Translate this page into:
Exopolysaccharide production by a new Lactobacillus lactis isolated from the fermented milk and its antioxidant properties
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Exopolysaccharide producing Lactobacillus lactis was identified. EPS production was optimized. Central Composite Design and Response Surface Methodology was used for optimization.
Abstract
Exopolysaccharides (EPSs) are synthesized various organisms, including bacteria. These EPSs are used as emulsifying agents in various industries and a search of novel food grade isolate is also a continuous process. In this line of investigation, EPS producing Lactobacillus lactis was used for this study. A potent exopolysaccharide producing Lacobacillus lactis was isolated from milk sources. EPS production was optimized by traditional method initially. Further EPS production was optimized by response surface methodology. Antioxidant properties of the isolated EPS were analyzed. Among various bacterial isolates, L. lactis showed ability to produced maximum amount of exopolysaccharides. Addition of 1% (w/v) maltose enhanced the production of EPS. The nitrogen sources (1%, w/v) such as, ammonium sulphate, casein, skim milk and oat meal stimulated the production of EPS. EPS yield was enhanced for the components of culture medium like gelatine (0.78%), copper sulphate (0.039%) and maltose (0.31%) in optimized medium using Central Composite Design. The isolated EPS showed antioxidant property. Results suggest that EPS and the organism have wide application in food industry.
Keywords
Extracellular polysaccharides
Lactobacillus sp.
Optimization
Antioxidant
1 Introduction
Polysaccharides synthesized by plants, bacteria and algae are mainly used as emulsifying agents and enhance the texture and consistency in milk processing industry. Exopolysaccharides (EPS) has much more attractive additive in food industry and widely used as food thickeners (Zhang et al., 2011; Ilavenil et al., 2015). Because of this property, it is widely used in food industry. Also, lactic acid bacteria (LAB) synthesized various types of exopolysaccharides (EPSs) and EPS play predominant role in yoghurt preparation. Lactobacillus is considered as the Generally Regarded as Safe (GRAS) organisms. Because of this tag, EPS produced by Lactobacillus sp. is widely used in food fermentation. This EPS is generally safe and widely used to improve the stability of various foods. EPS are also reported to have various novel therapeutic properties, such as antioxidant, antitumor, antibiofilm, cholesterol lowering, immunostimulatory and immunomodulatory activities (Adebayo-tayo and Onilude, 2008; Al-Dhabi et al., 2016; Al-Dhabi 2019a; Al-Dhabi et al., 2019c). The important source of LAB is milk and it synthesis various products other than lactic acid, like exopolysaccharides. EPS secreted by microorganisms has attained much more attention in recent years, because of the secretion of this polymer, improve the properties of various fermented food. Hence, screening of novel probiotic strains of commercial value for the formulation of various functional foods is of great importance (Jensen et al., 2012; Al-Dhabi et al., 2018; Al-Dhabi et al., 2019b). Probiotic bacteria mainly alter the microbial balance in the intestine because of the secretion of antibiotics and lactic acids, act as immune stimulant, enhance resistance and stimulate digestion process. Probiotic bacteria have been screened and isolated from various sources, including milk of goat (Setyawardani et al., 2013; Arasu et al., 2017; Arasu et al., 2019c; Arasu et al., 2013), sheep milk (Iranmanesh et al., 2012) and breast milk (Martin et al., 2005; Oda et al., 1983), has been reported earlier. Bacteria generally synthesized by various EPSs and these EPS have potent ecological and physiological function. LAB EPS is widely used in the preparation of fermented food products. Also LAB EPS has anti-ulcer, anti-tumor and reduce cholesterol (Nagaoka et al., 1994; Kitazawa et al., 1998). In some cases, EPS involved in undesirable ropiness of various products namely, beer, wine and cider.
LAB strains utilize various sugars for the production of EPS as the sole source of carbon (Xu et al., 2010). The culture medium components, mainly nitrogen and carbon sources supported EPS production (De Vuyst et al., 1998). LAB utilized various medium such as, sago starch, whey permeate medium and sugar beet molasses and these EPS have various applications in food processing industries (Yeesang et al., 2008). The bioprocess factors including pH of the medium, nitrogen source, carbon source, incubation temperature and ionic source effectively support the growth of LAB and EPS production. Traditional method (one variable at a time approach) has been widely used to optimize the physical and factors to enhance the production of EPS. However, this method has several demerits to locate optimum factors in bioprocess conditions. Hence, statistical methods have been widely used to optimize the bioprocess conditions (Lee and Gilmore, 2005). Among the statistical designs (Ruchi et al., 2008), Central composite design (CCD) is widely used for the production of various biomolecules (Mohana et al., 2008), including enzymes (Burkert et al., 2004) and polysaccharides (Zou, 2005). In this study, EPS production was optimized by statistical approach and antioxidant property was analyzed.
2 Material and methods
2.1 Bacterial strain and culture
In our study, Lactobacillus lactis was isolated by standard method from the fermented milk by MRS agar plates. Liquid culture was obtained by culturing the bacterial strain in M-17 broth for 18 h at 37 ± 2 °C and was stored at 2–8 °C. The bacterial strain was sub cultured periodically.
2.2 Biosynthesis and isolation of EPS
The selected bacterial strain was inoculated in skimmed milk medium and sterilized the culture medium. After that it was cooled and inoculated by incubating the Erlenmeyer flasks at 37 °C for 24 h. After 24 h of incubation the culture medium was incubated with trichloroacetic acid (12%) and incubated for overnight. Then the bacterial proteins and culture were removed from the extract by centrifugation (10,000×g, 15 min). Then EPS was separated from the cell free supernatant by adding ice cold ethanol (three volumes) and incubated for 2 days at ice cold conditions. Finally, precipitated EPS was centrifuged and added double distilled water. The pellet was further dissolved in water and dialyzed using 8–10 kDa molecular weight cut off membrane. The final product was lyophilized and has been used for other experiments.
2.3 Analysis of antioxidant property of EPS from LAB
In this study, antioxidant property was analyzed as suggested by Mitsuda et al. (1966). The reagent mixture containing ammonium molybdate (4 mM), sodium sulphate (28 mM), and sulphuric acid (0.6 M) in 250 mL double distilled water. EPS was diluted appropriately (5–7.5 mg/ml) and dissolved in 1 mL double distilled water. Finally the absorbance of the sample was read at 695 nm and compared with standard (ascorbic acid).
2.4 Optimization of process parameters by traditional method
In this study the EPS production was optimized by including 1% carbon and nitrogen sources, and 0.1% minerals. The culture medium was supplemented with pre calculated carbon, nitrogen and ions and sterilized. After sterilization, the bacterial strain was inoculated and incubated for 7 days. EPS was extracted and assayed as described previously.
2.5 Optimization of EPS production by statistical approach
The variables namely, maltose, gelatine and copper sulphate were selected for optimization of EPS by statistical approach based on the outcome of one-variable-at-a-time approach. The experiment was designed using Design-Expert software. CCD and RSM have been widely applied to determine optimum factors and this method is highly precise, economical, and efficient and saves time (Vijayaraghavan and Vincent, 2014, 2015). The selected independent variables for the production of EPS was evaluated at five different levels, which is tabulated (Table 1). The selected individual variables and codes were based on the following equation.
Variables
Symbol
Coded values
−α
−1
0
1
+α
Copper sulphate
A
−0.206
0.01
0.055
1.0
0.13
Maltose
B
−0.206
0.1
0.55
1
1.30681
Gelatin
C
−0.206
0.1
0.55
1
1.30681
This designed experimental model consists of 20 experimental runs and the designed matrix is tabulated in Table 2. Three independent experiments were performed and an average value was considered for analysis. The interactive relationship between the selected variables can be expressed by the following equation.
Run
Factor 1
Factor 2
Factor 3
EPS (mg/ml)
A:Moisture %
B:Maltose %
C:Gelatin %
1
0
0
0
11.2
2
0
1.30681
0
17.1
3
0
0
0
21.1
4
−1
1
−1
13
5
0
0
0
22.2
6
−1
−1
1
4.1
7
0
−0.206807
0
6.02
8
0
0
0
11.3
9
−1
1
1
1.7
10
1
−1
1
7
11
1
1
1
12.1
12
0
0
0
14.2
13
0
0
0
12.2
14
−1
−1
−1
1.1
15
100.227
0
0
5.2
16
0
0
1.30681
2.3
17
49.7731
0
0
1.8
18
0
0
−0.206807
8
19
1
1
−1
7.91
20
1
−1
−1
2.01
3 Results
3.1 Screening and identification of Lactobacillus lactis isolated from fermented milk
In this study, the bacterial strain, Lactobacillus lactis was isolated and screened for hyper production of EPS. The potent bacterial strain was identified by molecular approach and biochemical tests. Among various bacteria tested, this organism significantly produced large amount of polysaccharides. Subsequently this bacterial strain was used for further optimization studies.
3.2 One variable time method optimization to screen the process variables
The process parameters were initially tested by one variable at a time approach. Among the carbon sources, addition of maltose enhanced EPS production than other tested carbon sources (4.29 mg/ml) (Fig. 1). From the selected nitrogen sources, supplementation of gelatine significantly enhanced EPS production (5.13 mg/ml) (Fig. 2). The ions such as, Cu2+, Ca2+, Zn2+, Mn2+ and Mg2+ enhanced EPS production (Fig. 3).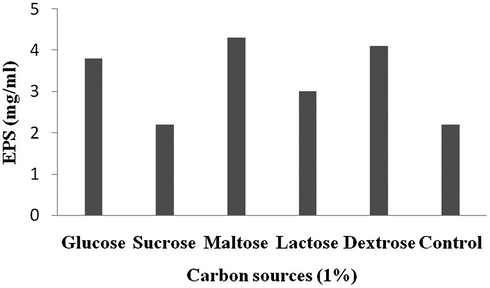
Effect of carbon sources on EPS production.
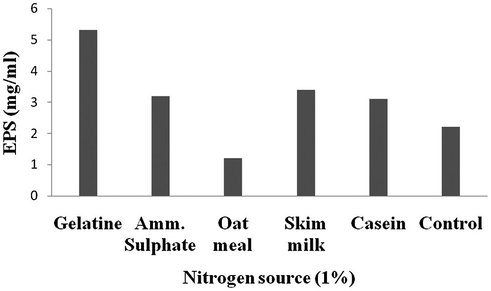
Effect of nitrogen sources on EPS production.
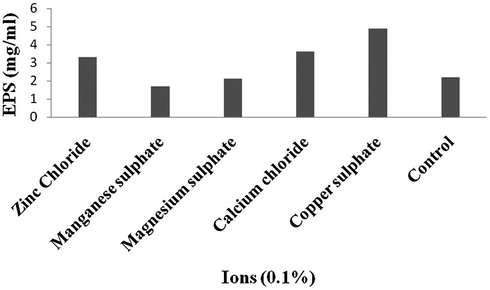
Effect of ions on EPS production.
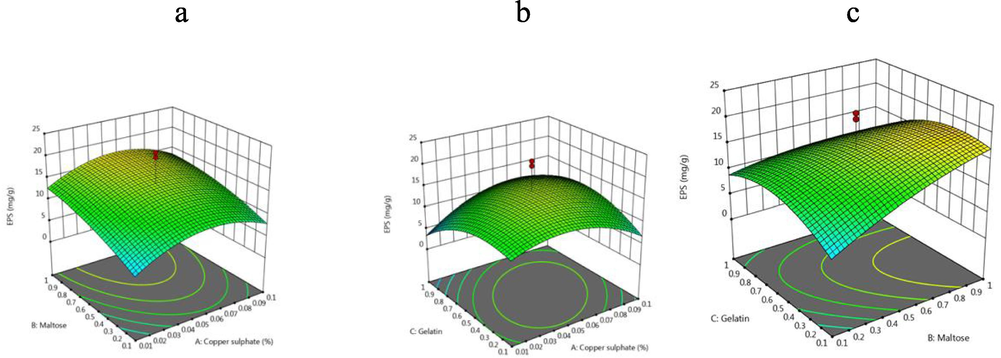
The 3D-response surface plots of EPS yield (mg/ml) between copper sulphate and maltose (a), gelatine and copper sulphate (b) and gelatine and maltose (c).
3.3 Optimization of EPS production by Central composite designs (CCD) and statistical analysis
The variables and levels were summarized in Table 3. In this study, experiment was designed for optimization of three variables using design expert software. The selected factors were copper sulphate (A), maltose, (B) and gelatine (C) and 20 experimental trials were performed (Table 1). Analysis of variance was performed to evaluate the significance of this model and the model F value 4.25 implied that the designed model was statistically significant (Table 3). The lack of fit of this designed model for the production of EPS was not significant.
Source
Sum of Squares
df
Mean Square
F-Value
p-Value
Model
6.14E+02
9
6.82E+01
4.25
0.0169
Significant
A-Moisture
1.61E+01
1
1.61E+01
1.01E+00
0.3396
B-Maltose
1.12E+02
1
1.12E+02
7
0.0245
C-Gelatina
5.55E+00
1
5.55E+00
0.35
0.5693
AB
2.80E−01
1
2.80E−01
0.018
0.8972
AC
3.82E+01
1
3.82E+01
2.38
0.1537
BC
2.85E+01
1
2.85E+01
1.78
0.212
A2
2.56E+02
1
2.56E+02
15.98
0.0025
B2
2.69E+01
1
2.69E+01
1.68
0.2242
C2
1.90E+02
1
1.90E+02
11.87
0.0036
Residual
1.60E+02
10
16.03
Lack of Fit
35.43
5
7.09
0.28
0.9034
Not significant
Pure Error
1.25E+02
5
24.97
Cor Total
7.74E+02
19
3.4 Analysis of antioxidant activity of EPS
EPS from Lactobacillus has antioxidant potential. The antioxidant potential of partially purified EPS was analyzed. At increasing concentration of EPS antioxidant activity enhanced. The selected dose of EPS was 5–7.5 mg/ml. The antioxidant potential of EPS exhibited dose dependent manner within the concentration range of 5–7.5 mg/ml. The antioxidant activity was comparable with the activity of ascorbic acid (Fig. 5).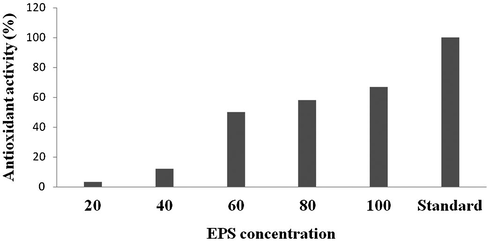
Antioxidant activity of EPS from Lactobacillus lactis.
4 Discussion
Biosynthesis of EPS is performed intracellularly and secreted out to the environment at various climatic conditions. Although EPS from bacteria has been studied widely, however the knowledge of bacterial EPS is limited (Sutherland, 2001). The traditional method of optimization of EPS production has been reported earlier, however statistical approach on EPS production is limited. In recent years, optimization of EPS production has been carried out by CCD, Plackett-Burman design, orthogonal matrix method, fractional factorial design and Box-Behnken design to enhance EPS production (Feng et al., 2010). In fermentation process, pH of the culture medium is an important factor which critically influenced on EPS production and has been reported by Wang and McNeil (1995). In EPS production, fungi required acidic pH value for enhanced EPS production (Arasu et al., 2019a; Arasu et al., 2019b; Arokiyaraj et al., 2015; Boovaragamoorthy et al., 2019); Ilavenil et al., 2015). The carbon source of the culture medium positively influenced on EPS production because the important component of EPS is composed of sugars. Although various sugars, including glucose, lactose, maltose, sucrose, xylose, galactose, fructose, mannitol, cellobiose for enhanced production of EPS, maltose, glucose and sucrose were most significant factors on EPS production. Among the nitrogen sources analyzed for theproduction of EPS, corn steep powder and yeast extract significantly influenced on EPS production (Mahapatra and Banerjee, 2013). The inorganic ions such as, ammonium sulphate, potassium nitrate, and diammonium oxalate monohydrate were frequently used for the production of EPS. Among the various nitrogen sources evaluated, the salts of ammonium were more effective than other salts from inorganic origin (Seviour et al., 1992). In a study, the salts such as, sodium nitrate was found to be highly suitable for the production of EPS by Epicoccum nigrum (Schmid et al., 2001; Gurusamy et al., 2019; Roopan et al., 2019; Valsalam et al., 2019; Ilavenil et al., 2015; Balachandran et al., 2015). Also, KH2PO4 and K2HPO4 enhanced EPS production in fungi (Mahapatra and Banerjee, 2013).
3D surface plot shows the influence of copper sulphate and maltose on EPS production. However, gelatine did not show any potential impact on EPS production. In this experiment the amount of EPS produced by the bacterial isolate was significantly enhanced by the addition of maltose and copper sulphate at certain level and declined at higher concentrations (Table 3). The significant impact of gelatine and maltose and their interactive effects on EPS production was shown in Fig. 4c. 3D response surface plot has been used earlier to analyze the optimized factors (Wu et al., 2007). The isolated EPS showed antioxidant activity and showed more than 60% activity compared with standard. Kullisaar et al. (2002) reported the significance of natural source of antioxidants to reduce oxidative stress. EPS also useful to prevent various diseases also showed antioxidant activity (Dinis et al., 1994).
5 Conclusion
In the present investigation, the important nutrient parameters were optimized by Central Composite Design and Response surface methodology. EPS produced by Lactobacillus lactis showed promising antioxidant activity. CCD and RSM significantly enhanced EPS production. New Lactobacillus sp. should be used to find their potential for maximum EPS production and antioxidant properties.
Acknowledgement
The author acknowledge the Deanship of Scientific Research at King Faisal University for the financial support under Nasher Track (Grant No. 186339).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Screening of lactic acid bacteria strains isolated from some Nigerian fermented foods for reproduction. World Appl. Sci. J.. 2008;4:741-747.
- [Google Scholar]
- Isolation, identification and screening of antimicrobial thermophilic Streptomyces sp. Al-Dhabi-1 isolated from Tharban hot spring, Saudi Arabia. Extremophiles. 2016;20:79-90.
- [Google Scholar]
- Environmental friendly synthesis of silver nanomaterials from the promising Streptomyces parvus strain Al-Dhabi-91 recovered from the Saudi Arabian marine regions for antimicrobial and antioxidant properties. J. Photochem. Photobiol. B: Biol.. 2018;189:176-184.
- [Google Scholar]
- Chemical profiling of Streptomyces sp. Al-Dhabi-2 recovered from an extreme environment in Saudi Arabia as a novel drug source for medical and industrial applications. Saudi J. Biol. Sci.. 2019;26:758-766.
- [Google Scholar]
- Chemical constituents of Streptomyces sp. strain Al-Dhabi-97 isolated from the marine region of Saudi Arabia with antibacterial and anticancer properties. J. Infect. Public Health 2019
- [CrossRef] [Google Scholar]
- Bioactivity assessment of the Saudi Arabian Marine Streptomyces sp. Al-Dhabi-90, metabolic profiling and its in vitro inhibitory property against multidrug resistant and extended-spectrum beta-lactamase clinical bacterial pathogens. J. Infect. Public Health. 2019;12:549-556.
- [Google Scholar]
- Antibacterial and antifungal activities of polyketide metabolite from marine Streptomyces sp. AP-123 and its cytotoxic effect. Chemosphere. 2013;90(2):479-487.
- [Google Scholar]
- Green chemical approach towards the synthesis of CeO2 doped with seashell and its bacterial applications intermediated with fruit extracts. J. Photochem. Photobiol., B. 2017;172:50-60.
- [Google Scholar]
- Essential oil of four medicinal plants and protective properties in plum fruits against the spoilage bacteria and fungi. Ind. Crops Prod.. 2019;133:54-62.
- [Google Scholar]
- Synthesis and characterization of ZnO nanoflakes anchored carbon nanoplates for antioxidant and anticancer activity in MCF7 cell lines. Mater. Sci. Eng., C. 2019;102:536-540.
- [Google Scholar]
- One step green synthesis of larvicidal, and azo dye degrading antibacterial nanoparticles by response surface methodology. J. Photochem. Photobiol., B. 2019;190:154-162.
- [Google Scholar]
- Green synthesis of Silver nanoparticles using aqueous extract of Taraxacum officinale and its antimicrobial activity. South Indian J. Biol. Sci.. 2015;2:115-118.
- [Google Scholar]
- Antimicrobial and cytotoxic properties of Streptomyces sp. (ERINLG-51) isolated from SouthernWestern Ghats. South Indian. J. Biol. Sci.. 2015;1:7-14.
- [Google Scholar]
- Clinically important microbial diversity and its antibiotic resistance pattern towards various drugs. J. Infect. Public Health 2019
- [CrossRef] [Google Scholar]
- Optimization of extracellular lipase production by Geotrichum sp. using factorial design. Bioresour. Technol.. 2004;91:77-84.
- [CrossRef] [Google Scholar]
- Production by and isolation of exopolysaccharides from Streptococcus thermophilus grown in a milk medium and evidence for their growth associated biosynthesis. J. Appl. Microbiol.. 1998;84:1059-1068.
- [CrossRef] [Google Scholar]
- Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys.. 1994;315:161-169.
- [CrossRef] [Google Scholar]
- Statistical optimization of media for mycelial growth and exo-polysaccharide production by Lentinus edodes and a kinetic model study of two growth morphologies. Biochem. Eng. J.. 2010;49:1-6.
- [CrossRef] [Google Scholar]
- Environmental friendly synthesis of TiO2-ZnO nanocomposite catalyst and silver nanomaterials for the enhanced production of biodiesel from Ulva lactuca seaweed and potential antimicrobial properties against the microbial pathogens. J. Photochem. Photobiol., B. 2019;193:118-130.
- [Google Scholar]
- Growth and metabolite profile of Pediococcus pentosaceus and Lactobacillus plantarum in different juice. South Indian J. Biol. Sci.. 2015;1:1-6.
- [Google Scholar]
- Isolation of lactic acid bacteria from Ewe milk, traditional yoghurt and sour buttermilk in Iran. Eur. J. Food Res. Rev.. 2012;2:79-92.
- [CrossRef] [Google Scholar]
- In vitro testing of commercial and potential probiotic lactic acid bacteria. Int. J. Food Microbiol.. 2012;153:216-222.
- [CrossRef] [Google Scholar]
- Phosphate group requirement for mitogenic activation of lymphocytes by an extracellular Phosphopolysaccharide from Lactobacillus delbrueckii sub sp. bulgaricus. Int. J. Food Microbiol.. 1998;40:169-175.
- [CrossRef] [Google Scholar]
- Two antioxidative lactobacilli strains as promising probiotics. Int. J. Food Microbiol.. 2002;72:215-224.
- [CrossRef] [Google Scholar]
- Formulation and process modelling of biopolymer polyhydroxy alkanoates, PHAs production from industrial wastes by novel crossed experimental design. Process Biochem.. 2005;40:229-246.
- [CrossRef] [Google Scholar]
- Fungal exopolysaccharide: production, composition and applications. Microbiol. Insight.. 2013;6:1-16.
- [CrossRef] [Google Scholar]
- Probiotic potential of 3 Lactobacilli strains isolated from breast milk. J. Hum. Lact.. 2005;21:8-17.
- [CrossRef] [Google Scholar]
- Antioxidative action of indole compounds during the autoxidation of linoleic acid. Eiyo to Shokuryo.. 1966;19:210-214.
- [CrossRef] [Google Scholar]
- Xylanase production by Burkholderia sp. DMAX strain under solid state fermentation using distillery spent wash. Bioresour. Technol.. 2008;99:7553-7564.
- [CrossRef] [Google Scholar]
- Antiulcer effects of lactic acid bacteria and their cell wall polysaccharides. Biol. Pharm. Bull.. 1994;17:1012-1017.
- [CrossRef] [Google Scholar]
- Antitumor polysaccharide from Lactobacillus sp. Agric. Biol. Chem.. 1983;47:1623-1625.
- [CrossRef] [Google Scholar]
- CuO/C nanocomposite: synthesis and optimization using sucrose as carbon source and its antifungal activity. Mater. Sci. Eng., C. 2019;101:404-414.
- [Google Scholar]
- Lipase from solvent tolerant Pseudomonas aeruginosa strain: production optimization by response surface methodology and application. Bioresour. Technol.. 2008;99:4796-4802.
- [CrossRef] [Google Scholar]
- Structure of epiglucan, a highly side-chain/branched (1–3;16)-β-glucan from the micro fungus Epicoccum nigrum Ehrenb. Ex Schlecht. Carbohyd. Res.. 2001;331:163-171.
- [CrossRef] [Google Scholar]
- Identification and characterization of probiotic lactic acid bacteria isolated from indigenous goat milk. J. Anim. Prod. 2013:57-63.
- [Google Scholar]
- Production of pullulan and other exopolysaccharides by filamentous fungi. Crit. Rev. Biotechnol.. 1992;12:279-298.
- [CrossRef] [Google Scholar]
- Microbial polysaccharides from Gram-negative bacteria. Int. Dairy J.. 2001;11:663-674.
- [CrossRef] [Google Scholar]
- Rapid biosynthesis and characterization of silver nanoparticles from the leaf extract of Tropaeolum majus L. and its enhanced in-vitro antibacterial, antifungal, antioxidant and anticancer properties. J. Photochem. Photobiol., B. 2019;191:65-74.
- [Google Scholar]
- Medium optimization for the production of fibrinolytic enzyme by Paenibacillus sp. IND8 using response surface methodology. Sci. World J.. 2014;2014:1-9.
- [CrossRef] [Google Scholar]
- A low cost fermentation medium for potential fibrinolytic enzyme production by a newly isolated marine bacterium, Shewanella sp. Biotech. Rep.. 2015;7:135-142.
- [CrossRef] [Google Scholar]
- pH effects on exopolysaccharide and oxalic acid production in cultures of Sclerotium glucanicum. Enzyme Microb. Technol.. 1995;17:124-130.
- [CrossRef] [Google Scholar]
- Optimization of extraction process of crude polysaccharides from boat-fruited Sterculia seeds by response surface methodology. Food Chem.. 2007;105:1599-1605.
- [CrossRef] [Google Scholar]
- Screening, identification and statistic optimization of a novel exopolysaccharide producing Lactobacillus paracasei HCT. Afr. J. Microbiol. Res.. 2010;4:783-795.
- [Google Scholar]
- Sago starch as a low cost carbon source for exopolysaccharide production by Lactobacillus kefiranofaciens, World. J. Microbiol. Biotechnol.. 2008;24:1195-1201.
- [CrossRef] [Google Scholar]
- Growth and exopolysaccharide production by Streptococcus thermophilus ST1 in skim milk. Braz. J. Microbiol.. 2011;42:1470-1478.
- [CrossRef] [Google Scholar]
- Optimization of nutritional factors for exopolysaccharide production by submerged cultivation of the medicinal mushroom Oudemansiella radicata. World J. Microbiol. Biotechnol.. 2005;21:1267-1271.
- [CrossRef] [Google Scholar]







