Translate this page into:
Exploration of the antiproliferative activity of lectin-like protein from seeds of Datura stramonium: An in vitro study
⁎Corresponding author. amitk.singh@sharda.ac.in (Amit Kumar Singh)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Lectins, glycoproteins with non-enzymatic and non-immunogenic origins, play essential roles in various biological processes. This study presents the purification and characterization of a lectin-like protein isolated from the seeds of Datura stramonium L., a member of the Solanaceae family. The purification of the lectin-like protein involved a dual method, employing both ammonium sulphate precipitation and anion exchange chromatography utilizing DEAE-Sepharose. Analysis via SDS-PAGE showed a distinct band, displaying an estimated molecular weight of approximately 30 kDa., indicative of the purified protein's homogeneity. Additionally, the purified protein exhibited hemagglutination activity towards human B and O red blood cells, underscoring its lectin-like properties. Periodic acid Schiff (PAS) staining confirmed the glycoprotein nature of the purified protein. Furthermore, its anti-proliferative activity against human cancer Caco-2 cell lines was evaluated through MTT assay, revealing promising results. This study elucidates the purification and characterization of a lectin-like protein from Datura stramonium L. seeds, providing valuable insights into its potential biological functions and diagnostic research applications.
Keywords
Lectin
SDS PAGE
Datura stramonium
PAS stain
Hemagglutination assay
MTT assay
- DEAE
-
Diethylamino ethyl cellulose
- DSL
-
Datura stramonium Lectin
- MTT
-
3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide
- RT
-
Room Temperature
- SDS-PAGE
-
Sodium dodecyl sulphate polyacrylamide gel electrophoresis
- PAS
-
Periodic acid Schiff
- CRD
-
Carbohydrate recognition domains
- DMEM
-
Dulbecco's Modified Eagle Medium
Abbreviations
1 Introduction
Datura stramonium, a widely recognized medicinal plant belonging to the Solanaceae family, often grows alongside important crops like soybeans, linseed, and wheat, potentially intermixing with them during harvest (Adamse et al., 2014). While it is acknowledged as an invasive weed, it also possesses poisonous properties due to the presence of tropane alkaloids, which can cause severe illness or even death upon high consumption (Soni et al., 2012). Despite its toxic effects at higher doses, Datura stramonium has been reported to exhibit excellent medicinal properties, including analgesic, anthelmintic, anti-inflammatory, and antiparasitic activities. Alongside alkaloids, it contains several crucial biomolecules such as carbohydrates and proteins with essential therapeutic functions.
Seeds of Datura species are known to contain multi-subunit lectins, a class of proteins widely distributed in organisms, particularly in plants (Broekaert et al., 1988; Mishra et al., 2019). These lectins are classified into various families based on primary and 3D structural similarities of their carbohydrate recognition domains (CRDs) (Dang et al., 2015). Their primary function is to identify biological molecules across a wide variety of species (Nascimento et al., 2012). Lectins serve diverse functions including cell–cell contact in multicellular organisms, host-parasite interactions, and interaction with sugars attached to other biomolecules (Albores et al., 2014; Ghazarian, Idoni, and Oppenheimer, 2011). Additionally, lectins play a crucial role in immune response and protein localization.The Datura Seed Lectin (DSL) is an extracellular protein characterized by electron microscopic immunocytochemistry. Immunofluorescent staining of seeds reveals a significant presence of DSL in the seed coat and seed epidermis (Konska et al., 2008).
The seeds of numerous plants contain lectins with sugar-binding properties, capable of agglutinating animal red blood cells. This characteristic renders plant lectins invaluable scientific tools for identifying and retrieving cancer cells from the bloodstream (Yau et al., 2015). Moreover, these lectins have demonstrated the ability to induce apoptosis in cancer cells. Additionally, lectins exhibit diverse medical and biological applications, including antiviral, antifungal, antibacterial, and antidiabetic properties, alongside immunomodulatory activities (Bah et al., 2013).
Despite the wealth of studies focusing on the alkaloid content of Datura, there is a notable dearth of reports investigating proteins such as DSL. While numerous reports detail the medicinal and biological applications of lectins from other plant sources, none specifically address lectins derived from Datura plants. Given that Datura stramonium is readily available as a weed and traditionally recognized for its medicinal properties, exploring the properties of its constituent components is of significant interest. Consequently, our study was designed to explore the structural and functional characterization of the lectin protein found in this plant, considering its potential cost-effectiveness and sustainability compared to edible sources of lectins. In this study, we purified the lectin present in D. stramonium seeds and examined its potential to inhibit cancer cell growth as a prospective anticancer agent (Sharon, 2007; Tang et al., 2015). We anticipate that our findings will encourage further investigation into the therapeutic properties of DSL, facilitating the development of sustainable and cost-effective therapeutics for cancer and other diseases.
2 Materials and methods
2.1 Extraction of proteins
The seeds of D. stramonium were collected from local areas of Greater Noida, Uttar Pradesh. Following a distilled water wash, the seeds (Fig. 1) were allowed to air dry and then kept at room temperature. Seeds were grinded and homogenized in an extraction buffer composed of 0.2 M sodium chloride and 50 mM sodium phosphate (PBS; pH 7.4). The homogenate was filtered by using a muslin cloth. The residue was discarded, and the filtrate was used for centrifugation at 10000 rpm for 30 min at 4 °C. After this, the pellet made up of cellular debris was discarded, and the supernatant was used for further protein precipitation (Sachin et al., 2021).
Seeds of Datura stramonium (sciencephotogallery.com/featured/thorn-apple-datura-stramonium-seeds).
2.2 Protein fractionation by ammonium sulphate precipitation method
The supernatant was used to do ammonium sulphate precipitation of proteins in different fractions. Ammonium sulphate was added to the crude sample to make it a 20 % saturated solution and kept overnight for the precipitation of proteins (Wingfield, 2001; Kirar, et al., 2023). Centrifugation was done further at 10000 rpm for 15 mins. at 4 °C. The pellet was dissolved in a 20 mM phosphate buffer, and the supernatant was further used for the addition of ammonium sulphate to make it 60 % saturated and was kept overnight. Centrifugation was done further at 10000 rpm for 15 mins. at 4 °C. The pellet was suspended in a 20 mM phosphate buffer (pH 7.4). The supernatant was further used to add ammonium sulphate to make it 60 % saturated and was kept overnight. Centrifugation was done further at 10000 rpm for 15 mins. at 4 °C. The pellet was suspended in a 20 mM phosphate buffer (pH 7.4). The supernatant was further used to add ammonium sulphate to make it 90 % saturated and was kept overnight. Centrifugation was done further at 10000 rpm for 15 mins. at 4 °C. After that pellet was dissolved in a 20 mM phosphate buffer (pH 7.4), and the supernatant was discarded. Dialysis was done for 20 %, 60 %, and 90 % dissolved pellet fractions to remove (NH4)2SO4 using 20 mM Phosphate buffer (pH 7.4). SDS-PAGE further analyzed the dialyzed sample.
2.3 Purification of targeted protein by ion exchange chromatography
Dialyzed fractions of 20 %, 60 %, and 90 % precipitated proteins were used for purification on anion exchanger-based resin DEAE-Sepharose Fast Flow column chromatography. 4 mL of DEAE resin was packed on a chromatographic column of dimension 40 × 1.5 cm and washed with water. Further, the resin was equilibrated with phosphate buffer pH 7.4. 40 mg/ml dialyzed protein was dissolved in 1 mL of equilibration buffer and loaded onto the column. To remove unbound proteins, protein-bound gel was washed with the equilibration buffer with a flow rate of 300 µl/min. The elution of bound proteins was done using increasing NaCl concentration (100, 200, 300 mM, and 1 M) in 50 mM phosphate buffer with pH 7.4. Eluted fractions were collected and evaluated for hemagglutination activity and for SDS-PAGE. Estimation of the purified sample was done by using Lowry’s methods (Lowry et al., 1951) along with a standard graph prepared by using 1 mg/ml of BSA.
2.4 Hemagglutination assay
Human erythrocytes of B and O blood groups were used to perform hemagglutination assay (13). 100 mM, 200 mM, 300 mM, and 1 M NaCl eluted protein fractions were used to perform hemagglutination assay. Protein fractions were serially diluted in PBS buffer (pH 7.4) on a U-shaped 96 well plate and 2 % erythrocyte suspension was added in all wells. After this incubation was done at 37 °C for 30 min. Hemagglutination titer was calculated as the reciprocal of the maximum dilution of protein which shows complete erythrocyte agglutination (Yagi et al., 2002).
2.5 Periodic acid Schiff’s staining
PAS (Periodic Acid Schiff) stain is the most flexible and commonly used carbohydrate visualization technique. Periodic acid – Schiff (PAS) is a staining method to identify polysaccharides in tissues. These can be glycogen and mucous substances such as glycoproteins, glycolipids, and mucins. 12 % SDS-PAGE was performed with the eluted Datura protein and the standard chick ovalbumin. The gel was immersed in 12 % TCA (tri-chloro acetic acid) for 30 min. as a fixative. TCA was removed and the gel was treated with mili Q water then transferred into the 1 % periodic acid reagent prepared with 3 % acetic acid and incubated for 1 h. After the incubation period, the gel was treated thoroughly with mili Q water for 90 min. with a routine change of water in each 10 min. The gel was transferred into the Schiff’s reagent for 1 h in the absence of light. After 1 h, the gel was washed with distilled water and incubated in 7 % acetic acid for 1 h and the gel was dried for the development of the bands.
2.6 Antiproliferative assay using MTT
Purified protein was tested in vitro against Caco-2 (colon) cancer cell lines to see if it has antiproliferative properties (Monks et al., 1991; Boleti et al., 2008). Optimum concentrations of cells were seeded in 96 well plates until the cell monolayer became confluent. The human cancer lines growing in DMEM with 10 % heat-inactivated FBS at 37 °C, 5 % CO2 in a CO2 incubator were treated for 72 hrs. with different concentrations of purified protein. Before treatment, protein solution at final concentrations of 0.25, 0.50, 1.0, 5.0, 10.0, 20.0, and 50.0 µg/ml was prepared in DMEM with heat-inactivated 10 % FBS.
As a control, some wells received the same concentration of cells and no protein in the DMEM medium. The MTT experiment was conducted using DMEM and 5 % fetal bovine serum, per the methodology provided by Heinrich et al., 2005. MTT assay calculates the reduction of yellow MTT by the action of enzyme succinate dehydrogenase to an insoluble, formazan which produces a dark purple colour, inside the mitochondria. In each experiment, 10 µl of MTT was added to the cells for 3 h incubation at 37 °C, 5 % CO2. Crystals of purple formazan were dissolved in isopropanol without water. At 37 °C, the dissolved purple solution was incubated for three hours. Using a spectrophotometer, the solubilized formazan reagent and 5 % CO2 are measured at 570 nm, with a background absorbance of 690 nm. An average of a few empty wells that contained only MTT solution, but no cells were used to serve as a background control (blank). Since the reduction of MTT occurs in live cells, it is used to measure cell viability.
3 Results
3.1 Extraction and ammonium sulphate precipitation
The Datura stramonium seed protein was extracted using a phosphate buffer at a pH of 7.4. The homogenized sample was filtered with a muslin cloth, followed by centrifugation at 10,000 rpm for 15 min at 4 °C. Ammonium sulphate ((NH4)2SO4) precipitation was performed using the supernatant. Samples with 20 %, 60 %, and 90 % ammonium sulphate saturation were obtained. The pellet from the 20 %, 60 %, and 90 % saturated sample was re-dissolved in 20 mM phosphate buffer (pH 7.4). Dialysis was performed to remove ammonium sulphate, and then SDS-PAGE was performed to visualize protein bands. Protein bands were observed in the crude sample of 60 % saturated fraction's pellet in the SDS-PAGE gel after staining the gel, as shown in Fig. 2. Therefore, protein purification was carried out from this 60 % fraction using ion-exchange chromatography.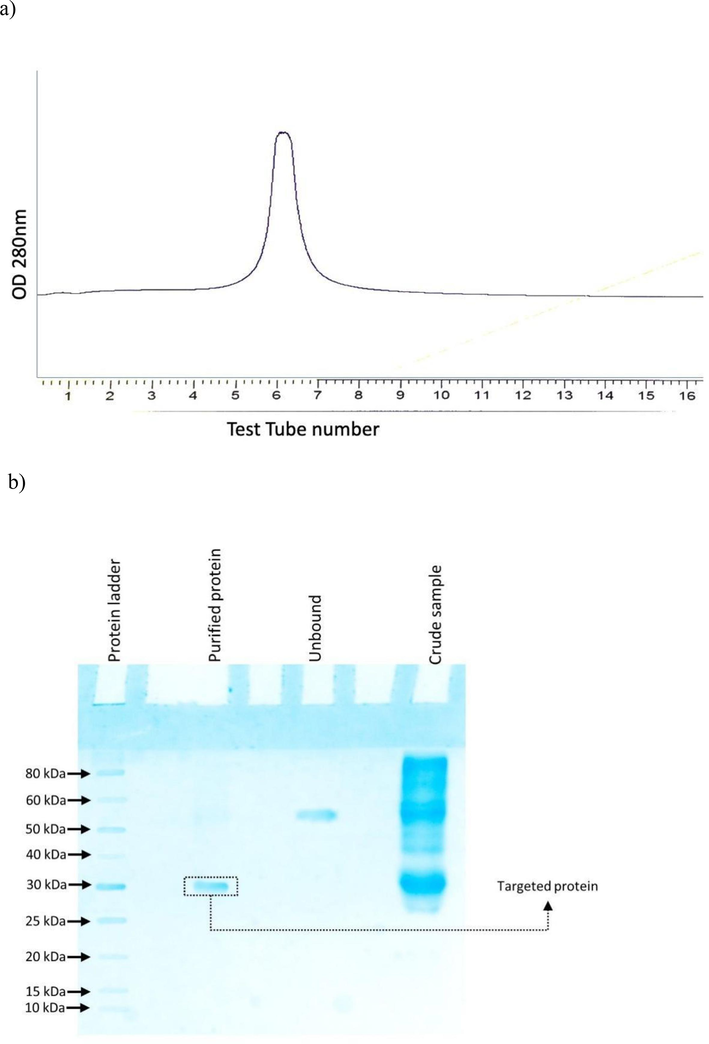
A) Elution peak with 200 mM NaCl and b) SDS-PAGE analysis showing crude, unbound, purified protein peak and protein ladder.
3.2 Protein purification
The dialyzed sample was utilized for anion exchange chromatography. 4 ml of DEAE resin was packed on a chromatographic column of dimension 40 × 1.5 cm and washed with water. The resin was equilibrated by using phosphate buffer pH 7.4. After equilibration 40 mg/ml of sample dissolved in the buffer was loaded onto the column. Unbound was collected from the column with the help of a phosphate buffer.
Bound proteins were eluted using NaCl, increasing the concentration (100, 200, 300 mM, and 1 M) in 50 mM phosphate buffer pH 7.4. A single peak was observed in 200 mM NaCl elute fraction as shown in Fig. 2a. SDS-PAGE revealed the presence of a single band of approximately 30 kDa, as shown in Fig. 2b. The elute peak was further tested for hemagglutination activity. The purified sample was estimated using Lowry’s methods (Lowry et al., 1951) along with a standard graph prepared by using 1 mg/ml of BSA. The concentration of purified protein was approximately 1 mg/ml.
3.3 Hemagglutination assay
Purified protein peak showed hemagglutination activity with human (B and O) erythrocytes as shown in Fig. 3. Hemagglutination titre was calculated as the reciprocal of the maximum dilution of protein which shows complete erythrocyte agglutination. Titre value of hemagglutination was observed at a minimum concentration of 15 µg/ml (B + ) and 3 µg/ml (O + ) of elute peak sample. This analysis predicts the lectin-like properties of purified protein samples. This protein sample showed a molecular mass of approximately 30 kDa on SDS-PAGE analysis. Further, the glycosylation nature of protein was determined by PAS staining.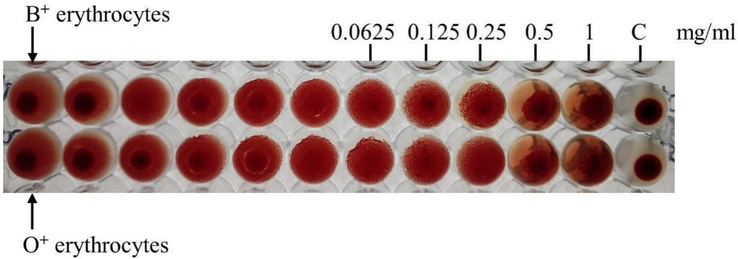
Hemagglutination assay of purified protein using human O and B blood cells.
3.4 Periodic acid Schiff’s staining
12 % SDS-PAGE were performed with the 200 mM NaCl eluted Datura protein and the standard as a chick ovalbumin (Leite et al., 2012). PAS stain of partially purified datura protein with ovalbumin as a positive standard shown in Fig. 4, the visible bands show the glycosylated nature of the protein which is 30 kDa DSL and the standard Ovalbumin with 42 kDa.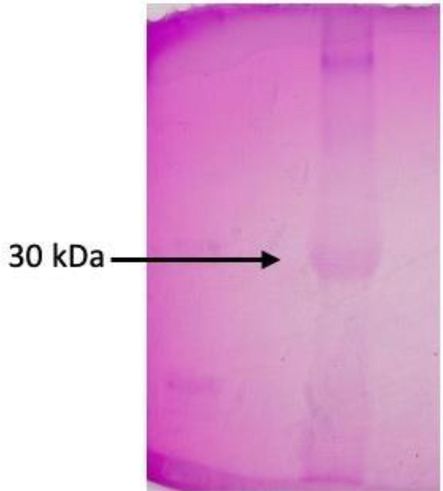
Periodic acid Schiff’s staining of purified protein with standard ovalbumin.
3.5 Cell viability assay by using MTT
To evaluate the anti-cancer property of isolated Lectin, Human cancer Caco-2 cells were treated in vitro conditions with purified protein and cell viability analysis was studied through MTT assay, and the results are shown in Fig. 5. Approximately 53 % growth inhibition of cells were recorded in this study.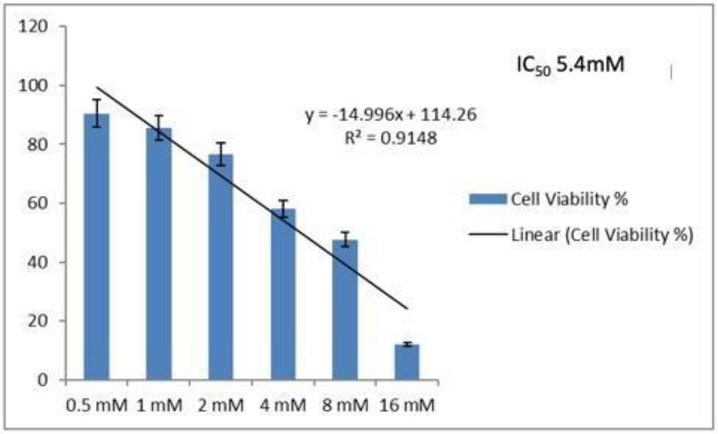
Cell viability assay of Caco-2 Cell lines treated with varying concentrations of purified protein.
4 Discussion
The results obtained from SDS-PAGE analysis revealed a singular band corresponding to a lectin termed DSL, with an estimated molecular weight of approximately 30 kDa (kDa). Furthermore, this DSL exhibited notable hemagglutination activity towards two pivotal human red blood cell types, namely B and O, indicating its ability to bind to specific carbohydrate moieties present on the surface of these cells. This lectin was further characterized through Periodic acid Schiff (PAS) staining, which confirmed its glycoprotein nature, suggesting the presence of carbohydrate residues essential for its biological activity and structural integrity.
In investigating the potential therapeutic utility of DSL, its anti-proliferative activity against human Caco-2 cancer cell lines was assessed using the MTT assay. The results demonstrated a significant inhibitory effect on the proliferation of Caco-2 cells, suggesting a potential role for DSL in cancer therapy. This inhibitory effect may be attributed to DSL's ability to interfere with signaling pathways crucial for cell growth and survival in the Caco-2 cell line.
We found only one lectin from D. stramonium, known as DSL, listed in the UniProt database (Pundir,Martin and O’Donovan, 2016; Wu et al., 2006), having mol wt. of approximately 29 kDa, which is specific for chitin, but structural information regarding DSL remains elusive. Therefore, to address this gap in knowledge, previous computational studies have been conducted to elucidate the three-dimensional structure of DSL (UniProt ID: A0A089ZWN7) and its interactions with various sugar molecules (Jain, Muthukumaran and Singh, 2020). Ongoing research endeavours aim to experimentally validate these computational findings, thereby providing insights into the structural basis of DSL's carbohydrate recognition and binding specificity. Furthermore, the broader literature on Datura species highlights the diverse bioactivities associated with extracts obtained from different parts of the plant, including antimicrobial, anti-inflammatory, anticancer, and anti-insecticidal properties (Sharma, et al., 2021). Previous studies on related species, such as Datura inoxia, have reported significant cytotoxicity and anticancer properties against specific cancer cell lines (Chinnasamy et al., 2014), further emphasizing the therapeutic promise of these botanical sources in drug discovery and development efforts.
Of particular significance, this study represents the first documentation of the anti-proliferative activity exhibited by a lectin-like protein isolated from Datura stramonium seeds. This finding expands our understanding of the pharmacological potential of Datura stramonium and underscores the importance of exploring its bioactive constituents for therapeutic applications.
5 Conclusion
In the present investigation, we isolated and characterized a lectin-like protein from the seeds of Datura stramonium, a member of the Solanaceae family. The purification process involved anion exchange chromatography utilizing DEAE-Sepharose resin. Subsequent analysis revealed that the purified lectin possesses a molecular weight of approximately 30 kDa (kDa). Notably, the lectin exhibited hemagglutination activity specifically targeting human B and O blood cells. Further characterization through Periodic Acid-Schiff (PAS) staining affirmed the glycoprotein nature of the purified protein. Moreover, we evaluated the potential antiproliferative properties of this lectin against Caco-2 human cancer cell lines utilizing the MTT assay. Results demonstrated a notable inhibition of cell proliferation, suggesting promising anti-cancer activity. However, to elucidate the underlying molecular mechanisms governing the inhibitory effects of DSL (Datura stramonium lectin) on tumor cell proliferation and differentiation, additional investigations are imperative. This study marks a significant advancement in understanding the extraction, isolation, purification, and functional attributes of DSL. Moreover, it underscores the potential of DSL as a valuable tool in cancer biology research, providing insights into its biological functions and paving the way for further exploration into its therapeutic applications.
CRediT authorship contribution statement
Monika Jain: Methodology, Writing – original draft. Mohd Amir: Methodology. Mohd Yousuf: Methodology. Manish Sharma: Resources. Sanjay Naik: Methodology. Sanjit Kumar: Methodology. Jayaraman Muthukumaran: Writing – review & editing. Mohd Sajid Ali: Methodology. Hamad A. Al-Lohedan: Funding acquisition. Amit Kumar Singh: Conceptualization, Writing – review & editing.
Acknowledgements
The authors thank Sharda University, Greater Noida, India for support. The authors acknowledge the financial support through the Researchers Supporting Project number (RSPD2024R724), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Adamse P., van Egmond H.P., Noordam M.Y., Mulder P.P.J., Nijs M. de. (2014), Tropane alkaloids in food: poisoning incidents. Quality Assurance and Safety of Crops & Foods. 2014;6(1):15–24.).
- Purification and applications of a lectin from the mushroom Gymnopilus spectabilis. Appl. Biochem. Biotechnol.. 2014;172(4):2081-2090.
- [Google Scholar]
- Purification and identification of bioactive protein from leaves of Datura inoxia P.mil. Biomed. Prev. Nutr.. 2014;4(2):143-149.
- [Google Scholar]
- Medicinal Applications of Plant Lectins. In: Fang E., Ng T., eds. Antitumor Potential and Other Emerging Medicinal Properties of Natural Compounds. Dordrecht: Springer; 2013.
- [CrossRef] [Google Scholar]
- a novel potential cytotoxic lectin-like protein with apoptosis-inducing activity in tumorigenic mammalian cells. Toxicon. 2008;51(8):1321-1330.
- [Google Scholar]
- Comparison of some molecular, enzymatic and antifungal properties of chitinases from thorn-apple, tobacco and wheat. Physiol. Mol. Plant Pathol.. 1988;33:319-331.
- [Google Scholar]
- A glycobiology review: carbohydrates, lectins and implications in cancer therapeutics. Acta Histochem.. 2011;113(3):236-247.
- [Google Scholar]
- Direct targeting of cancer cells: a multiparameter approach. Acta Histochem.. 2005;107(5):335-344.
- [Google Scholar]
- Jain, M, Muthukumaran J, Singh AK (2020). Structural and functional characterization of chitin binding lectin from Datura stramonium: insights from phylogenetic analysis, protein structure prediction, molecular docking and molecular dynamics simulation. J. Biomol. Struct. Dyn. 2020 Mar 11:1-19.
- Identification of Novel Protein from Datura stramonium Leaves with Bioinsecticide Potential Against Anopheles Stephensi. Int. J. Pept. Res. Ther.. 2023;29:49.
- [CrossRef] [Google Scholar]
- Possible application of lectins in diagnostics and therapy. Part I. Diagnostic application. Przegl. Lek.. 2008;65(4):189-194.
- [Google Scholar]
- Antinociceptive and anti-inflammatory effects of a lectin-like substance from Clitoriafairchildiana R. Howard seeds. Molecules (basel, Switzerland). 2012;17(3):3277-3290.
- [Google Scholar]
- Protein measurement with the Folin Phenol Reagent. J. Biol. Chem.. 1951;193:265-275.
- [Google Scholar]
- Structure-function and application of plant lectins in disease biology and immunity. Food Chem. Toxicol.. 2019;134:110827
- [CrossRef] [Google Scholar]
- Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J. Natl. Cancer Inst.. 1991;83:757-766.
- [Google Scholar]
- An overview of lectins purification strategies. J. Mol. Recognit.. 2012;25(11):527-541.
- [Google Scholar]
- Pundir, S., Martin, M. J., and O’Donovan, C. (2016). “UniProt Tools.” Current Protocols in Bioinformatics, 53,1.29.1-1.29.15.
- Anticoagulant and antiplatelet activities of novel serine protease purified from seeds of Cucumis maderaspatensis. 3 Biotech. 2021;11(1):30.
- [CrossRef] [Google Scholar]
- Phytochemistry, Pharmacology, and Toxicology of Datura Species-A Review. Antioxidants (basel, Switzerland). 2021;10(8):1291.
- [CrossRef] [Google Scholar]
- Lectins: carbohydrate-specific reagents and biological recognition molecules. J. Biol. Chem.. 2007;282(5):2753-2764.
- [Google Scholar]
- Pharmacological properties of Datura stramonium L. as a potential medicinal tree: an overview. Asian Pac. J. Trop. Biomed.. 2012;2(12):1002-1008.
- [Google Scholar]
- The detection and discovery of glycan motifs in biological samples using lectins and antibodies: new methods and opportunities. Adv. Cancer Res.. 2015;126:167-202.
- [Google Scholar]
- Protein precipitation using ammonium sulfate. Curr. Protoc. Protein Sci. 2001 Appendix-3F
- [Google Scholar]
- The Universal Protein Resource (UniProt): an expanding universe of protein information. Nucleic Acids Res.. 2006;34:D187-D191.
- [Google Scholar]
- The lectin from leaves of Japanese cycad, Cycas revoluta Thunb. (gymnosperm) is a member of the jacalin-related family. Eur. J. Biochem.. 2002;269(17):4335-4341.
- [Google Scholar]
- Lectins with potential for anti-cancer therapy. Molecules (basel, Switzerland). 2015;20(3):3791-3810.
- [Google Scholar]







