Translate this page into:
Exosomal miRNA-93 and miRNA-205 expression in endometrial cancer
⁎Corresponding author at: Department of Obstetrics and Gynecology, West China Second University Hospital of Sichuan University, Chengdu, Sichuan 610041, China. LacyJacobsonuph@yahoo.com (Xinru Liu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Noted exosomal miRNA -93 expression in endometrial cancer patients. Observed enhanced 1xosomal miRNA-205 expression in cancer patients. miRNA-205 expression is associated with smoking.
Abstract
Endometrial Carcinoma (EC) is one of the most common cancers of females’ reproductive system. It has been given first rank in the tumors of obstetrics and gynaecology and day by day the incidence of EC gradually increased in recent years, which has critically harmed the woman health. 100 cases of Endometrial cancer and equal number of female healthy subjects were included and serum were separated from blood sample collected in plain vials from all study participant. Total RNA was extracted from exosomes extracted from serum and cDNA were synthesised from 100 ng of total RNA. cDNA were subjected to quantitative real time PCR to study the microRNA-95 and micro-RNA205 expression among cased and controls using Taqman assay. 3.78 mean fold increased exosomalmicroRNA-95 expression was observed while 0.07 mean fold decreased exosomal microRNA was found in endometrial cancer patients. Increased expression of exosomal miRNA-93 expression were linked with smoking (p = 0.002), grade of tumor (p = 0.01), FIGO stage (p < 0.0001) and distance organ metastases (p < 0.0001). Decreased miRNA-205 (0.07 mean fold) expression of exosomal miRNA-205 was observed in EC patients compared to healthy controls and were found to associated with smoking (p = 0.01), lymph node involvement (p = 0.001), FIGO stage (p = 0.004). Poor overall median survival was observed to be associated with increase exosomalmicroRNA-95 and decreased microRNA-205 of endometrial cancer patients. ROC curves showed exosomal miRNA-93 at cut-off value of 3.25 fold increased in serum based exosomal microRNA-93 expression, sensitivity and specificity were 93% and 97%, respectively (AUC was 0.99, p < 0.0001). A negative correlation was observed between exosomal microRNA-93 and exosomal microRNA-205 expression (r = −0.20, p = 0.04).
Study concluded that exosomalmicroRNA-95 and exosomal microRNA-205 was associated with worseness of disease and could be a prognostic indicator for endometrial cancer patients.
Keywords
miRNA-93
miRNA-205
Expression
Cancer patients
ROC
1 Introduction
Endometrial Carcinoma (EC) is one of the most frequent malignant epithelial tumor reproductive system cancers of females. It has been given first rank in the tumors of obstetrics and gynaecology among Europe and America countries (Buhtoiarova et al., 2016). The incidence of EC in China, gradually increased in recent years, which has critically harmed the woman health (Hu et al., 2015). The diagnosis of EC is generally based on clinical symptoms, the history of disease and pathological outcome. However, the early symptoms of EC are not clear, and not easily diagnosed (Dijkhuizen et al., 2000), so the EC patient’s survival rate is not so good (Tangjitgamol et al., 2013). The early diagnosis is helpful to the standardized treatment to improve of the EC patient’s survival rate. At present, EC is still one of lethal gynaecological malignant tumors which cause the early death of EC patients. Early diagnosis of EC is very important to understand the pathogenesis of EC, early invasion and metastasis in term of better clinical management and care of patients (Prat, 2004). Micro-ribonucleic acid (miRNA) is a group of non coding RNA with a length of about 18–25 bp. miRNA can control gene regulation at the transcriptional level by degradation of mRNA, translational repression and other target RNAs and cause cell explosion, differentiation and apoptosis (Medina and Slack,2008). miRNA-93 is positioned chromosome number 7q.22.1 and produced by the transcription of miRNA-106b-25. miRNA-93 can contribute in many immune reactions and inflammatory through the interactions with target proteins MMP-2, integrin-β8 and E2F1 (Lyu et al., 2014). It has been revealed that MiR-93 expression has been associated in different cancer types as oncogenic role (Fu et al., 2012). miRNAs are normally deregulated in multiple human cancer and can perform as strong tumor suppressors and oncogenes (Esquela-Kerscher et al., 2006). miRNA-205 is the most commonly analysed miRNAs, exhibiting irregular expression in various human malignancies compared with healthy controls. The majority of studies have established that miRNA-205 behave as tumour suppressor microRNA (Hulf et al., 2013). However, few studies have given contradictory outcomes suggesting that miRNA-205 may function as an oncogene (Kalogirou et al., 2013). Hanna et al revealed that miRNA-205 upregulation suppresses growth of cell in vivo, cell senescence induction and cell proliferation reduction by targeting to down-regulate adenovirus E2 promoter-binding transcription factor 1 (E2F1) in melanoma patients (Hanna et al., 2012). MicroRNA-205 specifically targets and activates the tumour suppressor genes IL24 and IL32 by intracting with their promoter regions (Majid et al., 2010) and participates in transition of epithelial-mesenchymal by regulating zinc finger E-box-binding proteins (ZEB1/ZEB2) (Tran et al., 2013). Thus the present aimed to study analysed the serum based exosomal miRNA-93 and miRNA-205 among Endometrial cancer patients.
2 Materials and methods
2.1 Blood sample collection and exosomes isolation from serum
This study recruited newly diagnosed histopathologically confirmed 100 cases of Endometrial cancer. Patients previously with any history of metastasized cancer or any other body organ cancer were not included in study. 4 ml of patient’s peripheral blood were collected in plain vials after confirmed diagnosis as well as 4 ml of peripheral blood from healthy individuals. Blood samples collected in plain vials were centrifuged at 1500 rpm to separate the serum were collected and stored at −70 °C. Before exosomes isolation sample were thawed and centrifuged at 3000×g to for 10–20 min to pellet cells, debris and platelets to remove. 1.5 ml of serum were mixed in 600 μl of precipitation buffer A (miRCURY, Qiagen) and mixed for 30 s. Mixture was incubated for 60 min at 2–8 °C and centrifuges at 1500×g for 25 min at 20 °C. After centrifugation pellet were saved and resuspended in 200 μl of resuspension buffer to the tube containing pellet and further used for total exosomal RNA extraction.
2.2 Total RNA extraction, Polyadenylation and cDNA synthesis
Total RNA was extracted from exosomes suspended in resuspension buffer using Trizol and stored at −70 °C in RNase-free eppendorf tubes. The quality and purity of RNA were determined by the A260/280 ratio. 100 ng of total RNA was used for Polyadenylation and cDNA synthesis using advanced microRNA cDNA Synthesis Kit (TaqMan, Thermo Scientific, USA) by manufacturer protocol. Reverse Transcriptase enzyme and other essential reagents were added subsequently for cDNA synthesis to switch in poly (A) - tailed miRNAs into cDNA using an universal RT primer supplied with the manufacturer kit.
2.3 qPCR for miRNA-93 and miRNA-205 expression
Quantitative real-time PCR (qPCR) was performed to compute the serum based exosomal miRNA-93 and miRNA-205 expression level. Quantitative real time PCR was performed using advanced taqman master mix (4444556), Taqman probes (478768_mir) for miRNA-214 for quantification and hsa-miR-16-5p (477860_mir) were used as internal control as normaliser to calculate the expression.
2.4 Statistical analysis
All the data analysis was performed by using SPSS 20.0 and Graph Pad Prism 5 version of software. ΔΔct method was applied to compute the fold change in expression of miRNA-93 and miRNA-205 in EC patients. Parametric (Student t test and ANOVA) and nonparametric (Mann Whitney U test and Kruskal Wallis) test were performed to compare the various variables. The Kaplan–Meier analysis was used to compute the overall survival of EC patients. ROC curve was plotted to check the prognostic importance of miRNA-93 and miRNA-205 in EC patients and p value < 0.05 was considered to be statistically significant.
3 Results
3.1 Demographic and clinical characteristic of endometrial cancer patients
All the demographic and clinical characteristics were depicted in Table 1. Endometrial cancer patients in age group of <50 years were 48% while 52% were in age group of ≥50 years while in healthy controls in age group of <50 years were 50% and ≥50 years were 50%. Patients who were alcoholic were 45% while non alcoholic were 55% while in healthy controls 40% were alcoholic and 60% were non alcoholic, more details presented in Tables 1–3.
Variables
EC Cases (%)
Healthy controls (%)
Age (in years)
<50 years
48 (48)
50 (50)
≥50 years
52 (52)
50 (50)
Alcoholism
Yes
45 (45)
40 (40)
No
55 (55)
60 (60)
Smoking
Yes
34 (34)
30 (30)
No
66 (66)
70 (70)
Tobacco
Yes
24 (24)
20 (20)
No
76 (76)
80 (80)
Grade
G1
15 (15)
–
G2
42 (42)
–
G3
43 (43)
–
Lymph node involvement
Yes
20 (20)
–
No
80 (80)
–
FIGO stage
I & II
54 (54)
–
III & IV
46 (46)
–
Distant organ metastases
Yes
17 (17)
–
No
83 (83)
–
Variables
miRNA-93 expression (Mean ± SD)
P value
Overall expression
3.78 ± 1.39
–
Age (in years)
≤50 years
3.87 ± 1.52
0.64
>50 years
3.70 ± 1.28
Alcoholism
Yes
3.88 ± 1.41
0.58
No
3.74 ± 1.41
Smoking
Yes
4.42 ± 1.46
0.002
No
3.45 ± 1.24
Tobacco
Yes
3.96 ± 1.52
0.46
No
3.72 ± 1.36
Grade
G1
3.97 ± 1.41
0.01
G2
3.29 ± 1.17
G3
4.20 ± 1.46
Lymph node involvement
Yes
4.27 ± 1.42
0.07
No
3.66 ± 1.37
FIGO stage
I &II
3.22 ± 1.29
<0.0001
III & IV
4.44 ± 1.26
Distant organ metastases
Yes
5.10 ± 1.28
<0.0001
No
3.51 ± 1.26
Variables
miRNA-205 expression (Mean ± SD)
P value
Overall expression
0.07 ± 0.12
–
Age (in years)
≤50 years
0.06 ± 0.11
0.81
>50 years
0.08 ± 0.14
Alcoholism
Yes
0.07 ± 0.14
0.55
No
0.06 ± 0.11
Smoking
Yes
0.05 ± 0.11
0.01
No
0.08 ± 0.13
Tobacco
Yes
0.02 ± 0.02
0.05
No
0.08 ± 0.14
Grade
G1
0.10 ± 0.15
0.66
G2
0.05 ± 0.09
G3
0.07 ± 0.14
Lymph node involvement
Yes
0.04 ± 0.13
0.001
No
0.08 ± 0.12
FIGO stage
I &II
0.09 ± 0.13
0.004
III & IV
0.05 ± 0.11
Distant organ metastases
Yes
0.009 ± 0.01
<0.0001
No
0.08 ± 0.13
3.2 Exosomal microRNA-93 expression among endometrial cancer
3.78 mean fold increased exosomal miRNA-93 was observed in endometrial cancer patients compared to healthy control subjects. It has been observed that EC patients who were smokers showed 4.42 mean fold exosomal miRNA-93 expression while non smokers showed 3.45 mean fold exosomal miRNA-93 expression and differences among them was found to be significant (p = 0.002). EC patients with different grade such as grade 1, grade 2, grade 3, showed 3.97, 3.29, 4.20 mean fold exosomal miRNA-93 expression and differences among them was found to be significant (p = 0.01). EC patients who were in stage I&II had 3.22 mean fold exosomal miRNA-93 expression and patients in stage III&IV showed 4.44 mean fold exosomal miRNA-93 expression and differences among them was found to be significant (p < 0.0001). Patients with distant organ metastases showed 5.10 mean fold exosomal miRNA-93 expression while non metastatic patients showed 3.51 mean fold exosomal miRNA-93 expression and differences among them was found to be significant (p < 0.0001).
3.3 Exosomal miRNA-205 expression among endometrial cancer
Decreased miRNA-205 (0.07 mean fold) expression of exosomal miRNA-205 was observed in EC patients compared to healthy controls. EC patients who were smokers showed 0.05 mean fold exosomal microRNA-93 expression while non-smoker showed 0.08 mean fold expression and differences among them was found to be significant (p = 0.01). EC patients with lymph node involvement had 0.04 mean fold exosomal microRNA-93 expression while EC patients who did not showed any lymph node involvement had 0.08 mean fold exosomal microRNA-205 expression and differences among them was found to statistically significant (p = 0.001). EC patients in early stage (I & II) of disease had 0.09 mean fold of exosomal miRNA-205 expression while advanced stage (III & IV) of disease showed 0.05 mean fold of exosomal miRNA-205 expression and differences among them was found to be significant (p = 0.004).
3.4 Exosomal microRNA-93 expression and patients overall survival
Based on mean fold expression of exosomal microRNA-93 expression data, two group were formed such as ≤3 and >3 fold exosomal microRNA-93 expression and survival analysis were performed. It was observed that the EC patients who had ≤3 fold exosomal microRNA-93 expression showed 31.25 months of overall median survival while EC patients had >3 fold exosomal microRNA-93 expression showed 25.50 months of overall median survival and differences among them was found to be significant (p = 0.006, Fig. 1).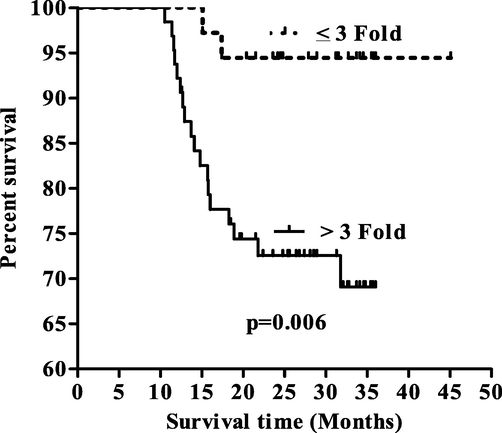
Kaplar Meier survival curve with respect to miRNA-93.
3.5 Exosomal miRNA-205 expression and patients overall survival
Based on mean fold expression of exosomal microRNA-205 expression data, two group were formed such as ≤0.07 and >0.07 fold exosomal microRNA-205 expression and survival analysis were performed. It was observed that the EC patients who had ≤0.07 fold exosomal microRNA-205 expression showed 24.80 months of overall median survival while EC patients had >0.07 fold exosomal microRNA-205 expression showed 33.25 months of overall median survival and differences among them was found to be significant (p = 0.01, Fig. 2).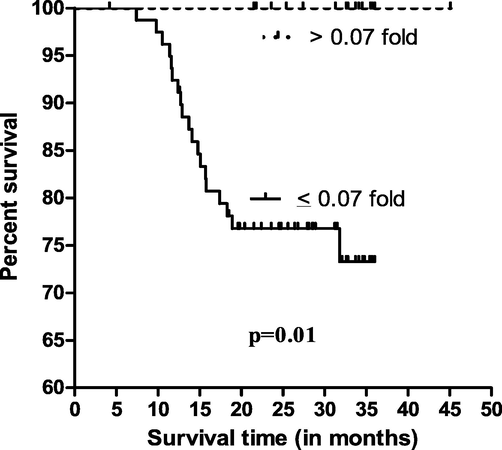
Kaplar Meier survival curve with respect to miRNA-205.
3.6 Prognostic importance of exosomal microRNA-93
Prognostic importance of exosomal microRNA-93 were calculated on the basis of mean fold and two group were formed (≤3 fold and >3 fold) (Fig. 3, Table 4). ROC curves plotted between ≤3 fold and >3 fold of exosomal miRNA-93at cut-off value of 3.25 fold increased in serum based exosomal microRNA-93 expression, sensitivity and specificity were 93% and 97%, respectively (AUC was 0.99, p < 0.0001).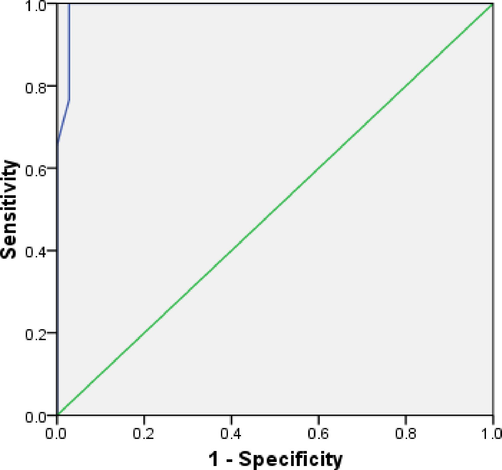
ROC curve with respect to miRNA-93.
AUC (95% Cl)
Sensitivity
Specificity
Cut off
P value
0.99
93%
97%
3.25 fold
<0.0001
3.7 Correlation of exosomal microRNA-93 and exosomal microRNA-205
A correlation analysis was performed between exosomal microRNA-93 and exosomal microRNA-205 expression (Fig. 4). A negative correlation (r = −0.20) was observed among them and was found to be significantly associated (p = 0.04).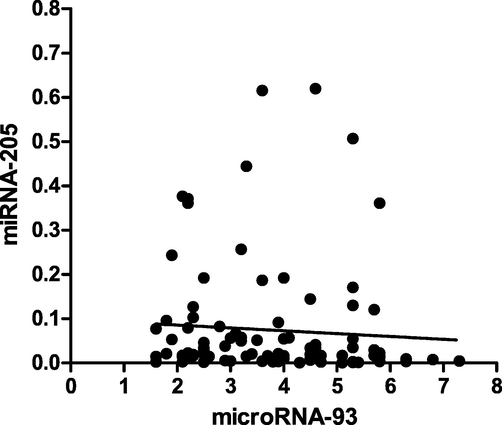
Spearman correlation of exosomal microRNA-93 and exosomal microRNA-205.
4 Discussion
Endometrial cancer has been one of the very common carcinoma in females’ reproductive system (Yeramian et al., 2013). The incidence rate of endometrial carcinoma has significantly increased over the past decades. Recurrence and metastasis and invasion have been major causes of death in patients with endometrial cancer (Meng et al., 2014). Micro RNAs may involve in several key cellular processes including apoptosis, proliferation and cell differentiation (Gammell, 2007; Carleton et al., 2007). Molecular characteristics of miRNA allow for not completely binding to the complementary target mRNAs. One miRNA could probably exert an effect on expression of many genes target (Rajewsky and Socci, 2004). It has been suggested that miRNAs may play significant role in carcinogenesis, cell expansion and cancer progression. Abnormal expression of miRNAs has been reported in a variety of human malignancies, including EC samples (Chung et al., 2009). MiRNAs’ indicated potential role in EC as a prognostic biomarker while data relating to prognostic role of miRNAs in EC is deficient.
Present study suggested that more 3.78 mean fold increased exosomal miRNA-92 was observed in EC patients. Increased exosomal miRNA-93 was observed in smokers compared to non smokers. It has been observed that patients in grade 3 had higher exosomal miRNA-93 expression compared to others grade such as grade1 and 2. It has found that patients in advanced FIGO stage and distant organ metastases showed higher exosomal miRNA-93 expression compared to early stage and without distant organ metastatic EC patients. Decreased exosomal miRNA-205 (0.07 mean fold) was observed in EC patients. It has been observed that smoking has been found to be linked with decreased exosomal miRNA-205 expression compared to non-smokers. Decreased expression was observed to be linked with the lymph node involvement, advanced FIGO stage and distant organ metastases among EC patients compared to without lymph node involvement, early FIGO stage and no distant organ metastatic patients. Survival of EC patients were found to be associated with increased exosomal miRNA over expression and linked with worse and decreased overall median survival of EC patients. It has been found that decreased or down regulation of exosomal miRNA-205 associated with poor EC patients survival. It has been suggested that the exosomalmiRNA-93 showed potential prognostic characteristic in term or AUC was 0.99 and showed high sensitivity and specificity at cutoff value of 3.25 fold. A negative correlation was found between exosomal miRNA93-and miRNA-205 among EC patients. It suggested that increase of exosomal miRNA-93 will lead to decrease the exosomalmiRNA-205, vice versa. Study by Meltzer PS in 2015 said that miR-93 was highly expressed in endometrial carcinoma samples and miR-93 overexpression promoted endometrial carcinoma cell migration and invasion (Meltzer, 2005). A study by Shuo Chen in 2015 said that miR-93 promotes endometrial carcinoma cell EMT, migration, and invasion via targeting down regulation of FOXA1 (Chen et al., 2016). MiR-93 expression has been implicated in various cancer types, implying an oncogenic role (Fu et al., 2012; Pineau et al., 2010; Yu et al., 2011). In breast cancer, its overexpression has been correlated with proliferation and tumor progression (Kim et al., 2012). Micro RNA array outcome demonstrated that the over expression of miR-93 was found in Osteosarcoma cell lines. Micro RNA-93 over expression played an vital role lung cancer cell growth promotion, angiogenesis, and metastasis, while its inhibition suppresses cell proliferation, migration, and colonisation (Du et al., 2014). Furthermore, additional in vitro and in vivo study showed that micro RNA-93 alteration involved in regulation of cancer cell growth and disease progression (Jiang et al, 2015). Markou et al presented that reduced miRNA-205 expression in breast cancer cell lines and decreased expression of miRNA-205 was associated with reduced disease-free survival (DFS) and overall survival (OS) during the early stages of breast cancer (Markou et al., 2014). Significantly reduced miRNA-205 expression was observed in glioma tissues compared with non-neoplastic brain tissues (Hou et al., 2013).
5 Conclusion
Increased exosomal microRNA-95, decreased microRNA-205 was found to be associated with endometrial cancer and associated with worse overall Endometrial cancer patients’ survival. Increased exosomal microRNA-95 could be a prognostic indicator for endometrial cancer patients.
Acknowledgement
Funding support: this study was supported by Sichuan provincial health and family planning commission scientific research popularization project (No: 17PJ298).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Endometrial carcinoma: role of current and emerging biomarkers in resolving persistent clinical dilemmas. Am. J. Clin. Pathol.. 2016;145:8-21.
- [Google Scholar]
- MicroRNA-93 promotes epithelial – mesenchymal transition of endometrial carcinoma cells. PLoS One. 2016;11(11):e0165776
- [Google Scholar]
- The accuracy of endometrial sampling in the diagnosis of patients with endometrial carcinoma and hyperplasia: a meta-analysis. Cancer. 2000;89:1765-1772.
- [Google Scholar]
- miR-93-directed downregulation of DAB2 defines a novel oncogenic pathway in lung cancer. Oncogene. 2014;33(34):4307-4315.
- [Google Scholar]
- Involvement of microRNA-93, a new regulator of PTEN/Akt signaling pathway, in regulation of chemotherapeutic drug cisplatin chemosensitivity in ovarian cancer cells. FEBS Lett.. 2012;586(9):1279-1286.
- [Google Scholar]
- MicroRNAs: recently discovered key regulators of proliferation and apoptosis in animal cells: Identification of miRNAs regulating growth and survival. Cytotechnology. 2007;53:55-63.
- [Google Scholar]
- In situ measurement of miR-205 in malignant melanoma tissue supports its role as a tumor suppressor microRNA. Lab. Invest.. 2012;92:1390-1397.
- [Google Scholar]
- Identification of microRNA-205 as a potential prognostic indicator for human glioma. J. Clin. Neurosci.. 2013;20:933-937.
- [Google Scholar]
- Aberrant promoter hypermethylation of p16 gene in endometrial carcinoma. Tumour Biol.. 2015;36:1487-1491.
- [Google Scholar]
- Epigenetic-induced repression of microRNA-205 is associated with MED1 activation and a poorer prognosis in localized prostate cancer. Oncogene. 2013;32:2891-2899.
- [Google Scholar]
- miR-93 promotes cell proliferation in gliomas through activation of PI3K/Akt signaling pathway. Oncotarget. 2015;6(10):8286-8299.
- [Google Scholar]
- MiR-205 is progressively down-regulated in lymph node metastasis but fails as a prognostic biomarker in high-risk prostate cancer. Int. J. Mol. Sci.. 2013;14:21414-21434.
- [Google Scholar]
- Identification of oncogenic microRNA-17-92/ZBTB4/specificity protein axis in breast cancer. Oncogene. 2012;31(8):1034-1044.
- [Google Scholar]
- TGFβR2 is a major target of miR-93 in nasopharyngeal carcinoma aggressiveness. Mol. Cancer. 2014;13:51.
- [Google Scholar]
- MicroRNA-205-directed transcriptional activation of tumor suppressor genes in prostate cancer. Cancer. 2010;116:5637-5649.
- [Google Scholar]
- Prognostic significance of metastasis-related microRNAs in early breast cancer patients with a long follow-up. Clin. Chem.. 2014;60:197-205.
- [Google Scholar]
- Knockdown of BAG3 induces epithelial-mesenchymal transition in thyroid cancer cells through ZEB1 activation. Cell Death Dis.. 2014;5:e1092
- [Google Scholar]
- miR- 221 overexpression contributes to liver tumorigenesis. Proc. Natl. Acad. Sci. U.S.A.. 2010;107(1):264-269.
- [Google Scholar]
- Endometrial carcinoma: clinical characteristic and survival rates by the new compared to the prior FIGO staging systems. J. Med. Assoc. Thai.. 2013;96:505-512.
- [Google Scholar]
- The p63 protein isoformΔNp63α inhibits epithelial-mesenchymal transition in human bladder cancer cells: role of MIR-205. J. Biol. Chem.. 2013;288:3275-3288.
- [Google Scholar]
- Endometrial carcinoma: molecular alterations involved in tumor development and progression. Oncogene. 2013;32:403-413.
- [Google Scholar]
- miR-93 suppresses proliferation and colony formation of human colon cancer stem cells. World J. Gastroenterol.. 2011;17(42):4711-4717.
- [Google Scholar]







