Translate this page into:
Exogenous glutathione revealed protection to bacterial spot disease: Modulation of photosystem II and H2O2 scavenging antioxidant enzyme system in Capsicum annum L
⁎Correspondence author. naveed.aslam@iub.edu.pk (Muhammad Naveed Aslam)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In a greenhouse, parallel two experiments were carried out for the estimation of the impact of exogenous GSH (reduced glutathione) on photosynthetic properties, the efficiency of the photosystem II, and the H2O2-scavenging mechanism in chilli (Capsicum annum L.) seedlings. On the bases of pathogenicity, resistant and susceptible verities were selected. The application of GSH increased the chlorophyll fluorescence parameters such as photochemical quantity yield (YII). It declined the excitation pressure (1-qP) of photosystem and production of non-regulated energy dissipation [Y (NO)] in Xanthomonas campestris treated chilli leaves. In the addition of GSH, accumulation of reactive oxygen species was down and maintained the activity of ROS-scavenging enzymes (enzymatic antioxidants) in resistant and susceptible plants. Consequently, GSH supplementation relieves inhibition of production of bacterial toxin and photosynthesis primarily by overwhelming stomatal limitations, enhancing the performance of photosystem, and modulating the antioxidant protection mechanism to defend chloroplasts from oxidation. The Present study confirmed that seed priming with GSH in chilli plants to relieve biotic stress.
Keywords
Capsicum annum
Antioxidant enzymes
Glutathione
Chlorophyll fluorescence
1 Introduction
The chilli is ranked second most popular solanaceous vegetable worldwide. It is cultivated in Pakistan on a wide scale as a cash crop (Annonymous, 2000). Xanthomonas campestris bacterial leaf spot is a severe disease and a critical factor in reducing chilli production worldwide. Infected plant displays numerous symptoms like leaf and fruit spots and resulting in decreased physiological processes, such as photosynthesis (Horváth et al., 2015). Photosynthesis is a crucial mechanism in plant metabolism, and its maintenance plays a pivotal role in plant defence against biotic stress. Chlorophyll fluorescence parameters (Photo System II) are a powerful tool to investigate photosynthesis activity at the cellular level, leaf and plant scale, enabling plant phenotyping (Baker, 2008; Murchie and Lawson, 2013). Besides, Chl-F's sensitivity to even subtle changes in plant metabolism provides an ideal technique for providing correlations between host plant-stress factors. Similarly, (Rohacek and Bartak, 1999) reported that Chlorophyll fluorescence parameters indicates about pathogen location and describes how photosynthesis from leaf to crop level is regulated. Although pathogenic bacteria cause substantial yield losses in crops, several scholars have attempted Chl-FI to investigate the effects of bacterial infection relative to other forms of infectious agents. Glycine max treated with Pseudomonas syringae pv's avirulent form, Glycine displayed lower maximal photochemical efficiency of PSII (Fv/Fm) and PSII values and strengthened non-photochemical quenching coefficient (NPQ) before symptoms formed. (Zhang et al., 2015; Bonfig et al., 2006). Infection with X. oryzae (bacterial blight) in rice induces photosynthesis disruption and a decline in Fv/Fm, PSII and Ft could be observed (Šebela et al., 2018).
Luckily, plants contain a variety of active defence responses which offer resistance to various pathogenic agents. Systematic acquired resistance to different plant pathogens is efficient in plants (Schreiber et al., 2004). Previously, researchers used various plant growth hormones which are act as signals and activates plant defence responses (Devoto and Turner, 2005).
Nowadays, Glutathione (GSH) is a non-enzymatic antioxidant and critical part of the ascorbate–glutathione cycle, a process controlling the levels of H2O2 in plant cells. It also has biological activity against biotic stress (Hu et al., 2014; Kuzniak and SkLodowska, 2004). The presence of cysteine confers antioxidant function of GSH during its participation in cell redox homeostasis. (Nedbal et al., 2005) reported that GSH plays a crucial role in various cellular processes such as development, growth or environmental response in plants. Several scientific studies have documented regulation of GSH in plants contaminated by multiple pathogens (Foyer and Noctor, 2011; Meyer et al., 2001). Also, numerous experiments have documented the protective roles of extracellular GSH enhancing nutrient levels and heavy metal stress tolerance in the various plant species, which GSH triggered reactive oxygen species synthesis and also improves absorption and metabolism of nutrients or metals (Cai et al., 2011; Mostofa et al., 2014).
Besides, few studies on GSH’s role to control the excess energy excitation, redox state and antioxidant potential of plant cells under X. campestris stress were reported. Considering GSH's ability to relieve biotic stress, we believe that seed priming with GSH will modulate the capacity for photosynthesis, as well as the antioxidant defence mechanism in chilli seedlings.
To the best of authors’ information, there seem to be no studies on the beneficial roles of foliar application of GSH as a means of enhancing tolerance to biotic stress (X. campestris) in chilli was reported previously. Thus in the present study, we investigated the effect of exogenous GSH on leaf photosynthetic performance, photosystem II efficiency, and the antioxidant system of C. annum plant under biotic stress. Meanwhile, to further determine the role of GSH in balancing the excess excitation energy and modulating the ROS scavenging system in stressed plants. We found that modulating photosynthetic activity at tissue levels and ROS metabolism at organelle levels by adding exogenous GSH was an important mechanism for mitigating the inhibition of plant growth and photosynthesis caused by biotic (X. campestris) stress.
2 Materials and methods
2.1 Plant materials, growth conditions and treatments
Different varieties of Chilli were used for pathogenicity tests in a greenhouse at The Islamia University of Bahawalpur, Pakistan. The soil used comprised bhal (50%), organic matter (20%), and sandy loam (30%). The soil was sterilized for 15–20 days in the sunlight. Plastic pots (1 kg) were used in the experiments. Seeds were sterilized using NaOCl (0.1%) for 2 min and rinse thoroughly with distilled water twice. Seedlings (21 days old) was transplanted in pots. Seedlings were divided into six groups and inoculated with 10−7 CFU X. campestris and 0, 0.5 and 1.0 mM GSH respectively. Seedlings were introduced to respective treatments while uninoculated plants were used as control. The same experiment was repeated three times and given 15 days interval after harvesting of each experiment (Table 1). In the experimental pots the Hoagland solution was supplemented with 200 mL full strength twice a weak
Treatments
Treatment Code
Time of application after emergence
Duration after treatment
Control positive (without any treatment)
C
21 Days
35 days
Control negative (Exogenous inoculation of bacteria)
B
21 Days
21 days
Glutathione 1.0 mM → 10−7 CFU Bacteria
T1
21 days → 22 days
30 Days
Glutathione 0.5 mM → 10−7 CFU Bacteria
T2
21 days → 22 days
30 Days
Glutathione 1.0 mM + 10−7 CFU Bacteria
T3
21 Days
30 Days
Glutathione 0.5 mM + 10−7 CFU Bacteria
T4
21 Days
30 Days
2.2 Isolation of bacterial pathogens inoculum preparation, spot index and severity
X. campestris was collected from the infected chilli plants from the greenhouse of the department of plant pathology, Islamia University of Bahawalpur. The pathogenicity of X. campestris on chilli was checked by the application on chilli seedling in a pathology lab. Pure colonies of X. campestris was streaked separately on nutrient agar plates and then incubated at temperature 30 ± 1 °C for 24. Single colony picked after 24 h from each of tested bacteria, X. campestris was injected individually into flasks containing nutrient broth and then placed them in the incubator at temperature 32 ± 1C° for three days (72 h). Cell density was determined by using spectrophotometer (Pg Instruments UV–VIS-T60). This culture was use as source of inoculum. The suspension was diluted by adding water (serial dilution) and prepared 10^7 cfu/ml. Leaves were pinched with sterilized syringe and suspension was sprayed over leaves. Disease index. (0–5) scale was used for bacterial spot index, where 0 shows no symptoms of the disease and 5 = severe disease/highest spot index. Aerial and underground factors of the plant were dried at 75% for overnight. Both parts of the plants were weighted after the drying, and the rate of relative growth measured by the given equation of (Van et al., 2016).
2.2.1 Estimation of MDA and H2O2
For melondialdehyde (MDA) content, the thiobarbituric acid test have done for lipid peroxidation measurement, a degradation product of polyunsaturated fatty acid oxidation, as described earlier by Horváth et al. (2015) was performed·H2O2 content was assessed using previously mentioned method (Diao et al., 2014).
2.2.2 Determination of the redox state
The contents of total glutathione (GSH + GSSG) and oxidized glutathione (GSSG) in the leaves were evaluated using a method described earlier (Van et al., 2016). Calculated GSH content by deducting GSSG content from GSH + GSSG, and then calculating the proportion of GSH/GSSH.
2.3 Assay of antioxidant enzymes activity
The presence of superoxide dismutase (SOD; EC:1.15.1.1) was calculated using the Giannopolitis and Reis (1997). Activity of SOD activity was assessed by monitoring its ability to prevent nitro blue tetrazolium's photochemical reduction. Mixture contained sodium phosphate (50 mM (pH7.8), methionine (13 mM), riboflavin (2 mM), NBT (75 mM), EDTA (100 nM), and extracted enzyme solution (100 mL). The absorbance adjustments had been read at 560 nm. Super oxide dismutase activity unit has been described as the quantity of enzyme that inhibits the photoreduction of NBT by 50 percent.
Peroxidase activity (POD; EC:1.11.1.7) was calculated by calculating the H2O2 peroxidation as an electron donor with guaiacol (Chance and Maehly, 1955). Increase in absorption intensity for 4 min due to tetraguaiacol production @ 470 nm. One value of the enzyme was considered to be the sum of the enzyme responsible for increasing the POD value by 0.01 in 1 m.
Ascorbate peroxidase (APX; EC:1.11.1.11) occurrence was evaluated by measuring the ascorbic acid absorbance reduction at 290 nm (extinction coefficient 2.8 mM L−1 cm−1) in a reaction mixture (1 mL) comprising phosphate buffer (50 mM (pH 7.6), Na-EDTA (0.1 mM), H2O2 (12 mM), ascorbic acid (0.25 mM) and plant extract as described in (Cakmak, 1994).
Catalase activity (CAT; EC:1.11.1.6) was estimated as earlier described (Chance and Maehly, 1955) to calculate the rate of conversion of hydrogen peroxide to water and oxygen molecules. The behavior was measured in a reaction solution (3 mL) consisting of phosphate buffer (7.0 pH 50 mM) containing H2O2 extract (5.9 mM) and enzyme (0.1 mL). Consumption of H2O2 was determined the catalase activity by a decrease in absorbance at 240 nm after every 20 s for 1 min. The occurrence was determined using a 39.4 mol L−1 cm−1 extinction coefficient.
Glutathione reductase (GR; EC: 1.6.4.2) the reduction in absorption coefficient at 340 nm due to NADPH oxidation was recorded for 1 min. The occurrence was measured using the 6.2 mmol L−1 cm−1 absorbance value.
Glutathione peroxidase (GPX: EC:1.11.1.9) NADPH oxidation was observed at 340 nm for 1 min, and the activity was measured using a 6.62 mmol L−1 cm−1 extinction coefficient.
2.4 Measurement of photosynthetic pigments
For the estimation of chl a and chl b pigments leaf samples (0.5 gm) from each treatment group were homogenized by the addition of 80% (v/v) acetone. Then, filtered the homogeneous. The absorbance of the mixture solution was calculated by spectrophotometer at 663, and 645 nm, respectively for Chl a and Chl b (Lichtenthaler et al., 1987).
2.4.1 Measurement of chlorophyll fluorescence parameters
Chlorophyll (Chl) fluorescence was recorded with a portable PhotoSyn Q.V.2.0 at room temp among 10:00 and 12:00 after 15 min of dark-adaptation of the attached leaves (Yang et al., 2008).
2.5 Statistical analysis
Using the statistical programme SPSS v. 2.0, all data collected were subjected to a one-way variance analysis (ANOVA). Means of treatments were measured using the Duncan test at α = 0.05 level of significance. Data set shows the mean of triplicates (n = 3), and as a mean ± standard error (SE) was represented.
3 Results
3.1 GSH reduced bacterial spot disease
Compared to the control, inoculated plants shown varying degree of symptoms. We selected two chilli varieties Serrano and Desi on the base of susceptibility. Serrano ranked as moderate susceptible, whereas Desi is susceptible. No variation in both varieties was observed based on the relative growth rate of the aerial and underground part when inoculated with X. campestris alone. Exogenous application of GSH before inoculation increased the relative growth rate of both varieties compared to simultaneous inoculation which showed not significantly difference. Serrano exhibited 24% suppression in plant dry weight while other variety Desi showed dry weight suppression higher than 35% in plants. But exogenous GSH application before bacterial inoculation significantly increased the plant dry weight with no and least symptoms respectively when compared with a simultaneous application (P < 0.05).
3.2 Chlorophyll pigments
The decline of photosynthetic pigments caused by X. campestris pathogen (Table 2) relative to control plants. In case of exogenous application of GSH before pathogen inoculation significantly increased the value of photosynthetic pigments were observed. However slightly increased level of pigments were recorded in case of simultaneous treatments of GSH and pathogen (P < 0.05).
Chl. Fluorescence parameters
Terms used
Formula
The initial fluorescence
F0
Measuring beam (<0.05 µmol m−2 s−1)
The maximal fluorescence
Fm
saturating pulse (12000 µmol m−2 s−1)
Maximum quantum yield of PSII
FV/Fm
(Fm − F0)/Fm
PSII excitation pressure
1-qP
(F − F0/)/(Fm/ − F0/)
PS II efficiency
ΦPSII (Phi2)
(F′m − Fs)/F′m
Photochemical quenching
qP
(F′M − Ft)/(F′M − F′0)
Non-photochemical quenching
NPQ
(FM − F′M)/F′M
qN
1 − (F′M − F′0)/(FM − F0)
Sunburn
PhiNO
Fs/Fm
Complementary with the photochemical quantum yield
Y(II) + NPQ + NO = 1
Linear electron flow
LEF
–
Relative chlorophyll
PQSPAD
–
Fraction of photon energy absorbed in PSII and dissipated via thermal energy
D%
(1 − FV//Fm/) × 100
Photon energy absorbed in PSII utilized for photosynthetic electron transport
P%
Fv//Fm/ × qP × 100
Fraction of excess excitation energy not dissipated in PSII and nor utilized in photosynthesis
EX
FV//Fm/ × (1 − qP)
Photon activity distribution coefficient of PSII and PSI
β and α
1(1 + f) and f/(1 + f)
f
Fm/ − Fs)/(Fm/ − F0/)
Relative deviation between PSII and PSI
β/α − 1
(1 − f)/f
3.3 GSH improved photosynthetic efficiency
Compared to controls, Chlorophyll fluorescence parameters viz., PhiNPQ, NPQt, ql, LEF, FS, RFD, ΘPSII and qP values were significantly reduced in bacterial inoculated seedlings of Desi followed by Serrano. Bacterial inoculated Serrano variety significantly increased the Fv/Fm value. A similar value of qP was observed in both inoculated seedlings. Application of exogenous GSH before bacterial inoculation exhibited increased the values of all fluorescence parameters in Desi except Fv/Fm, LEF, ΘPSII, qP and RFd parameters which were increased in Serrano (P < 0.05). The usage of exogenous GSH, by comparison before bacterial inoculation significantly increased all photosynthetic fluorescence parameters except NPQt, and FS (Fig. 1A) values in the leaves of Serrano under T1 and T2 treatments. While in case of Desi significantly increased NPQt, PQSPAD, FS, and qP values and decreased qL, Fv/Fm, LEF, RFd, and ΘPSII parameters compared to bacterial inoculated seedlings (Fig. 1B). In contrast, the simultaneous exogenous application of GSH + X. campestris significantly increased ql, Fv/Fm, LEF, and PQSPAD and decreased NPQt values (P < 0.05) in Serrano. Compared with Desi variety simultaneous application of GSH with virulent bacteria noticeably improved NPQt, ql, and qP values whereas remaining fluorescence parameters were decreased (P < 0.05).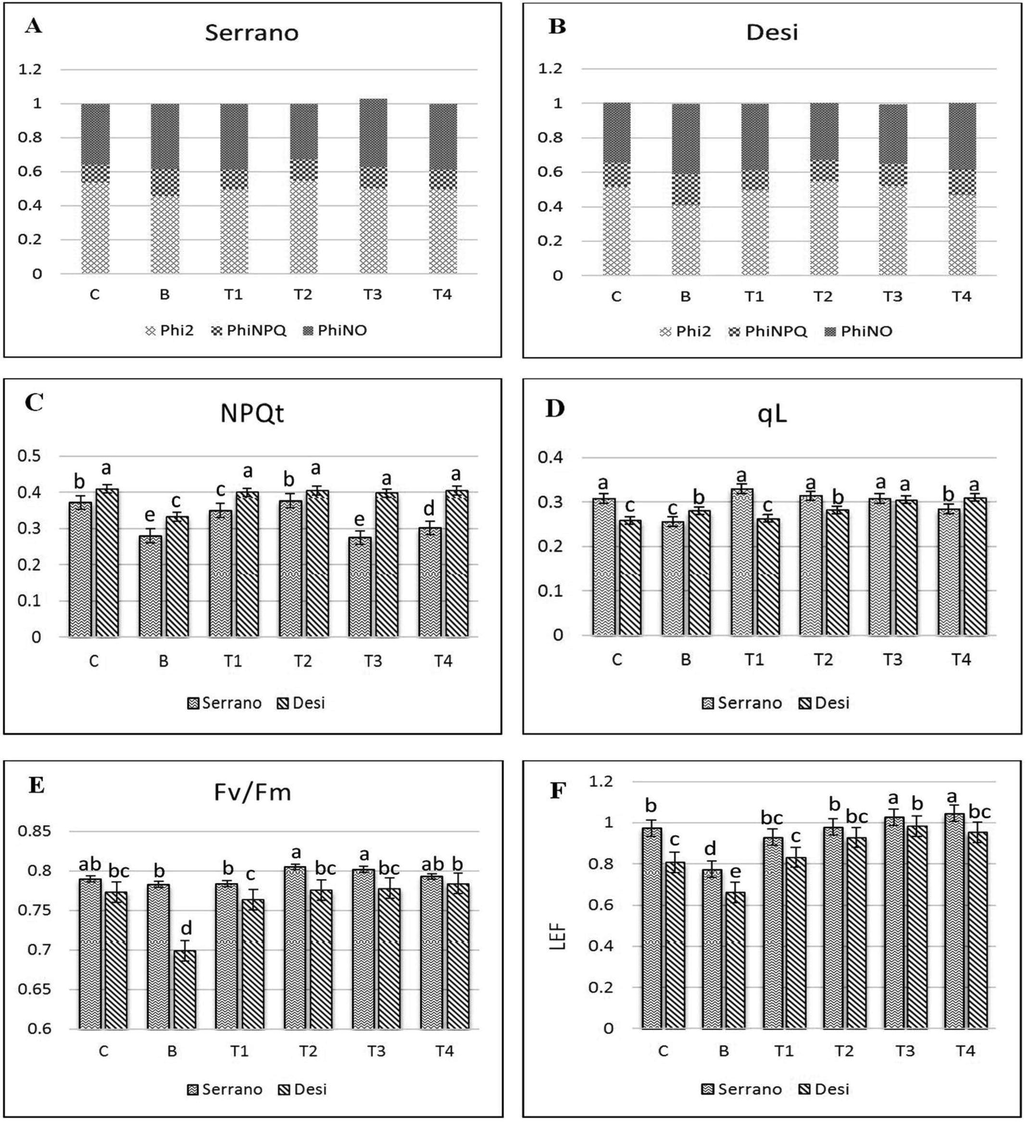
(A and B). Values of photosynthetic parameters (A)Phi2, PhiNPQ and PhiN0, (B) Phi2, PhiNPQ and PhiN0, (C) NPQT, (D) qL, (E) Fv/Fm, (F) LEF, (G) PQSPAD, (H) FS, (I) RFD, (J) ΦPSII, (K) qP, in Xanthomonas campestris (X.c) moderate susceptible and susceptible chilli seedlings as treated by exogenous reduced Glutathione (GSH). Control, not inoculated, no sprayed GSH, and B, inoculated with X.c, T1, First sprayed with 1.0 mmol GSH later X.c, T2, First sprayed with 0.5 mmol GSH later X.c, T3, simultaneous sprayed and inoculated with 1.0 mmol GSH + X.c and T4, simultaneous sprayed and inoculation with 0.5 mmol GSH + X.c respectively. Error bars indicate Sd values (n = 3).
(A and B). Values of photosynthetic parameters (A)Phi2, PhiNPQ and PhiN0, (B) Phi2, PhiNPQ and PhiN0, (C) NPQT, (D) qL, (E) Fv/Fm, (F) LEF, (G) PQSPAD, (H) FS, (I) RFD, (J) ΦPSII, (K) qP, in Xanthomonas campestris (X.c) moderate susceptible and susceptible chilli seedlings as treated by exogenous reduced Glutathione (GSH). Control, not inoculated, no sprayed GSH, and B, inoculated with X.c, T1, First sprayed with 1.0 mmol GSH later X.c, T2, First sprayed with 0.5 mmol GSH later X.c, T3, simultaneous sprayed and inoculated with 1.0 mmol GSH + X.c and T4, simultaneous sprayed and inoculation with 0.5 mmol GSH + X.c respectively. Error bars indicate Sd values (n = 3).
3.4 Allocation of absorbed light
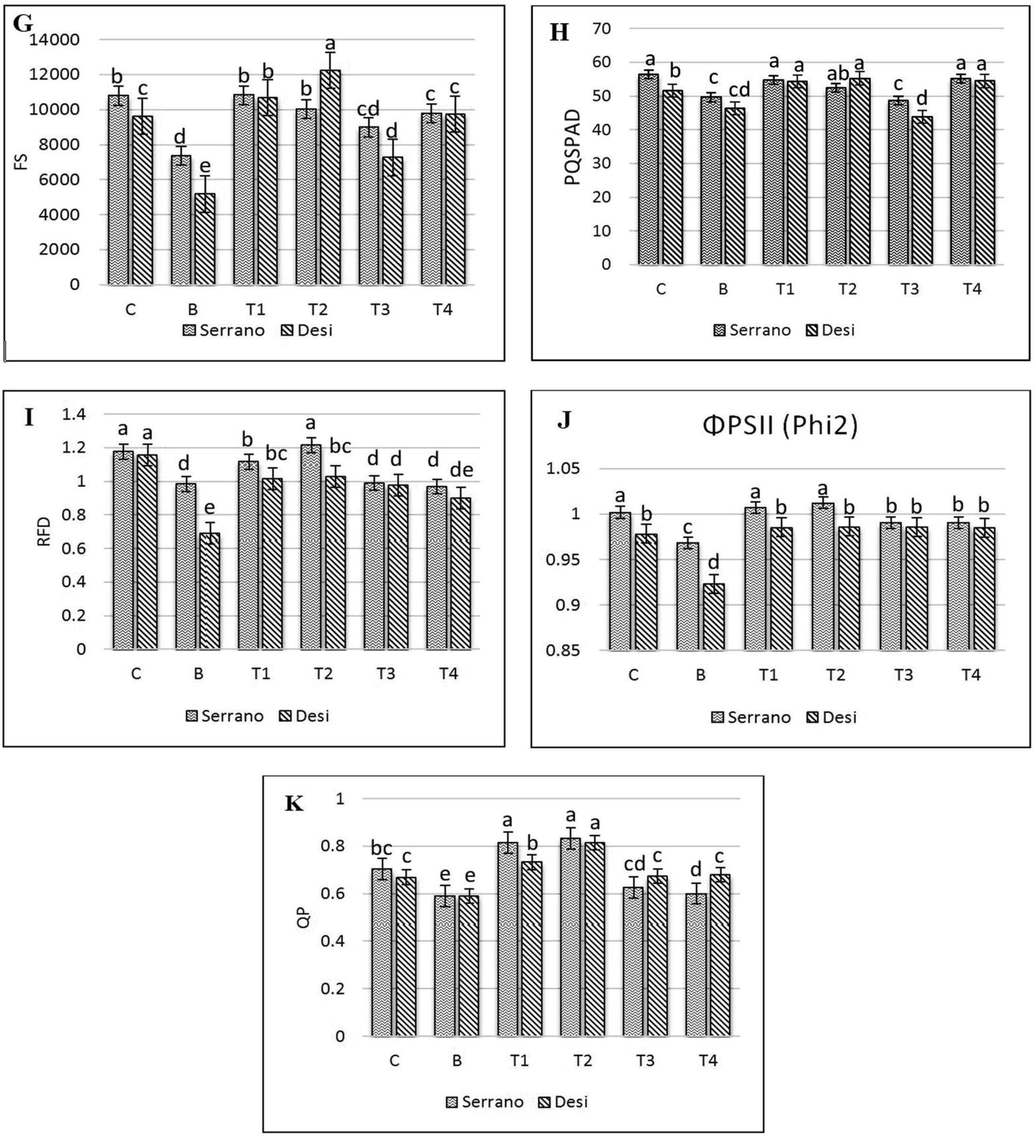
Treatment with bacteria has declined significantly the fraction of photon energy absorbed in PSII and utilized for photosynthesis (P) in both varieties whereas, PSII absorbed photon energy which dissipated via thermal energy termed as D% only decrease in Serrano (Fig. 2) and Ex and β/α−1 in the leaves of chilli increased significantly (P < 0.05). When 1.0 and 0.5 mM GSH applied before X. campestris, increased substantially in P and D% compared to bacterial treated seedlings except in Desi at 0.5 mM GSH before bacterial inoculation and had no significant impact on D% in both varieties. The D value in T2 (0.5 mM GSH before X. campestris) was significantly increased in Serrano. The Ex and β/α-1 values in T1 and T2 were significantly decreased by GSH application before bacterial inoculation. Simultaneous application of 1.0 and 0. 5 mM GSH with bacteria had not to effect on D value in both varieties compared to bacterial treated seedlings. The P-value was relatively high, and the values of Ex and β/α−1 in both simultaneous treatments (T3 & T4) were reduced significantly relative to bacterial exposed plants.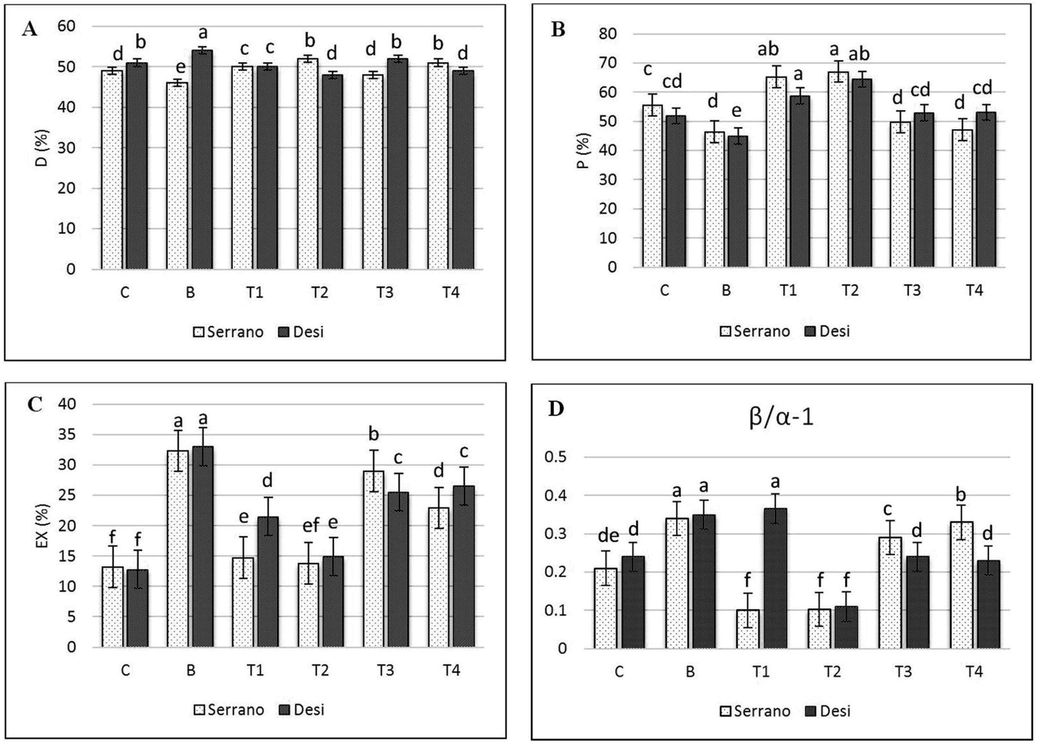
Values of Absorbed light allocation (A) D%, (B) P%, (C) Ex %, and (D) β/α-1 in Xanthomonas campestris (X.c) moderate susceptible and Susceptible chilli seedlings as treated by exogenous reduced Glutathione (GSH). Control, not inoculated, no sprayed GSH, and B, inoculated with X.c, T1, First sprayed with 1.0 mmol GSH later X.c, T2, First sprayed with 0.5 mmol GSH later X.c, T3, simultaneous sprayed and inoculated with 1.0 mmol GSH + X.c and T4, simultaneous sprayed and inoculation with 0.5 mmol GSH + X.c. Error bars indicate Sd values (n = 3).
3.5 GSH reduces reactive oxygen species
The plants suffered severely from bacterial disease, as indicated by increasing ratio of H2O2 and MDA in the chilli leaves (Table 3). Fortunately, the concentration of H2O2 and MDA in Xanthomonas-stressed seedlings was significantly reduced by GSH application. On the other hand, exogenous application of GSH before inoculation of X. campestris decreased remarkably reactive oxygen species compared to plants that had been treated simultaneously with GSH and X. campestris.
Treatments
Chl a (µmol m−2)
Chl b (µmol m−2)
C Serrano
12.5 ± 0.075b
6.5 ± 0.040c
Desi
12.0 ± 0.075b
5.9 ± 0.047c
B Serrano
8.3 ± 0.04 e
6.2 ± 0.040 d
Desi
7.9 ± 0.04 e
4.8 ± 0.025 e
T1 Serrano
14.1 ± 0.04 a
7.8 ± 0.025b
Desi
12.7 ± 0.027 a
6.9 ± 0.028b
T2 Serrano
12.8 ± 0.075b
8.3 ± 0.025 a
Desi
11.6 ± 0.028c
8.0 ± 0.028 a
T3 Serrano
10.2 ± 0.062c
6.6 ± 0.028c
Desi
9.3 ± 0.047 d
5.9 ± 0.025c
T4 Serrano
9.0 ± 0.028 d
6.0 ± 0.028 e
Desi
9.0 ± 0.028 d
5.5 ± 0.047 d
3.6 GSH modulate endogenous GSH content and redox state
Chilli seedlings exposure to X. campestris significantly reduced intrinsic GSH level and also the ratio of GSH/GSSG (Table 3). Endogenous GSH contents increased by the exogenous application of GSH. Correspondingly, compared to pathogen inoculated seedlings, GSH and GSH/GSSG ratio were significantly reduced in the simultaneously treated seedlings (Table 4). Before pathogen inoculation, exogenous application of GSH increased the endogenous contents of GSH and GSH/GSSG ratio, compared to simultaneously treated plants.
Treatments
H2O2 content
(µmol mg−1 protein)MDA content
(µmol mg−1 protein)GSH content
(µmol mg−1 FW)GSH/GSSG ratio
C Serrano
12.0 ± 0.04c
1.5 ± 0.04c
2.5 ± 0.028c
0.66 ± 0.004 a
Desi
16.0 ± 0.04c
1.8 ± 0.04c
2.2 ± 0.04b
0.62 ± 0.004 a
B Serrano
23.3 ± 0.085 a
3.9 ± 0.02 a
1.5 ± 0.04f
0.24 ± 0.004 e
Desi
28.5 ± 0.085 a
4.1 ± 0.04 a
0.98 ± 0.008f
0.18 ± 0.004 e
T1 Serrano
9.5 ± 0.028 d
1.2 ± 0.04 d
2.8 ± 0.04b
0.44 ± 0.004c
Desi
10.3 ± 0.028 e
1.6 ± 0.02 d
2.0 ± 0.04c
0.38 ± 0.003c
T2 Serrano
7.3 ± 0.04 e
0.98 ± 0.007 e
3.0 ± 0.04 a
0.51 ± 0.004b
Desi
9.7 ± 0.11f
1.0 ± 0.028 e
2.7 ± 0.04 a
0.45 ± 0.004b
T3 Serrano
12.2 ± 0.064c
2.5 ± 0.028b
2.0 ± 0.04 e
0.38 ± 0.003 d
Desi
13.0 ± 0.075 d
2.8 ± 0.040b
1.2 ± 0.04 e
0.29 ± 0.004 d
T4 Serrano
16.8 ± 0.04b
1.9 ± 0.04c
2.2 ± 0.04 d
0.41 ± 0.004c
Desi
17.5 ± 0.04b
2.1 ± 0.04c
1.87 ± 0.04 d
0.37 ± 0.003c
3.7 GSH modulated enzymatic antioxidants
SOD, POD, CAT and APX activities were significantly decreased in Desi seedlings under bacterial stress as opposed to control (P < 0.05) (Fig. 3A−D). While in Serrano bacteria did not effect on antioxidant enzymes relative to the control seedlings (P < 0.05) (Fig. 3A−D). Treatment 1 (1.0 mM GSH) before bacterial inoculation seedlings shows enhanced activities of SOD, POD, and CAT enzymes by 70, 52 and 57% respectively, in Serrano relative to Desi cultivar. In T2 (0.5 mmol GSH) significantly increased activities of SOD and POD were observed in both varieties whereas CAT and APX were decreased compared with bacterial treated seedlings. Simultaneous application of 1.0 mmol GSH with X campestris (T3) increased the SOD, POD, CAT and APX activities in Serrano followed by Desi. In contrast, combined the application of 0.5 mmol GSH + X. campestris (T4) improved activities of SOD, POD, CAT and APX in Serrano by 70, 47, 58 and 80% respectively, relative to the Desi seedlings (Fig. 3A−D)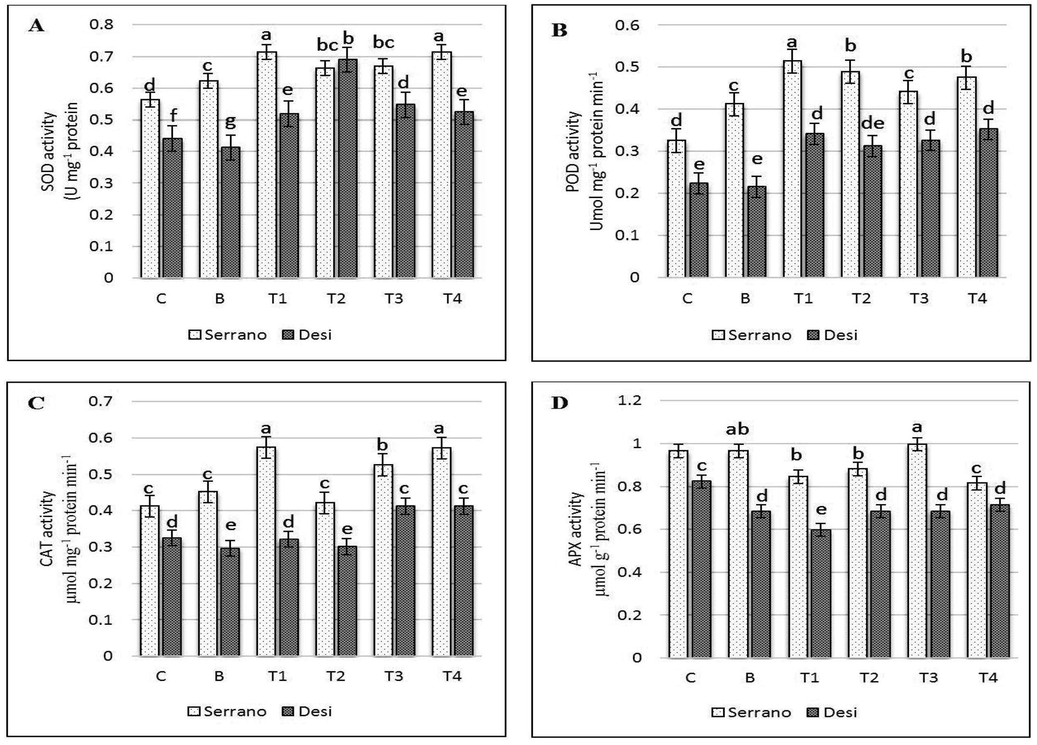
Antioxidant enzyme activities (A) Superoxide dismutase (SOD), (B) peroxidase dismutase (POD), (C) catalase (CAT) and (D) ascorbate peroxidase (APX) in Xanthomonas campestris (X.c) moderate susceptible and Susceptible chilli seedling leaf as treated by exogenous reduced Glutathione (GSH). Control, not inoculated, no sprayed GSH, and B, inoculated with X.c, T1, First sprayed with 1.0 mmol GSH later X.c, T2, First sprayed with 0.5 mmol GSH later X.c, T3, simultaneous sprayed and inoculated with 1.0 mmol GSH + X.c and T4, simultaneous sprayed and inoculation with 0.5 mmol GSH + X.c. Error bars indicate Sd values (n = 3).
4 Discussion
Chlorophyll fluorescence metrics testing of the potential of photosynthetic activity, distribution in the plant leaves. This method also provides a chance to estimate time-based modifications and the kinetics of mechanisms that affect photosynthetic efficiency. The quantum performance of PSII and the rate of photoinhibition is used constantly to ensure the energy distribution condition in the thylakoid membrane (Hu et al., 2014; Zhang et al., 2015). In this study, on the base of pathogenicity test of chilli varieties to X. campestris we selected two chilli varieties (moderately susceptible and susceptible). Besides, the improvements in pathogen infection were associated with the effect of the GSH.
Fluorescence parameters used conventionally in plant physiology are tested routinely, and the one with the better contrast is used to assess the regional stress. (Nedbal et al., 2005; Chaerle et al., 2001; Mostofa et al., 2014; Šebela et al., 2018; Berger et al., 2004). Numerous researchers reported that Fv/Fm, ETR, Qp and 1-Qp values distinguished physiological status of stressed plant (Baker and Rosenqvist, 2004; Rolfe and Scholes, 2010; Van et al., 2016). This study confirmed that biotic stress, Xanthomonas bacteria, decreased Fv/Fm, Qp, ETR parameters (Fig. 1). The declines in Fv/Fm indicated inhibition of the photosynthetic apparatus induced by photoinhibition in the direct vicinity of the PSII reaction centers (Noctor et al., 2012). A decline in photosynthesis, mostly calculated as an effective quantity of PSII, was also recorded when biotrophic and necrotrophic pathogens were infected in various plant species (Balachandran et al., 1997; Noctor et al., 2012; Chaerle et al., 2001; Mostofa et al., 2014; Berger et al., 2004). In contrast, pathogen attack had a direct and indirect effect on NPQ. The impact on NPQ will rely on how much tissue damage there is. In non-heavily damaged tissues, NPQ may raise as a protective mechanism, based on stimulated electron flow (Schreiber et al., 2004).
Simultaneous application of GSH with X. campestris decreased in photosynthesis whereas exogenous application of glutathione before bacterial inoculation increased photosynthesis. Glutathione has a low molecular weight and directly acts as an antioxidant, maintains homeostasis among different components of the cellular antioxidant and plays a central role in the scavenging and detoxification of toxic material in the chloroplast (Foyer and Noctor, 2005; András et al., 2012). The present study revealed that antioxidant molecule GSH generated from the plant often exerts similar effects on fluorescence parameters. Due to their reactivity, the rapid decrease in Fv/Fm in response to GSH suggests a direct impact on PSII. This is in line with the earlier identification and induction of protection against avirulent strain (Dong et al., 1991; Baker et al., 1997). Exogenous GSH stimulates plant growth and improve tolerance against the stress through the detoxification of reactive oxygen species (Meyer et al., 2001; Cao et al., 2015; Ding et al., 2016). Further, our findings reported that the performance of exogenous GSH was more useful and inhibited the generation of reactive oxygen species.
Superoxide dismutase delivers front line defense against reactive oxygen species by removing O2 (Hasanuzzaman et al., 2012). In our present research a significant increase in superoxide dismutase enzyme action was detected in T1, and T4 (Fig. 3). Kuzniak and SkLodowska (2004) observed similar findings in 2010; GSH was founded to be the most significant antioxidant thiol in plant-pathogen interactions. Similarly increase the defenses against ROS generated by pathogen and which supports the results of an earlier study which confirmed that GSH increases under biotic stress (Kuzniak and SkLodowska, 2004; Clemente-Moreno et al., 2013).
In this study, increased POD activity was observed in T1 followed by simultaneous exogenous application > 0.5 mM > bacterial inoculation > control. (Diao et al., 2014) reported that this reduction was due to POX catalysis of hemicellulose feruloylation and Insolubilization of glycoproteins rich in hydroxyproline, causing cell-wall rigidity. Likewise reduced oxidizing stress with reduced POD activity was observed due to lack of H2O2 and lipid hydroperoxidase, which involved in protection against oxidative stress.
GSH is an essential constituent of the Ascorbate/Glutathione cycle linked with H2O2 foraging (Foyer and Noctor, 2011; Goraya and Asthir, 2016). In the present study CAT activity in the chilli plant decreased in control and bacterial treated plants and significantly increased when simultaneously sprayed glutathione and bacteria in all varieties except Desi. (Hasanuzzaman et al., 2012) observed similar results, 2012 revealed that inducing GSH activity under abiotic stress conditions has been a significant factor in the development of stress tolerance in plants. The direct scavenging of H2O2 to H2O is also responsible for ascorbate peroxidase activity (Foyer and Noctor, 2005; Hasanuzzaman et al., 2012). The results of present study showed that bacterial stress produced higher Ascorbate peroxidase activity in Serrano and Hybrid. In simultaneous inoculation of glutathione and bacteria (Fig. 3), followed by 0.5 mM glutathione with bacteria inoculation in Padron and Desi (Fig. 3) which agrees with previous findings where abiotic stress caused higher APX activity in different plants (Alam et al., 2013).
5 Conclusion
Bacterial spot disease inhibit plant development and photosynthesis reduction have been ameliorated by exogenous use of GSH on infected cultivars. Glutathione application prompt plant growth by modulating photosynthesis of susceptible chilli seedlings, Improving the efficiency of light utilization and excitation energy dissipation in the PSII, regulating the absorbed light distribution and maintaining redox homeostasis of plants as well as activating plant antioxidant protection system to prevent chloroplast from oxidative harm. Exogenous application of GSH can be a useful tool for enhancing growth and tolerance against pathogen attack.
Acknowledgments
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for the funding of this research through the Research Group Project No. RG-1441-484.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Exogenous salicylic acid ameliorates short-term drought stress in mustard (Brassica juncea L.) seedlings by up-regulating the antioxidant defense and glyoxalase system. Aust. J. Crop Sci.. 2013;7:1053-1063.
- [Google Scholar]
- The ascorbate-glutathioneα-tocopherol triad in abiotic stress response. Int. J. Mol. Sci.. 2012;13:4458.
- [Google Scholar]
- Annonymous, 2000 Achievements, All India Coordinated research project on Agroforestry. Division of Forestry, Technical Document No 13.
- Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu. Rev. Plant Biol.. 2008;59(1):89-113.
- [CrossRef] [Google Scholar]
- Applications of chlorophyll fluorescence can improve crop production strategies: an examination of future possibilities. J. Exp. Bot.. 2004;55:1607-1621.
- [Google Scholar]
- Concepts of plant biotic stress. Some insight into the stress physiology of virus infected plants, from the perspective of photosynthesis. Physiol. Plant.. 1997;100:203-213.
- [Google Scholar]
- Complex regulation of gene expression, photosynthesis and sugar levels by pathogen infection in tomato. Physiol. Plant.. 2004;122:419-428.
- [CrossRef] [Google Scholar]
- Infection with virulent and avirulent P. syringae strains differentially affects photosynthesis and sink metabolism in Arabidopsis leaves. Planta. 2006;225:1-12.
- [Google Scholar]
- Genotypic dependent effect of exogenous glutathione on Cd-induced changes in proteins, ultrastructure and antioxidant defense enzymes in rice seedlings. J. Hazard. Mater.. 2011;192(3):1056-1066.
- [Google Scholar]
- Activity of ascorbate-dependent H^Oj scavenging enzymes and leaf chlorosis are enhanced in magnesium- and potassium-deficient leaves, but not in phosphorus-deficient leaves. J. Exp. Bot.. 1994;45:1259-1266.
- [Google Scholar]
- Effects of silicon on absorbed light allocation, antioxidant enzymes and ultrastructure of chloroplasts in tomato leaves under simulated drought stress. Sci. Hortic.. 2015;194:53-62.
- [CrossRef] [Google Scholar]
- Thermal and chlorophyll-fluorescence imaging distinguish plantpathogen interactions at an early stage. Plant Cell Physiol.. 2001;45:887-896.
- [CrossRef] [Google Scholar]
- Chloroplast protection in plum pox virus-infected peach plants by L-2-oxo-4-thiazolidine-carboxylic acid treatments: effect in the proteome. Plant, Cell Environ.. 2013;36(3):640-654.
- [Google Scholar]
- Jasmonate regulated Arabidopsis stress signaling network. Physiol. Plant.. 2005;123:161-172.
- [Google Scholar]
- Selinium promotes the growth and photosynthesis of tomato seedlings under salt stress by enhancing chloroplast antioxidant defense system. J. Plant Growth Regul.. 2014;33:671-682.
- [Google Scholar]
- Exogenous glutathione improves high root-zone temperature tolerance by modulating photosynthesis, antioxidant and osmolytes systems in cucumber seedlings. Sci. Rep.. 2016;6(1)
- [CrossRef] [Google Scholar]
- Induction of Arabidopsis defense genes by virulent and a virulent Pseudomonas syringae strains and by a cloned avirulence gene. Plant Cell. 1991;3:61-72.
- [Google Scholar]
- Oxidant and antioxidant signalling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant, Cell Environ.. 2005;28(8):1056-1071.
- [Google Scholar]
- Ascorbate and glutathione: the heart of the redox hub. Plant Physiol.. 2011;155(1):2-18.
- [CrossRef] [Google Scholar]
- Superoxide dismutase I. Occurrence in higher plants. Plant Physiol.. 1997;59:309-314.
- [Google Scholar]
- Magnificant role of intracellular reactive oxygen species production and its scavenging encompasses downstream processes. J. Plant Biol.. 2016;59(3):215-222.
- [CrossRef] [Google Scholar]
- Exogenous nitric oxide alleviates high temperature induced oxidative stress in wheat ('Triticum aestivum'L.) seedlings by modulating the antioxidant defense and glyoxalase system. Aust. J. Crop Sci.. 2012;6(8):1314.
- [Google Scholar]
- Hardening with salicylic acid induces concentrationdependent changes in abscisic acid biosynthesis of tomato under salt stress. J. Plant Physiol.. 2015;183:54-63.
- [Google Scholar]
- Photosynthesis, chlorophyll fluorescence characteristics, and chlorophyll content of soybean seedlings under combined stress of bisphenol A and cadmium. Environ. Toxicol. Chem.. 2014;33:2455-2462.
- [Google Scholar]
- Differential implication of glutathione, glutathione-metabolizing enzymes and ascorbate in tomato resistance to pseudomonas syringae. J. Phytopathol.. 2004;152(10):529-536.
- [CrossRef] [Google Scholar]
- Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. In: Douce R., Packer L., eds. Methods in Enzymology. New York (NY): Academic Press Inc; 1987. p. :350-382.
- [Google Scholar]
- Inhibition of photosynthesis by Colletotrichum lindemuthianum in bean determined by chlorophyll fluorescence imaging. Plant Cell Environ.. 2001;24:947-955.
- [CrossRef] [Google Scholar]
- Exogenous sodium nitroprusside and glutathione alleviate copper toxicity by reducing copper uptake and oxidative damage in rice (Oryza sativa L.) seedlings. Protoplasma. 2014;251(6):1373-1386.
- [CrossRef] [Google Scholar]
- Chlorophyll fluorescence analysis: a guide to good practice and understanding some new applications. J. Exp. Bot.. 2013;64:3983-3998.
- [Google Scholar]
- Photosynthesis in dynamic light: systems biology of unconventional chlorophyll fluorescence transients in Synechocystis sp. PCC 6803. Photosynth. Res.. 2005;84:99-106.
- [Google Scholar]
- Glutathione in plants: an integrated overview. Plant, Cell Environ.. 2012;35(2):454-484.
- [Google Scholar]
- Roháček K, Barták M., 1999. Technique of the modulated Chlorophyll Fluorescence: basic concepts, useful parameters, and some applications.
- Chlorophyll fluorescence imaging of plant–pathogen interactions. Protoplasma. 2010;247(3-4):163-175.
- [CrossRef] [Google Scholar]
- Pulse amplitude modulation (PAM) fluorometry and saturation pulse method: an overview. In: Papageorgiu G., Govindjee,, eds. Chlorophyll a Fluorescence. A Signature of Photosynthesis. Dordrecht, The Netherlands: Springer; 2004. p. :279-319.
- [Google Scholar]
- Chlorophyll fluorescence and reflectance-based non-invasive quantification of blast, bacterial blight and drought stresses in rice. Plant Cell Physiol.. 2018;59:30-43.
- [CrossRef] [Google Scholar]
- Functional response (FR) and relative growth rate (RGR) do not show the known invasiveness of Lemna minuta (Kunth) PLoS ONE. 2016;11:1-18.
- [Google Scholar]
- Comparison of effects of salt and alkali stresses on the growth and photosynthesis of wheat. Photosynthetica. 2008;46(1):107-114.
- [CrossRef] [Google Scholar]
- Effects of Bisphenol A on chlorophyll fluorescence in five plants. Environ. Sci. Pollut. Res.. 2015;22:17724-17732.
- [Google Scholar]







