seed germination and seedling growth parameters. Data obtained causing a disruption of osmotic as well as in ionic balance. The alkaline stress has the stronger effect as compared with saline and combined stress which contributes in the increase of pH of the media surrounding the roots causing root damage and reducing their function in absorption and transportation of water. Also, the higher concentrations above 100 mmol/L stops seed germination, however it found that lower salt concentrations have stimulating role for sum morphological characters. The results also indicate the role of this plant to reduce the effect of salt by increasing the accumulation of osmolytes in the cytoplasm in order to balance the os-motic potential of the Na+ and Cl−.
4 Discussion
The response of the plant to environmental conditions such as salinity and soil alkalinity is a key factor to determine the ability of the plant to complete its life cycle (Lin et al., 2016). The obtained results showed that the germination rate of Arta seeds gradually decreased with the increase in the concentration of stress factors, as the germination rates of salt stress reached 42, 41, 12, 6 % and alkaline stress, the most harmful effect was the alkaline stress treatment, whereas the seed germination inhibited at concentrations higher than 100 mmol/L in all the three stress types. These results are in agreement with (Al-Otaibi and Ebid, 2015) who mentioned that germination stopped completely at a concentration higher than 100. mmol/L, so the arta cannot be considered a halophytic plant that has the ability to germinate and grow in environments where the salt concentration exceeds 200 mmol/L (Flowers and Colmer, 2008). The percentage of seed germination significantly reduced by increasing the concentration of salts in the different treatments compared to the corresponding control, and the percentage of germination in the alkaline stress treatments was the most influenced. These are coincident with the studies of (Zhanwu et al., 2011) on the effects of Different salt (saline, alkaline and combined), where stress reduced the germination rate and then stopped it at different concentrations of salts, and alkaline stress was the most influential on germination rate.
The high effect of alkaline stress on seed germination can be explained by the fact that the sodium salts used in this study engage the same cation, Na+, the results of (Zhang and Mu, 2009) indicate that the inability of Lathyrus quinquenervius seeds to restore their ability to germinate after stopping the alkaline stress treatments indicates the death of the seeds exposed to stress. It was mentioned by (Bordenave et al., 2019) in their study on Lotus japonicus, that each salt stress, whether alkaline, saline, or combined, had different effects on the plant, and that the effect of the combined stress was greater than the saline or alkaline stress alone in opposite of our results, where the combined effect was moderate than other factors. Ionization of ions that are absorbed in large quantities (Hu et al., 2018). Perhaps the reason for the lower germination percentage of Arta seeds in filter paper by 4 % is due to a physical hard coat in the seeds. Therefore, this dormancy was broken by treating Arta seeds with 96 % sulfuric acid, and this is in agreement with the study of11 on the three species of the genus Callignum, including C. comosum, during which it was found that treating the seeds with 96 % sulfuric acid led to the breaking of the seed dormancy, as well as in a study of (Ren and Tao, 2004) on ten types of the same genus, where they explained that the best way to break the dormancy in these species is by mechanically scratching the seeds and then treating them with sulfuric acid. The improvement of the seeds germination of the Arta plant under field conditions in sandy soil without the need to break the dormancy may be due to their aeration and good water retention, which in turn may contribute to washing out the chemical inhibitors of germination secreted by the embryo in the soil. In water, or the improvement may be due to alternating temperatures and washing these inhibitors together. This is consistent with what was found in the seeds of many plants whose germination was better in sandy soil than in the laboratory (Ren and Tao, 2004). Gairola (Gairola et al., 2011) on the plant Jatropha curcas L. found that the germination percentage in sandy soil was 82 % compared to filter paper (55 %), and this was explained by the quality of aeration in sandy soil and its ability to hold the water. The seedling stage is one of the critical stages in the life of a plant exposed to environmental stresses. The radicle and plumule lengths decrease with the increase in the concentration of stress factors, a study has shown. Radicle length was 72 % and the length of the plumule 52 %, and the effect of stress on the radicle is greater than that of the plumule, these results are consistent with the studies of (Zhang et al., 2017) and (Hu et al., 2018). hat the effect is due to a disruption in the various growth processes, a change in the rigidity of the protoplasm, and the inhibition of transfer processes between cells, which results in a reduction in cell elongation and division, and that's may be considered as a type of adaptation to saline and alkaline stress to reduce the absorbent surface area between the radicle and its surrounding environment (Chai et al., 2012). The results in this study also indicated that saline stress has a different effect in reducing the fresh and dry weight of seedlings which were 78 % & 77 for saline stress, 79 % & 86 for alkaline stress, and 68.7 % & 67.9 % for combined stress, respectively. This effect is due to the reduction of the plant's ability to absorb water and transport nutrients necessary for the growth of the embryo inside the seed. These results are in agreement with those of (Prasad et al., 2014) on rice plants in which alkaline stress has a greater effect on the plant compared to other stresses and also with embryo's growth that was result of (Shuai et al., 2015) globally, 33 % of the total irrigated agricultural area is subjected to salt stress. This figure is increasing rapidly by an average of 10 % per year (Abbas et al., 2019).
CRediT authorship contribution statement
Amnah S. Alshahwan: . Mohammed N. Alyemeni: . Mona S. Alwahibi: Writing – review & editing, Writing – original draft, Visualization, Investigation, Funding acquisition, Data curation, Conceptualization. Nadi Awad Al-Harbi: . Salem Mesfir Al-Qahtani: . Abdullah Alaklabi: .
Acknowledgment
The authors extend their appreciation to the Researchers Supporting Project Number (RSP2024R173), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Halotolerant PGPR: A hope for cultivation of saline soils. J. King Saud Univ.-Sci.. 2019;31(4):1195-1201.
- [Google Scholar]
- The effect of some heavy metals accumulation on physiological and anatomical characteristic of some Potamogeton. L. plant. J. Ecol. Environ. Sci.. 2013;4(1):100-108. The effect of some heavy metals accumulation on physiological and anatomical characteristic of some Potamogeton (uobasrah.edu.iq)
- [Google Scholar]
- Ecological study on Dobera glabra Forssk. at Jazan region in Saudi Arabia. J. Horticult. For.. 2009;1(10):198-204. Ecological study on Dobera glabra Forssk. at Jazan region in Saudi Arabia (academicjournals.org)
- [Google Scholar]
- Chlorophyll a fluorescence analysis reveals divergent photosystem II responses to saline, alkaline and saline–alkaline stresses in the two Lotus japonicus model ecotypes MG20 and Gifu-129. Acta Physiol. Plant.. 2019;41:1-13.
- [CrossRef] [Google Scholar]
- Effects of saline-alkaline stress on early growth strategy and colonization success of Flaveria bidentis (l.) Kuntze (Asteracae)-a new exotic plant in northern China. Pol. J. Ecol.. 2012;60(3):559-565. Effects of saline-alkaline stress on early growth strategy and colonization success of Flaveria bidentis(l.) Kuntze (Asteracae)-a new exotic plant in northern (researchgate.net)
- [Google Scholar]
- Chaudhary, S. A., & Al-Jowaid, A. A. A. (1999). Vegetation of the kingdom of Saudi Arabia. Vegetation of the Kingdom of Saudi Arabia (fao.org).
- Effects of some seed-coat dormancy breaking treatments on germination of three Calligonum species occurring in Southern desert of Tunisia. Ecologia Mediterranea. 2012;38(1):19-27. Effects of some seed-coat dormancy breaking treatments on germination of three Calligonum species occurring in Southern desert of Tunisia (persee.fr)
- [Google Scholar]
- Effect of temperatures and germination media on seed germination of Jatropha curcas Linn. Adv. Biores.. 2011;2(2):66-71. Effect of temperatures and germination media on seed germination of Jatropha curcas Linn (researchgate.net)
- [Google Scholar]
- Seed germination of hemp (Cannabis sativa L.) cultivars responds differently to the stress of salt type and concentration. Ind. Crop. Prod.. 2018;123:254-261. Seed germination of hemp (Cannabis sativa L.) cultivars responds differently to the stress of salt type and concentration (sciencedirect.com)
- [Google Scholar]
- Germination responses of the halophyte Chloris virgata to temperature and reduced water potential caused by salinity, alkalinity and drought stress. Grass Forage Sci.. 2016;71(3):507-514.
- [CrossRef] [Google Scholar]
- Anti-inflammatory and anti-ulcer activity of Calligonum comosum in rats. Fitoterapia. 2001;72(5):487-491.
- [CrossRef] [Google Scholar]
- Biological activity of Calligonum comosum extracts as antibacterial and antioxidant. J. Pure Appl. Microbiol. 2014;8(2):529-534. Biological activity of Calligonum comosum extracts as antibacterial and antioxidant (researchgate.net)
- [Google Scholar]
- Ethnobotanical magnitude towards sustainable utilization of wild foliage in Arabian Desert. J. Tradit. Complement. Med.. 2016;6(3):209-218.
- [CrossRef] [Google Scholar]
- Effect of alkalinity on germination efficiency index, seedling growth, free proline and sugar during early seedling growth of rice. Afr. J. Agric. Res.. 2014;9(44):3251-3257. Effect of alkalinity on germination efficiency index, seedling growth, free proline and sugar during early seedling growth of rice (academicjournals.org)
- [Google Scholar]
- Effects of different pre-sowing seed treatments on germination of 10 Calligonum species. For. Ecol. Manage.. 2004;195(3):291-300.
- [CrossRef] [Google Scholar]
- Physiological and biochemical responses of Jerusalem artichoke seedlings to mixed salt-alkali stress conditions. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2015;43(2):473-478. Physiological and biochemical responses of Jerusalem artichoke seedlings to mixed salt-alkali stress conditions (notulaebotanicae.ro)
- [Google Scholar]
- Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann. Bot.. 2015;115(3):433-447.
- [Google Scholar]
- Mechanisms of combined effects of salt and alkaline stresses on seed germination and seedlings of Melilotus officinalis (Fabaceae) in Northeast of China. Pak. J. Bot. 2015;47(5):1603-1611. Mechanisms of combined effects of salt and alkaline stresses on seed germination and seedlings of Melilotus officinalis (Fabaceae) in Northeast of China (pakbs.org)
- [Google Scholar]
- Alkalinity and salinity tolerance during seed germination and early seedling stages of three alfalfa (Medicago sativa L.) cultivars. Legume Research-An. Int. J.. 2017;40(5):853-858. Alkalinity and salinity tolerance during seed germination and early seedling stages of three alfalfa (Medicago sativa L.) cultivars (indianjournals.com)
- [Google Scholar]
- Effects of saline and alkaline stresses on the germination, growth, photosynthesis, ionic balance and anti-oxidant system in an alkali-tolerant leguminous forage Lathyrus quinquenervius. Soil Sci. Plant Nutr.. 2009;55(5):685-697.
- [CrossRef] [Google Scholar]
- Germination responses of Alfalfa (Medicago sativa L.) seeds to various salt-alkaline mixed stress. Afr. J. Agric. Res.. 2011;6(16):3793-3803. Germination responses of Alfalfa (Medicago sativa L.) seeds to various salt-alkaline mixed stress (academicjournals.org)
- [Google Scholar]
Further reading
- Aspects of the autecology of arta (Calligonum comosum L. Her) a medical plant from arid region of Saudi Arabia. J. Biodivers. Environ. Sci.. 2015;6(3):248-255. Aspects of the autecology of arta (Calligonum comosum L. Her) a medical plant from arid region of Saudi Arabia (psu.edu)
- [Google Scholar]
- Reproductive stage tolerance to salinity and alkalinity stresses in rice genotypes. Plant Breed.. 2008;127(3):256-261.
- [CrossRef] [Google Scholar]







