Translate this page into:
Exploration of Cladosporium uredinicola GRDBF21 and Bipolaris maydis GRDBF23 in biodegradation of the organophosphorus pesticide chlorpyrifos
⁎Corresponding author at: Department of Medical Laboratory Sciences, College of Applied Medical Sciences, Majmaah University, Saudi Arabia. m.palanisamy@mu.edu.sa (Palanisamy Manikandan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives
The present study explored the ability of lignin-degrading enzyme-producing fungal isolates, Cladosporium uredinicola GRDBF21 (Accession No. KJ913698) and Bipolaris maydis GRDBF23 (Accession No. KJ913699) in the biodegradation of structurally complex chlorpyrifos (CPS).
Methodology
Axenic cultures of the test isolates were acclimatised to CPS in Czapek Dox broth (CDB) containing 50 mgL−1 followed by 100 mgL−1 of CPS and subsequently with 200 mgL−1 of CPS as the only carbon source. A comparative evaluation of biomass production by the isolates in the presence and absence of CPS was made. The biodegradation of CPS by the isolates was evaluated by Gas Chromatography-Mass Spectrometry (GC–MS).
Results
The Minimum Inhibitory Concentrations (MICs) of CPS against C. uredinicola GRDBF21 and B. maydis GRDBF23 were 700 mg/L and 1300 mg/L, respectively. In the presence of CPS, C. uredinicola GRDBF21 and B. maydis GRDBF23 exhibited biomass production and maximum laccase activity of 4.2 g/L & 5.15 g/L and 290.14 U L−1 & 164.91 U L−1, respectively. The GC–MS analysis proved an effective biodegradation of CPS, as only a negligible and unquantifiable amount of CPS was present after 20 d of incubation.
Conclusion
These results are indicative that the test isolates could be effectively used in the bioremediation of recalcitrant environmental pollutants, especially organophosphate pesticides like CPS.
Keywords
Laccase
Lignolytic fungi
Neuro and respiratory system
Cardiovascular
Organophosphate pesticide
Chlorpyrifos (CPS)
Biodegradation
1 Introduction
In recent decades, a significant number of harmful xenobiotic pollutants have been released into the environment because of human activity, industrialisation, and rapid development. Chemical pesticides, in addition to their use in agriculture, are widely used in residential, urban, municipal, and industrial settings for maintaining plantings, removing weeds, controlling pests and mites, handling goods with hygienic practices, preserving wood, and preventing mould growth in paper mills (Mörner et al., 2002). Organophosphate pesticides are extremely toxic compounds with a broad spectrum of activity. Organophosphorus chemicals are used often and widely, which has led to the contamination of various ecosystems, the annual poisoning of 3 million people and the death of 200,000 people (Tse et al., 2004)
CPS [O, O-diethyl O-(3, 5, 6-trichloro-2-pyridyl) phosphorothioate] with a structural formula of C9H11Cl3NO3PS is a versatile, non-systemic, toxic, and persistent broad-spectrum organophosphate insecticide. It is used all over the world effectively against a wide range of agricultural pests, veterinary pests, mosquitoes, flies, termites, animal parasites and domestic pests due to their stability and effectiveness (Anwar et al., 2009). The respiratory, cardiovascular, and central neurological systems are all impacted by CPS intoxication (Slotkin, 2006).
The fate and behavior of CPS in soil have been intensively researched due to its extended persistence in soil environments and adverse impacts on species (Vejares et al., 2010). Due to the parent compound's conversion to metabolites, which are more persistent and/or equally toxic to non-target organisms, conventional treatment options, such as physicochemical methods for detoxification and elimination of toxic pesticide residues, are extremely inefficient, out-of-date, and sometimes not practical. Therefore, to remove them from the environment, efforts should be made to develop effective and affordable bioremediation techniques (Singh and Walker, 2006).
Several studies have reported the capability of microorganisms in the bioremediation of pesticides (Yongliang et al., 2013). Microorganisms play a crucial role in the degradation of the parent compound and the subsequent metabolism of its breakdown products. Fungal isolates like Aspergillus spp., Trichoderma harzianum and Penicillium brevicompactum have been reported to use CPS as a phosphorus and sulfur source (Omar, 1998). The present study was aimed at exploring lignin-degrading enzyme-producing fungal isolates, C. uredinicola GRDBF21 (Genebank Accession No. KJ913698) and B. maydis GRDBF23 (Genebank Accession No. KJ913699) in the biodegradation of structurally complex pesticide, CPS.
2 Materials and methods
2.1 Chemicals
The organophosphate pesticide, CPS [O, O-diethyl O-(3,5,6-trichloro-2- pyridyl) phosphorothioate; MW: 350.62], commercial grade CPS, Predator (50 %) was procured from Dow AgroSciences India Pvt. Ltd., Ahmedabad, Gujarat, India.
2.2 Fungal strains
Two lignolytic and constitutive laccase-producing fungal strains isolated from decaying wood bark C. uredinicola GRDBF21 (GenBank accession number: KJ913698) and B. maydis GRDBF23 (GenBank accession number: KJ913699) were used for the study. Both the isolates were morphologically and molecularly characterised and were determined for their capability to produce lignolytic enzymes.
2.3 Acclimatization studies
Initially, 1 mL of spore suspension (108 spores mL − 1) was inoculated into 100 mL of CDB containing 50 mgL−1 of CPS and incubated on a rotary shaker (100 rpm) at 28 ± 2 °C for two weeks. Following the incubation, 5 mL of culture of inoculated into CDB containing 100 mgL−1 of CPS and incubated as mentioned above. Thereafter, 2 subsequent transfers of 5 mL inoculum from previous incubation were made in CDB with CPS (200 mgL−1) as the only carbon source and incubated for 2–4 weeks. Following incubation, the isolates were maintained on CDA slopes containing 100 mgL−1 of CPS and used for further studies.
2.4 Determination of minimum inhibitory concentrations (MICs) of CPS against the test isolates
Sterile CDA plates with increasing concentrations of CPS (100 mgL−1 to 1500 mgL−1) were inoculated with the test isolates (0.2 cm disc from the CDA plate). The plates were incubated at 28 ± 2 °C for 7 d. After incubation, the plates were examined for colony growth. The MIC was noted as the least concentration of CPS that inhibited the fungal growth on test agar plates.
2.5 Effect of CPS on the biomass production by the test fungal isolates
Spore suspension (1 mL; 108 spores ml−1) of the test fungal isolates were inoculated to 100 mL CDB with and without CPS (200 mg/L) and incubated at 28 ± 2 °C on a rotary shaker at 120 rpm for 10 d. Using Whatman filter paper No. 1, the mycelial mass from each flask was extracted and then washed with distilled water. The fungal biomass was dried in a pre-weighed aluminium foil, until it reached a consistent weight at 80°C. The dry weight of the biomass was determined in g L − 1.
2.6 Screening of pesticide-acclimatized fungal isolates for laccase production
CDA plates with 2,2-azino-bis-3-ethylbenzothiazoline-6-sulphonic acid (ABTS; 0.1 g/L) and CPS (50 mg/L) were inoculated with 0.2 cm mycelial disc of the test fungal isolates. The plates were incubated at 28 ± 2 °C for 7 d. A positive activity was indicated by the formation of bluish–green colouration around the growth. For broth assay, sterile mineral salt medium (MSM) with CPS (200 mg/L) was inoculated with 0.2 cm mycelial disc and incubated at 28 ± 2 °C on a rotary shaker at 120 rpm for 15 d. The culture supernatant from the flasks was periodically assayed for laccase production.
2.7 Analysis of biodegradation of CPS by the fungal isolates
CPS degradation by the test fungal isolates was studied in MSM with CPS as the only carbon source. Sterile MSM (100 mL) containing CPS (100 mg/L) was inoculated with 1 mL spore suspension (108 spores mL−1 and incubated at 28 ± 2 °C on a rotary shaker at 120 rpm for 20 d. The mycelial mass and the culture filtrate were separated by filtration and were subjected to an extraction process with dichloromethane. The mycelial mass obtained was suspended in dichloromethane and subsequently, pesticide residues were extracted. The aqueous phase obtained was discarded and the pooled organic layer (obtained as an upper layer in a separating funnel) was dried over anhydrous sodium sulphate to remove moisture. The contents were evaporated to dryness on a rotary vacuum evaporator and the obtained residue was dissolved in n-hexane for further analysis.
The extracted samples were analyzed for the presence of CPS residues and their degradation products using a Gas Chromatography-Mass Spectrometry (GC–MS) system equipped with an electron capture detector (ECD) at South Indian Textile Research Association (SITRA), Coimbatore, Tamilnadu, India. The chromatograms were recorded and compared with the standard library of NIST-07 mass spectral data.
3 Results
In the present study, two isolates of constitutive laccase-producing lignolytic fungi, C. uredinicola GRDBFF21 and B. maydis GRDBF23 isolated from wood bark samples were screened for their potential to degrade the organophosphate pesticide CPS. The isolates recorded good growth during the acclimatization process with CPS and considerable growth was observed on CDA slopes containing CPS (100 mgL−1).
3.1 MIC studies
The MIC was established as the lowest possible concentration of any substance that capable of fully suppressing the visible germination of fungal spores. When there was a complete absence of germination of spores/growth at a specific concentration, the isolate was determined to be susceptible and that specific concentration of substance was taken as the ‘breakpoint’ for that particular isolate. MICs of CPS against C. uredinicola GRDBF21 and B. maydis GRDBF23 were 700 mg/L and 1300 mg/L, respectively (Table 1). Presence of the fungal growth (+); Absence of fungal growth (−); Values are based on the growth of triplicate plates.
Chlorpyrifos
(mg/L)
Growth of C. uredinicola GRDBF21
Growth of
B. maydis GRDBF23
100
+
+
200
+
+
300
+
+
400
+
+
500
+
+
600
+
+
700
−
+
800
−
+
900
−
+
1000
−
+
1100
−
+
1200
−
+
1300
−
−
3.2 Effect of CPS on biomass production
Considering the growth response and tolerance of C. uredinicola GRDBF21 and B. maydis GRDBF23 in the presence of various concentrations of CPS, a concentration of 200 mg/L of CPS was selected for studying the growth pattern of fungal test isolates in the presence of pesticide. From Fig. 1 and Fig. 2, it could be inferred that the growth and biomass production of the fungal isolates exposed to CPS were higher when compared to the growth in the medium without CPS. B. maydis GRDBF23 exhibited a higher production of mycelial biomass (515 mg/100 mL) than C. uredinicola GRDBF21 (420 mg/ 100 mL).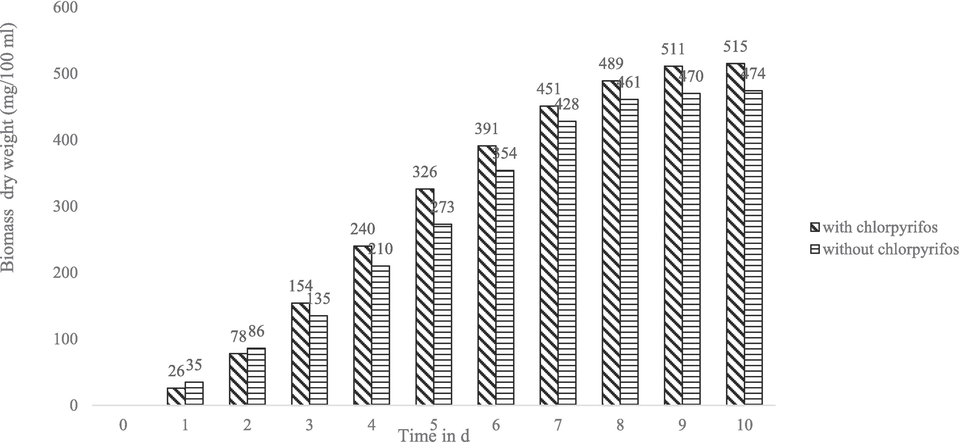
Growth pattern of B. maydis GRDBF23 in the presence and absence of chlorpyrifos (200 mg L − 1).
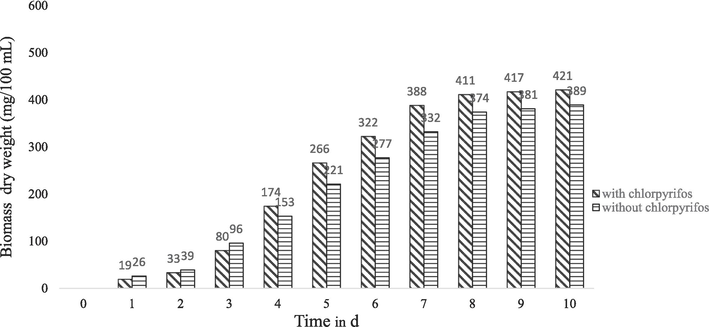
Growth pattern of C. uredinicola GRDBF21 in the presence and absence of chlorpyrifos (200 mg/L).
3.3 Laccase studies
The laccase production by the test isolates was qualitatively determined on CMA with ABTS. The test isolates exhibited green colouration on the test plates (Fig. 3). In the quantitative broth assay it was observed that the laccase activity of the test fungal isolates increased with the incubation time. The enzyme activity declined after the 7th and 9th day of incubation for B. maydis GRDBF23 and C. uredinicola GRDBF21, respectively. B. maydis GRDBF23 exhibited a maximum laccase activity of 290.14 UL−1 on the 7th day of the incubation period in the presence of CPS. However, in the control sample (without CPS), a maximum activity of 201.51 UL−1 was observed on the 9th day of incubation. Similarly, C. uredinicola GRDBF21 exhibited a maximum laccase activity of 164.91 UL−1 on the 9th day of incubation in the presence of CPS and showed a maximum of only 130.2 UL−1 on the same day in the control without CPS and the enzyme activity of both the isolates in the presence and absence of CPS declined on further incubation period (Table 2). Values are means of duplicate assays.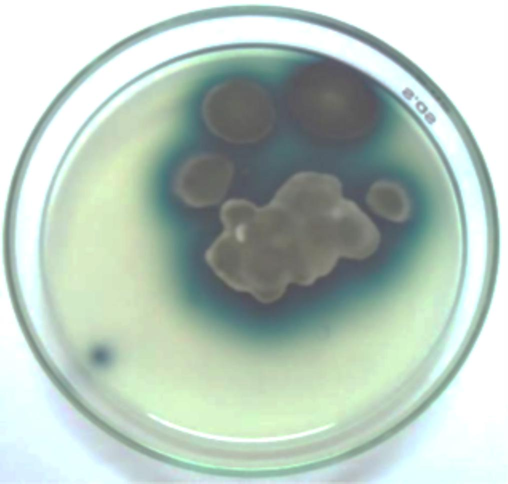
Bluish-green colour formation around the colony on CDA with ABTS indicating laccase production. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Incubation
time (d)
Enzyme activity of fungal isolates (UL−1)
C. uredinicola GRDBF21
B. maydis GRDBF23
Control (without CPS)
Test(with CPS)
Control (without CPS)
Test(with CPS)
1
0.461
1.04
29.51
36.46
3
7.29
6.52
46.28
71.79
5
28.33
35.75
80.33
118.62
7
78.62
90.08
179.62
290.14
9
130.2
164.91
201.51
233.86
11
81.46
120.79
168.38
194.20
13
54.81
88.69
121.78
164.78
15
40.02
57.04
93.57
123.57
3.4 Analysis of CPS biodegradation
The GC–MS analysis of the CPS standard showed a peak at retention time (RT) of 22.74 min representing CPS (Fig. 4). The peak was identified based on its mass spectra and the NIST database. The GC–MS analysis conducted on the 1st day of incubation showed the same findings as that of untreated culture filtrate. This peak dropped subsequently with further incubation. The formation of other new peaks at various retention times suggested CPS degradation. C. uredinicola GRDBF21 exhibited a considerable change in the peak pattern after 20 d of incubation and only a negligible amount of CPS was detected after this period and was not sufficient for quantification (Fig. 5). It confirmed that C. uredinicola GRDBF21 degraded CPS almost completely. Further, the peaks obtained for the control sample remained unchanged even after 20 d of incubation. This showed that abiotic and environmental factors did not affect CPS. Analysis of residual CPS in the B. maydis GRDBF23 treated medium showed the complete degradation of CPS after 20 d of incubation (Fig. 6).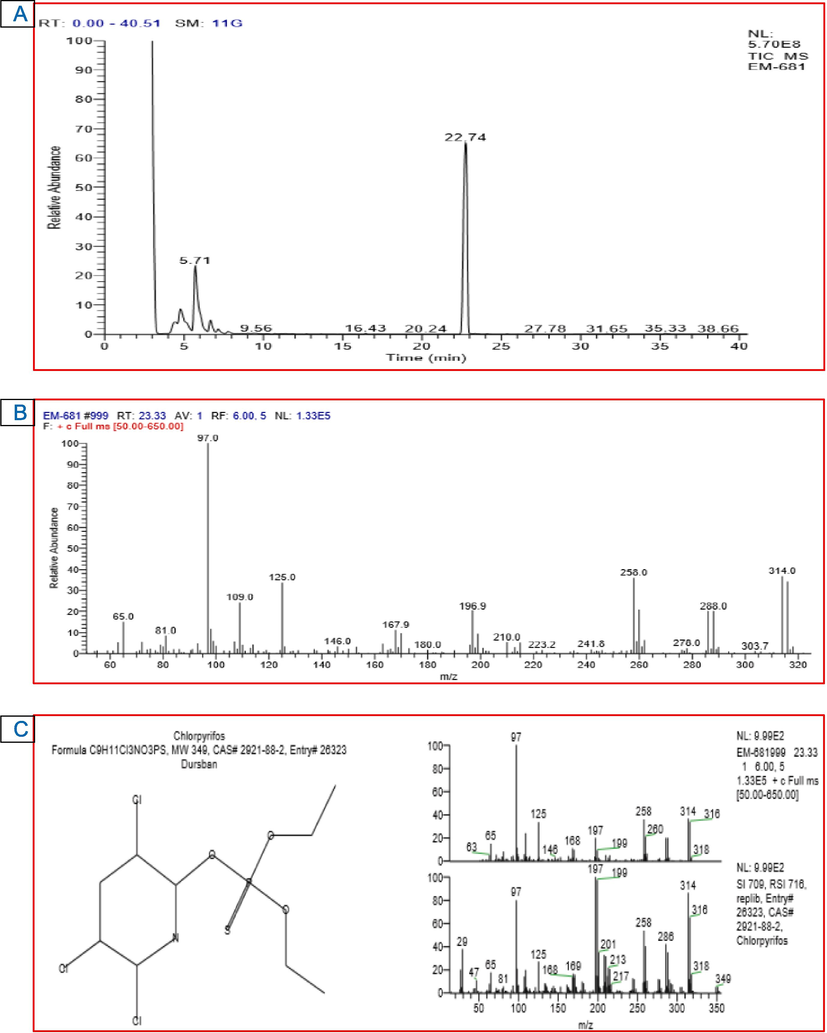
GC–MS chromatogram of CPS standard (A); Mass spectrum of CPS (B); NIST library database search result (C).
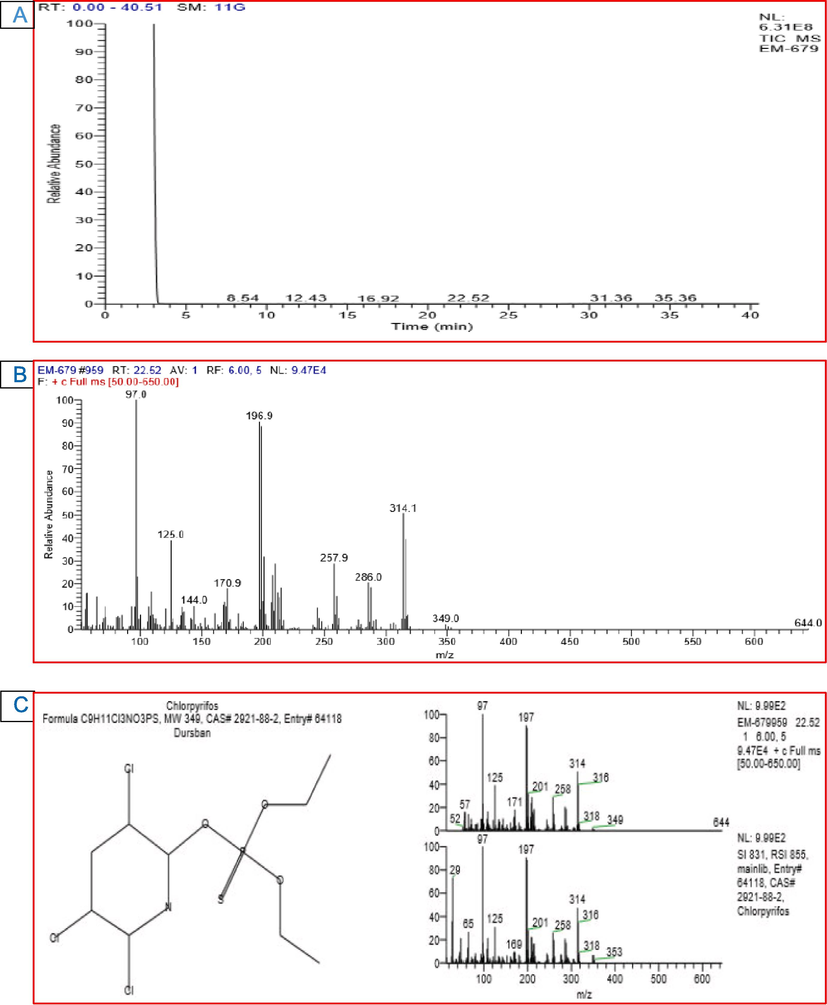
GC–MS chromatogram of CPS treated with C. uredinicola GRDBF21 showing the disappearance of peaks indicating the degradation of pesticide in medium (A); Mass spectrum of CPS (B); NIST library database search result (C).
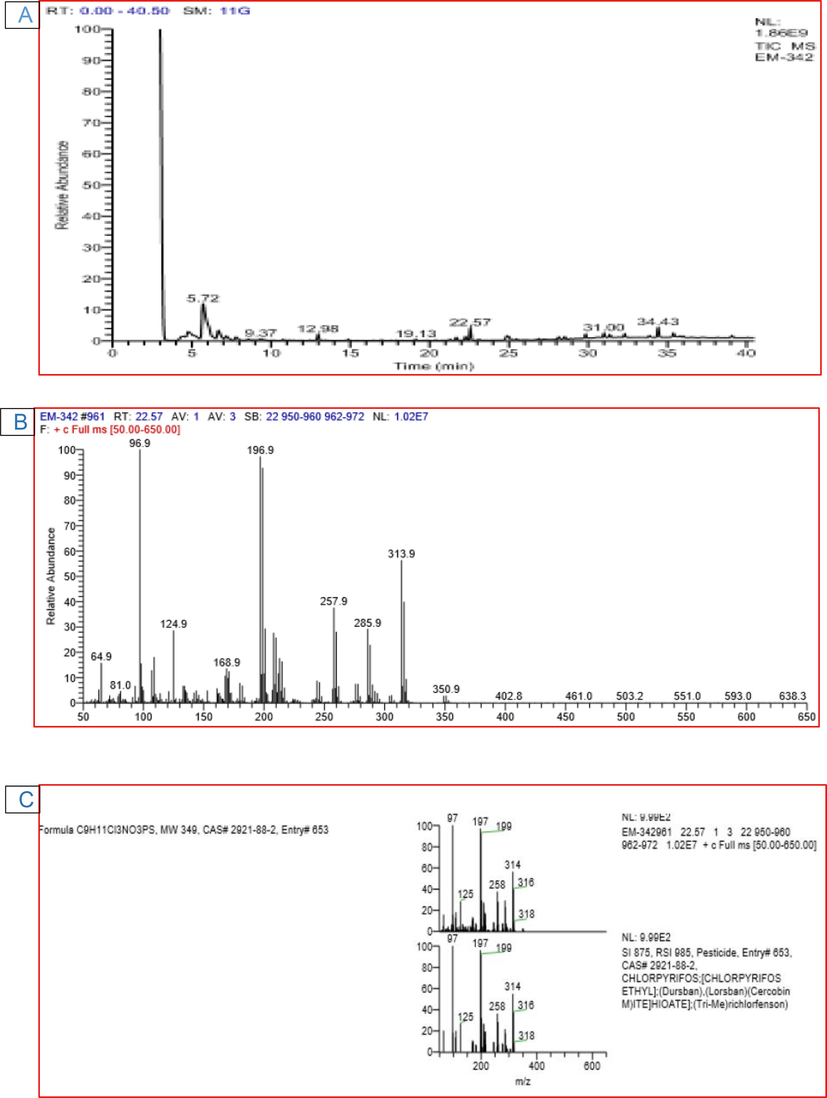
GC–MS chromatogram of CPS treated with B. maydis GRDBF23 showing the difference in peak area and pattern appeared indicating the degradation of pesticide in medium (A); Mass spectrum of CPS (B); NIST library database search result (C).
4 Discussion
There is a growing interest in the degradation and removal of pollutants from the environment using biological methods. Biological methods have no negative effects when compared to the physicochemical methods of removing pollutants and they do not affect the natural ecosystems and have low cost (Corso and Maganha de Almeida, 2009). Lignolytic fungi and their enzymatic system have been extensively studied to explore their potential application in the bioremediation of xenobiotics such as polycyclic aromatic hydrocarbons (PAH), polychlorinated biphenyl (PCB), pesticides, herbicides, synthetic dyes, and various industrial effluents (Tišma et al., 2010). The majority of the wood-colonizing fungi are ligninolytic and the wood-degrading fungi are the only organisms able to completely mineralize recalcitrant lignin polymer (Anastasi et al., 2013). With the aid of extracellular ligninolytic enzymes of broad substrate specificity and high adaptability, these fungi establish the grounds for the bioremediation and detoxification of various recalcitrant toxic xenobiotic compounds of anthropogenic origin (Reddy, 1995; Atalla et al., 2010).
The current analysis thus employed lignolytic fungal isolates viz., C. uredinicola GRDBFF21 and B. maydis GRDBF23 and their ability to be utilized for the biodegradation of structurally complex pesticide, CPS was analysed. The MICs analysis showed that the isolates had good tolerance towards CPS and their growth response to the pesticide was influenced by pesticide concentration. It was reported that several groups of fungi belonging to the class Ascomycotina were involved in the degradation of organophosphate compounds (Maya et al., 2012). The findings of the present study were comparable to those of Bhalerao and Puranik (2009), where A. oryzae ARIFCC 1054 was able to tolerate an organophosphate pesticide, monocrotophos up to a concentration of 900 mg/L and grew well up to 500 mg/L.
The ability of these test fungi to grow in the presence of pesticides, as well as their ability to produce degradative enzymes, determines their suitability for bioremediation. Hence, laccase production by C. uredinicola GRDBF21 and B. maydis GRDBF23 in the presence of CPS were thus analysed by both plate assay and broth assay methods. The results indicated that the presence of CPS did not hinder the laccase production by both the test fungal isolates. Lignolytic enzymes viz., lignin peroxidase, manganese dependent peroxidase and laccase are directly involved not only in the degradation of lignin in their natural lignocellulosic substrates but also in recalcitrant environmental pollutants such as crude oil wastes, textile effluents, organochloride agrochemicals and pulp effluents which are as cause of serious environmental pollution (Kiiskinen et al., 2004; Mtui and Nakamura,). It is critical to monitor the effect of compounds on the enzymatic profile of the microbes as the growth and lignolytic enzymes are produced mainly in environmentally stressful conditions. The growth and lignolytic enzyme production by the fungi could be favoured as well as promoted in the bio-mixture containing pesticides (Castillo et al., 2007). In this respect, it was reported that the laccase from Pleurotus ostreatus in the presence of ABTS exhibited a complete oxidative degradation of organophosphorus insecticide and nerve agent. The present study recorded an increase in laccase production with incubation time in the medium amended with CPS. Similarly, Maciel et al. (2013) reported that the addition of xenobiotics has a stimulating effect on laccase production. An increase in the laccase enzyme activity could be attributed to the oxidative stress on fungi caused by CPS, as observed by Cupul et al. (2014). Thus, exposure of C. uredinicola GRDBF21 and B. maydis GRDBF23 to CPS stimulated their lignolytic enzyme production. Based on the outcomes, it could be concluded that CPS had no inhibitory effect on the laccase production of both the test fungal isolates. Thus, exposure of C. uredinicola GRDBF21 and B. maydis GRDBF23 to CPS stimulated their lignolytic enzyme production.
GC- MS analysis of the solvent-extracted culture filtrate was conducted to analyze the degradation of CPS as well as the residual concentration of the CPS in the liquid medium and also to elucidate the structural changes of CPS after test fungal treatment. The GC–MS results indicated an effective biodegradation of the CPS (100 mg/L) by both the test fungal isolates. Further, the toxic degradation product, 3,5,6-trichloro-2-pyridinol (TCP) was also not detected during the study. It could be due to the complete utilization of this compound as the energy source by the test fungal isolates. TCP is the major degradation product of CPS (Meikle et al., 1978; Tomlin, 2003). Another degradation product, diethyl thiophosphoric acid (DETP) formed due to hydrolysis of phosphor-ester linkage in CPS was also detected. Singh et al. (2003 & 2004) reported the biodegradation of CPS into DETP and TCP by microorganisms in a liquid medium and DETP could also be used as a source of energy by microbes when further incubated. The occurrence of unidentified peaks in the chromatograms of the present study could represent the degradative byproducts of TCP requiring further investigation.
The mechanism of CPS degradation in bacteria and fungi is fairly understood and several degradation products such as diethylthiophosphoric acid, TCP, chlorodihydro-2-pyridone, dihydroxy pyridine, tetrahydro-2pyridone, and maleamide semialdehyde have been identified (Singh and Walker, 2006). Chen et al. (2012) & Gao et al. (2012) have also analysed the degradation of CPS by C. uredinicola. Xie et al. (2013) reported that laccase-producing Pichia pastoris showed excellent degradation of CPS. Harish et al. (2013) reported that T. harzianum was able to degrade 70 – 80 % of CPS in 21 days of incubation. Abraham and Silambarasan (2013) reported 100 % degradation of CPS and TCP in the soil added with nutrients as well as in the mineral medium by the fungal strain A. terreus JAS1. Geetha and Fulekar (2008) demonstrated the complete degradation of two CPS metabolites, i.e., TCP and benzo pyridine into simpler organic compounds and benzene derivatives in a CPS concentration of 50 mg/L.
Continuous research on lignolytic white rot fungi has proved that the lignolytic enzymes involved in the lignin degradation are extracellular, highly non-specific about their substrate range and free radical based. Because of this peculiar feature, lignin-degrading fungal enzymes have been shown as capable of the transformation and mineralization of a wide range of highly recalcitrant xenobiotic pollutants with structural similarities to lignin (Pointing, 2001; Pozdnyakova et al., 2022). Very importantly, these fungi do not need previous exposure to the pollutant. Furthermore, an enzyme system can be induced even at low concentrations of the pollutant and degradation of the pollutant with additional nutritional sources (Pointing, 2001). In addition, the lignolytic enzyme system is extracellular, insolubility and impermeability of the pollutant are not an obstacle for biodegradation.
Conclusively, the two novel lignolytic fungal strains − C. uredinicola GRDBF21 and B. maydis GRDBF23, were specifically assessed for their effectiveness in the biodegradation of organophosphate pesticide CPS. The results of the analyses proved an effective biodegradation of the CPS by both the test fungal isolates.
5 Conclusions
The MICs of CPS against the test fungal isolates C. uredinicola GRDBF21 and B. maydis GRDBF23 were 700 mg/L and 1300 mg/L, respectively. In the presence of CPS, test fungal isolates C. uredinicola GRDBF21 and B. maydis GRDBF23 exhibited a biomass production of 4.2 g/L and 5.15 g/L, respectively. C. uredinicola GRDBF21 and B. maydis GRDBF23 exhibited a maximum laccase activity of 290.14 U L−1 and 164.91 U L−1 in the presence of CPS, respectively. The GC–MS analysis proved an effective biodegradation of the CPS by both the test fungal isolates.
CRediT authorship contribution statement
Jameson T. Joseph: Investigation. Coimbatore Subramanian Shobana: Conceptualization, Supervision, Writing - Original Draft. Dhivya Sekhar: Investigation. Sreeram Suresh: Investigation. Saritha Poothenchery: Investigation. Kanesan Panneer Selvam: Validation, Supervision. Saleh Abdullah Aloyuni: Project administration, Formal analysis. Ayoub Al Othaim: Project administration, Formal analysis. Bader Alshehri: Project administration, Formal analysis. Ahmed Abdel-Hadi, Ahmed Ismail: Project administration, Formal analysis, Data curation. Palanisamy Manikandan: Funding acquisition, Writing - Review and Editing, Conceptualization, Validation.
Acknowledgments
The authors extend their appreciation to the deputyship for Research & Innovation, Ministry of education in Saudi Arabia for funding this research work through the project number (IFP-2022-09).
Funding
This research was funded by the deputyship for Research & Innovation, Ministry of education in Saudi Arabia through the project number (IFP-2022-09).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Biodegradation of chlorpyrifos and its hydrolyzing metabolite 3,5,6-trichloro-2-pyridinol by Sphingobacterium sp. JAS3. Process. Biochem.. 2013;48:1559-1564.
- [CrossRef] [Google Scholar]
- Anastasi A., Tigini V., Varese G.C., 2013. the bioremediation potential of different ecophysiological groups of fungi., in: Goltapeh E.M., Danesh Y.R., Varma A., (Eds.). Fungi as Bioremediators. Volume 32. Soil Biology, Springer; Berlin/Heidelberg, Germany, pp. 29–49.
- biodegradation of chlorpyrifos and its hydrolysis product 3,5,6-trichloro-2-pyridinol by Bacillus pumilus strain C2A1. J. Hazard. Mater.. 2009;168(1):400-405.
- [Google Scholar]
- Microbial degradation of monocrotophos by Aspergillus oryzae. Int. Biodeterior. Biodegradation. 2009;63(4):503-508.
- [CrossRef] [Google Scholar]
- Effect of Biobed Composition, Moisture, and Temperature on the Degradation of Pesticides. J. Agric. Food Chem.. 2007;55:5725-5733.
- [CrossRef] [Google Scholar]
- Biodegradation of chlorpyrifos and its hydrolysis product 3,5,6-trichloro-2-pyridinol by a new fungal strain Cladosporium cladosporioides Hu-01. PLoS One.. 2012;7(10):e47205 Epub 2012 Oct 8. PMID: 23056611; PMCID: PMC3466218
- [CrossRef] [Google Scholar]
- Bioremediation of dyes in textile effluents by Aspergillus oryzae. Microb. Ecol.. 2009;57:384-390.
- [CrossRef] [Google Scholar]
- Response of ligninolytic macrofungi to the herbicide atrazine: dose-response bioassays. Rev. Argent Microbiol.. 2014;46:348-357.
- [CrossRef] [Google Scholar]
- Purification and characterization of a novel chlorpyrifos hydrolase from Cladosporium cladosporioides Hu-01. PLoS One.. 2012;7(6):e38137 Epub 2012 Jun 5. PMID: 22693630; PMCID: PMC3367910
- [CrossRef] [Google Scholar]
- Bioremediation of pesticides in surface soil treatment unit using microbial consortia. African J. Environ. Sci. Technol.. 2008;2:036-045.
- [Google Scholar]
- Biodegradation of organophosphate pesticide by soil fungi. Adv. Bio Tech.. 2013;12:4-8.
- [Google Scholar]
- Screening for novel laccase-producing microbes. J. Appl. Microbiol.. 2004;97(3):640-646.
- [CrossRef] [Google Scholar]
- Response of Ganoderma lucidum and Trametes sp. to the herbicide picloram: Tolerance, antioxidants and production of ligninolytic enzymes. Pestic. Biochem. Physiol.. 2013;105:84-92.
- [CrossRef] [Google Scholar]
- degradation kinetics of chlorpyrifos and 3,5,6-trichloro-2-pyridinol (TCP) by fungal communities. Bioresour. Technol.. 2012;126:216-223.
- [CrossRef] [Google Scholar]
- The hydrolysis rate of chlorpyrifos, O-O-diethylO-(3,5,6-trichloro-2-pyridyl) phosphorothioate, and its dimethyl analog, chlorpyrifos-methyl, in dilute aqueous solution. Arch. Environ. Contam. Toxicol.. 1978;7:13-22.
- [CrossRef] [Google Scholar]
- Reducing and eliminating the use of persistent organic pesticides. Geneva: United Nations Environment Programme (UNEP); 2002. p. :5-6.
- Feasibility of bioremediation by white-rot fungi. Appl. Microbiol. Biotechnol.. 2001;57(1–2):20-33. PMID: 11693920
- [CrossRef] [Google Scholar]
- Widespread Ability of Ligninolytic Fungi to Degrade Hazardous Organic Pollutants as the Basis for the Self-Purification Ability of Natural Ecosystems and for Mycoremediation Technologies. Appl. Sci.. 2022;12(4):2164.
- [CrossRef] [Google Scholar]
- The potential for white-rot fungi in the treatment of pollutants. Curr. Opin. Biotechnol.. 1995;6(3):320-328.
- [CrossRef] [Google Scholar]
- Microbial degradation of organophosphorus compounds. FEMS Microbiol. Rev.. 2006;30(3):428-471.
- [Google Scholar]
- Effects of soil pH on the biodegradation of chlorpyrifos and isolation of a chlorpyrifos-degrading bacterium. Appl. Environ. Microbiol.. 2003;69(9):5198-5206. PMID: 12957902; PMCID: PMC194978
- [CrossRef] [Google Scholar]
- Biodegradation of chlorpyrifos by enterobacter strain B-14 and its use in bioremediation of contaminated soils. Appl. Environ. Microbiol.. 2004;70(8):4855-4863. PMID: 15294824; PMCID: PMC492451
- [CrossRef] [Google Scholar]
- Slotkin, T.A., 2006. Developmental neurotoxicity of organophosphates. In: Gupta, R., C (Ed.) Toxicology of organophosphate & carbamate compounds, in: Toxicology of organophosphate & carbamate compounds. Elsevier, pp. 293–314.
- White-rot fungi in phenols, dyes and other xenobiotics treatment – a brief review. Croat. J. Food Sci. Technol.. 2010;2(2):34-37.
- [Google Scholar]
- The Pesticide Manual: A World Compendium (13th ed.). 2003.
- Method for the determination of organophosphate insecticides in water, sediment and biota. Chemosphere.. 2004;54(1):41-47.
- [Google Scholar]
- Tissue-specific inhibition and recovery of esterase activities in Lumbricus terrestris experimentally exposed to chlorpyrifos. Comp. Biochem. Physiol. C Toxicol. Pharmacol.. 2010;151(3):351-359.
- [Google Scholar]
- Production of a recombinant laccase from Pichia pastoris and biodegradation of chlorpyrifos in a laccase/vanillin system. J. Microbiol. Biotechnol.. 2013;23(6):864-871. PMID: 23676909
- [CrossRef] [Google Scholar]
- A Review on the detoxification of organophosphorus compounds by microorganisms. African J. Microbiol. Res.. 2013;7(20):2127-2134.
- [Google Scholar]







