Translate this page into:
Evaluation of molecular mechanisms of heparin-induced angiogenesis, in human umbilical vein endothelial cells
⁎Corresponding author. safi.nust@yahoo.com (Sher Zaman Safi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Chitosan and heparin-based hydrogels possess potent properties such as air permeability, absorption and water retention. Our group has previously reported heparin-loaded, freeze-dried porous hydrogels, using chitosan/PVA and variable concentrations of PCL. Significant angiogenic effect of these hydrogels was observed. In this follow-up study, we have investigated the underlying molecular mechanisms of increased angiogenesis due to these heparin-releasing chitosan-based hydrogels, in human umbilical vein endothelial cells (HUVECs). Cells were cultured in complete ECM media and supplemented with 10% FBS and 1% Penicillin-Streptomycin. HUVECs were treated with heparin-releasing chitosan-based hydrogels in different concentrations (H4, H8 and H16) for 1, 3 and 7 days. RNA was extracted using trizol and quantified by nanodrop. Primers were designed for the target genes including VEGF, VEGFR, Ap-1, BCL-2, HIF-1, BAX, IL-1 and TNF-α. Gene expression analysis were performed using PCR. Gel electrophoresis was used to visualize results. MTT and DCFH-DA assays were performed to check cytotoxicity and reactive oxygen species (ROS) respectively. Hydrogels H8, had significantly high effect on the expression of VEGF and VEGFR, as compared to H4 and H16. At day 1, most of the target genes showed higher expression. HIF-1 showed high expression at day one, with a gradual decrease at day 3 and 4. We observed no expression of AP-1 with hydrogels H4, H16 and control, but a high expression with hydrogels H8, at day 1 and 3. BCL-2, IL-1and TNF-α also revealed high expression with hydrogels H8. A low expression of BAX was observed in all cases. Our study demonstrates high expression of genes with H4 probably due to high concentration of heparin. Similarly, at day 1, the high expression is attributed to the fact that these hydrogels release maximum heparin in the first 24 h.
Keywords
Molecular mechanisms
Cell biology
Biomaterials
Chitosan
Heparin
Angiogenesis
1 Introduction
Chitosan and heparin are promising biomaterials for wound healing. Hydrogels which contain these materials possess exceptional properties such as air permeability, absorption, water retention and absorption of wound exudates (Liu et al., 2018). According to the literature, a broad range of biomedical applications of chitosan-based hydrogels have been reported (Kweon et al., 2003; Jeon et al., 2008). Heparin binds and activates VEGF which regulates the proliferation and migration of endothelial cells and enhance the process of angiogenesis (Harmey, 2004; Safi et al., 2014).
Angiogenesis, formation of new blood vessels, is an important aspect of normal biological processes including growth, reproduction, development and repair of damaged tissue (Bauer et al., 2005). There are several soluble growth factors such as Vascular Endothelial Growth Factor (VEGF) and Fibroblast Growth Factor (FGF), that regulate the initiation of angiogenesis (Costa et al., 2007; Safi et al, 2015; Nissen et al., 1998). VEGF, is a significant signaling protein of various cascades but mainly involved in vasculogenesis and angiogenesis (Nör et al., 2001). Its activity is mainly constrained to vascular endothelial cells, although studies have shown that it has effect on several other cell lines. Other than angiogenesis in adults, VEGF also plays an important role during embryonic development and damage repair after injury (Baffert et al., 2006). Signaling cascade of VEGF-induced angiogenesis, mediates endothelial cell migration and proliferation (Zachary and Gliki, 2001; Brandner et al., 2006; Uciechowska-Kaczmarzyk et al., 2018; Simons et al., 2016).
It is well known that VEGF is regulated by heparin, a natural glycosaminoglycan, found on cell surfaces and in extra cellular matrix of nearly all mammalian cells (Safi et al., 2014). It is present in wounds at high concentrations where it regulates various proangiogenic factors such as VEGF and FGF (Gigliobianco et al., 2015). Balance between diffusion and retention of VEGF is also maintained by heparin (Ruhrberg et al., 2002). Heparin contains negatively charged specific motifs on its side chain that can bind specific proteins, growth factors, and regulate their diffusion (Zhao et al, 2012; Alajmi et al., 2021).
VEGF and VEGFR-mediated angiogenesis involve a number of genes and other biological factors such transcription factors and reactive oxygen species (ROS) generation. IL-1α, an inflammatory cytokine also known as hematopoietin 1, belongs to interleukin 1 family, encoded by IL1A gene in humans (Nicklin et al., 1994). It is mainly produced by activated endothelial cells, epithelial cells, macrophages and neutrophils. Interleukin 1 plays an important role in regulation of many biological processes including angiogenesis, immune and inflammation. TNF-α is a cytokine, a cell signaling protein, involved in inflammatory responses. It is a potent activator of angiogenesis and activates fibroblast growth factor (FGF) and platelet activating factor (PAF) in endothelial cells (Bankers-Fulbright et al., 1996; Safi et al., 2018; Ryuto et al., 1996; Karar et al., 2021). It has been observed that TNF-α interacts with VEGF-A, FGF, and IL-1, to induce angiogenesis in endothelial cells (Yoshida et al., 1997; Defilippi et al., 1991; Safi et al., 2016a, 2017; 2016b;; Koolwijk et al., 1996).
In endothelial cells, migration, proliferation and angiogenesis are induced when VEGF binds to its specific receptors VEGFR-1 and VEGFR-2. Studies have reported that transcription factor AP-1 plays an important role in cell proliferation and differentiation, consequently regulating VEGF in response to various cytokines (Mar et al., 2015). Expression of AP-1 is stimulated by various physiological and pathological factors including growth factors, cytokines, microbial infections, stress signals and oncogenes (Hess et al., 2004; Safi et al., 2016a; 2016b). BCL-2 is a regulatory protein considered as an oncogene which regulates cell death, apoptosis, either by inducing or inhibiting apoptosis, proliferation and angiogenesis (Karimian et al., 2015a, 2015b; Stein et al., 1995; Dias et al., 2002). BAX is apoptosis regulator protein which is known as Bcl2 like protein, which belongs to BCL-2 gene family. BAX functions as an apoptotic activator when forms heterodimer with BCL-2. (Jaafar et al., 2012).
A composite of polymers, chitosan, polyvinyl alcohol (PVA) and polycaprolactone (PCL) was developed to get a hydrogel with greater heparin binding ability by our group (Yar et al., 2016; Metwally et al., 2021; Arif et al., 2021). These hydrogels exhibited a great potential as a pro-angiogenic factor, however the underlying molecular mechanisms were not investigated. To explain the molecular basis of heparin/chitosan-induced angiogenesis, we conducted this study in HUVECs and included some of the key genes involved in the process of angiogenesis, including VEGF, VEGFR, TNF-α, IL-1, HIF-1, Ap-1, regulatory proteins BCL-2 and BAX. We have also examined ROS generation in the presence of these hydrogels, as ROS generation also mediates the gene expression and the progression of angiogenesis.
2 Materials
Human Umbilical Vein Endothelial Cells (HUVECs), MTT reagent (Cat. No. 30-1010 K), Trizol Reagent (Cat. No. 15596026), Dichloro-dihydro-fluorescein diacetate (H2DCFDA) reagent (Cat. No. D399), DNA ladder (Cat. No. 15628019), cDNA synthesis (M0253) and Taq DNA Polymerase (M0273) kits.
3 Methods
3.1 Preparation of hydrogels
Hydrogels (Control, H4, H8 and H16) were prepared at Interdisciplinary Research Centre in Biomedical Materials, COMSATS University Islamabad, Lahore Campus, by our group (37). The formulations of hydrogels are given in Table 1.
Product
Chitosan (g/10 ml Solution
PVA (g)/10 ml Solution
PCL(g) + heparin (mg)/10 ml solution
CS:PVA:PCL (wt%)
H4
0.3
0.7
0.4 + 1
21.4/50/28.6
H8
0.3
0.7
0.8 + 1
16.7/38.9/44.4
H16
0.3
0.7
1.6 + 1
11.5/26.9/61.5
Control
0.3
0.7
0.4 + 0
21.4/50/28.6
3.2 Cell culture
Human Umbilical Vein Endothelial Cells (HUVECs) were thawed at 37 °C in water bath. Cells were cultured in gelatin coated T-25 flasks, containing 4 ml of media, supplemented with 10% Fetal Bovine Serum (FBS) and 1% of pen/strep. Upon 80% confluency, cells were sub cultured using Trypsin/EDTA and incubated at 37 °C with 5% CO2. Media was changed every 2 to 3 days. Upon 70% confluency, HUVECs were exposed to hydrogels (Control, H4, H8 and H16) and incubated at 37 °C, with 5% CO2, for day 1, day 3 and day 7.
3.3 RNA extraction
Total RNA was extracted from set-1 of four T-25 flasks containing control, H4, H8 and H16 at day 1, followed by the extraction of other sets at day 3 and day 7. Briefly, cells were washed with 1 ml PBS followed by addition of 1 ml TRIzol Reagent. 200 µl of chloroform/1ml Trizol was added to each Eppendorf tube, followed by vertexing for 15 s. RNA was carefully transferred into new tubes followed by the addition of 0.5 ml of isopropyl alcohol. Tubes were centrifuged at 10,000 rpm for 10 min which resulted into gel like pellet formation. Supernatant was removed and RNA pellet was washed with 75% ethanol and again vortexed for 15 s followed by another centrifugation at 14000 rpm for 5 min. Ethanol was removed and RNA pellet was air dried. RNA was dissolved in 50 µl of DEPC treated water by pipetting several times.
3.4 RNA quantification
The amount and purity of extracted RNA was measured by NanoDrop Spectrophotometer. The absorption was recorded at 260/280 while amount was taken in ng/µl.
3.5 cDNA synthesis
cDNA was synthesized according to the manufacturer’s guideline. The components of the kit were thawed on ice and mixed by inverting the tubes several times. All the components were mixed and incubated at 42o C for 1 h followed by denaturation of enzyme at 80oC for 5 min.
3.6 Primer designing
Primers were designed for VEGF, VEGFR, TNF-α, IL-1, HIF-1, Ap-1, BCL-2 and BAX using Primer3. The primers used for each gene are provided in Table 2.
Gene
Forward primer
Reverse primer
TNF-Α
5′-CTGGGCAGGTCTACTTTGGG-3′
5′-CTGGAGGCCCCAGTTTGAAT-3′
BAX
5′-GTCTTTTTCCGAGTGGCAGC-3′
5′-GGAGACAGGGACATCAGTCG-3′
BCL-2
5′-CCTTTGTGGAACTGTACGGC-3′
5′-CCGGCCAACAACATGGAAAG-3′
IL-1A
5′-ACTGGAAAACCAGGCGTAGG-3′
5′-CTTCATCTTGAGGCTCGGCT-3′
Beta-Actin
5′-AGAGCTACGAGCTGCCTGAC-3′
5′-AGCACTGTGTTGGCGTACAG-3′
HIF-1A
5′-GTGGTGGTTACTCAGCACTT-3′
5′-CCAGAAGTTTCCTCACACGC-3′
AP-1
5′-CAGCCAGGTCGGCAGTATAG-3′
5′-GGGACTCTGCCACTTGTCTC-3′
VEGFA
5′-TGTCTAATGCCCTGGAGCCT-3′
5′-CTTCCGGGCTCGGTGATTTA-3′
3.7 Polymerase chain reaction (PCR)
DNA template amplification was made through PCR by following manufacturer’s guideline. All the components including Taq DNA Polymerase, Template DNA, dNTPs, Buffer, MgCl2, Nuclease-free water and primers were added in the PCR mixture. PCR’s basic conditions are given in Table 3, while annealing temperature was randomly changed between 50 and 66 °C untill the expression was achieved.
Step
Temperature
Time
Initial Denaturation
95 °C
30 s
35 Cycles
95 °C
30 s
40–65 °C
45 s
72 °C
40 s
Final Extension
72 °C
5 min
Hold
4 °C
∞
3.8 Gel electrophoresis
Agarose was added in 1X TAE buffer in order to prepare 1% Agrose Gel which was then heated for 1 min in a microwave. This was followed by addition of 2 µl of ethidium bromide (EtBr) to it. Agarose solution was poured into a gel tray having a wells comb. Tray with liquid gel was then placed at room temperature for solidification. PCR samples were loaded with 100 bp ladder. 2 µl of loading dye was added to each PCR sample and results were observed on UV trans illuminator.
3.9 MTT assay
MTT reagent is used to determine the number of viable and metabolically active cells. Hydrogels (Control, H4, H8 and H16) treated HUVECs were cultured in 96 wells plate for day-1, day-3 and day-7. In brief, 10 µl of MTT reagent with final concentration was added to each well and incubated at 37 °C for 4 h followed by the addition of 100 µl of detergent reagent. The plate was gently swirled and again incubated. The absorbance was measured at 570 nm in a plate reader.
3.10 Dichloro-dihydro-fluorescein diacetate (DCFH-DA) assay
2′7′-dichloro-dihydro-fluorescein diacetate (DCFH-DA) reagent, the ROS indicator was used to measure intracellular reactive oxygen species (ROS) in HUVECs. HUVECs were seeded in 96 wells-plate treated with hydrogels and readings were taken at 485 nm excitation and 530 nm emission.
4 Results and discussion
4.1 Effect of heparin releasing chitosan-based hydrogels on cytotoxicity
Before gene expression analysis, we conducted MTT to check if these hydrogels have any cytotoxic effect on HUVECs. We observed no significant effect. The number of viable cells in case of heparin loaded hydrogels was not decreased much as compared to control hydrogel, except a slight difference (Fig. 1A, 1B and 1C).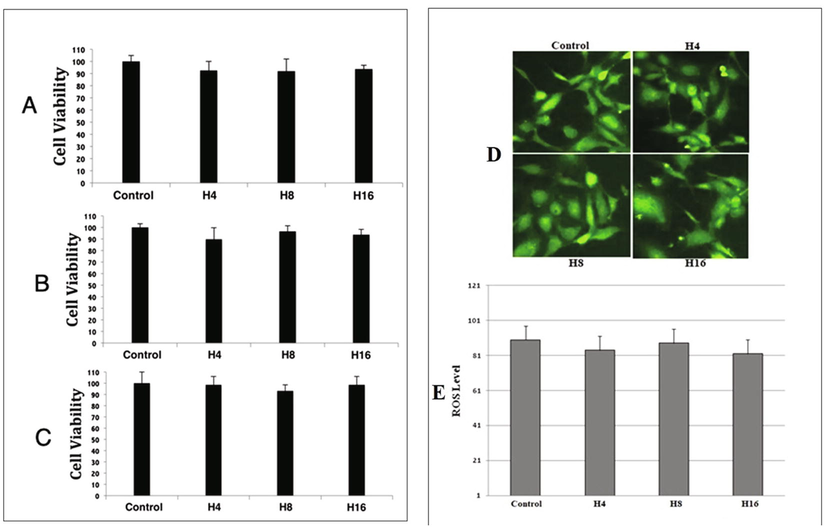
Graphical representation of cells viability after exposing them to our hydrogels. (A) Represents HUVECs treated with hydrogels at day 1, (B) day 3 and (C) day 7. Fig. 1D and 1E shows level of reactive oxygen species (ROS).
4.2 Heparin loaded hydrogels have no effect on ROS generation
Heparin loaded hydrogels exhibited increased angiogenesis. Reactive oxygen species play a key role in the process of angiogenesis, thus we decided to assess the amount of ROS in our hydrogels treated HUVECs. As shown in Fig. 1D and 1E, we observed no elevation of ROS generation in H4, H8 and H16, as compared to control.
4.3 Heparin releasing chitosan-based hydrogels significantly increased the expression of VEGFA and VEGFR
We observed a high expression of VEGFA and VEGFR in HUVECs, treated with heparin loaded hydrogels (H4, H8 & H16), as compared to control. The expression was maximum at day 1, as compared to day 3 and day 7. Hydrogels H8, showed highest effect on both VEGFA and VEGFR, while a gradual decrease was observed in cells treated with H4 and H16. In case of VEGFR, H16 exhibitted low expression at both day 3 and day 7. VEGFA also revealed low expression at day 7 when treated with H16 (Fig. 2A and 2B).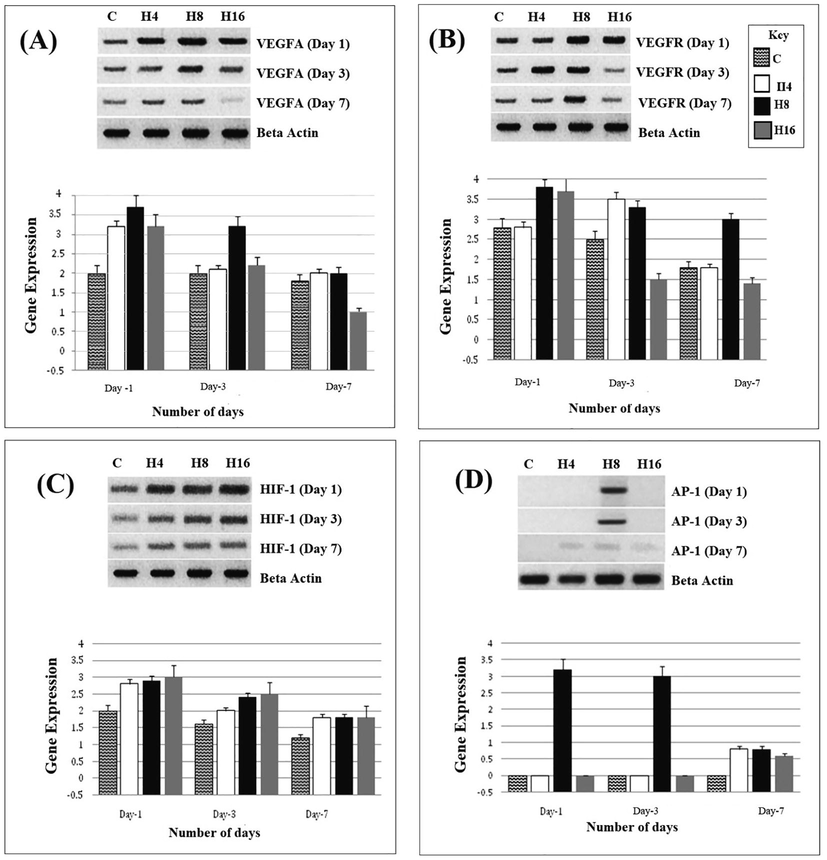
Shows the expression of VEGFA (2A), VEGFR (2B), HIF-1(2C) and AP-1(2D) in H4, H8 and H16 treated HUVECs, at day 1, day 3 and day 7.
In our previous, report, the maxiumu angiogenesis was seen in hydrogels H8, which has more heaprin as compared to H4 and H16. The fact that VEGFA and VEGFR showed higher expression in H8, rather than H4 and H16, attributes to the presence of heparin. Heparin appears to be the key component regulating VEGFA and VEGFR which inturn stimulate the angiogenesis. At day 1, the expressions of these genes are maximaly high, and that again confirms the role of heparin, which has maxim release during the first 24 h. At day 3, and day 7, the expression is comparatively low, which demonstrates less release during the following days. Heparin acts as a scaffold which conveniently delivers the growth factor to its receptors and protects it from degradation. Heparin released from hydrogel helps in mimicking extracellular matrix, where VEGFA-heparin complex binds with VEGFR and initiates the signaling cascades for angiogenesis.
4.4 Up-regulation of HIF-1 in HUVECs, treated with heparin loaded hydrogels
Hypoxia Inducible Factors (HIFs) has two isoforms HIF-1α and HIF-2α. HIF-1α or HIF-1 is mainly responsible for inducing angiogenesis by upregulating the VEGFA in response to hypoxia. Our results revealed up-regulation of HIF-1 gene. At day 1, there was maximum expression while at day 3, it showed reduced expression, which was further reduced at day 7. At each time period (day 1, 3 and 7), H4, H8 and H16 exhibited same pattern of expression. There was slight difference in the expression of control, which probably depicts error in loading during electrophoresis (Fig. 2C).
In hypoxic conditions oxygen dependent proteolysis of HIF-1alpha is avoided as hydroxylation is inhibited and HIF-1 dependent VEGFA transcription is up regulated which enhances the angiogenesis. High expression of HIF-1 in our study demonstrates that H8, which contain more heparin, positively mediates the expression of HIF-1 which in turn regulates a high expression of VEGFA and VEGFR.
4.5 AP-1 expresses in HUVECs, treated with H8
AP-1, a transcriptional factor which plays an important role in cell proliferation, differentiation and regulating gene expression of VEGF in response to various cytokines. In our results AP-1 exhibited interesting results. Only H8 induced expression in HUVECs which shows the role of heparin in mediating AP-1. It has already been reported that heparin activates the transcription of various genes such as HIF-1, VEGFA and GLUT-1. H8 contains more heparin, and that is why a higher expression of AP-1 is seen as compared to H4 and H8. Interestingly, AP-1 was expressed only at day 1 and day 3, with no expression at day 7, which reconfirms the notion that heparin has minimum release at day 7 (Fig. 2D).
4.6 Hydrogels H8 induce TNF-α and IL-1 expressions
TNF-α is a cytokine (a cell signaling protein), involved in inflammatory responses. It is a potent activator of angiogenesis and activates the angiogenic growth factors which regulate angiogenesis. It has been observed that TNF-α interacts with VEGF-A, FGF, and IL-1 to induce angiogenesis in endothelial cells.
We investigated the role of cytokines TNF-α and IL-1 in the heparin loaded hydrogel-induced angiogenesis, and both are apparently regulated by the heparin presence (Fig. 3A and Fig. 3B). Heparin-induced up-regulation of these two cytokines is mediated by MAPKs and NF- κB which is inturn mediated through VEGF-A and VEGFR signaling. Pro-angiogenic potentials of these hydrogels could also be attributed to the fact that TNF-α regulates the expression of several integrins, and a higher expression of integrins could lead to improved adhesion and increased angiogenesis. Inflammation caused by cytokines such as TNF-alpha and IL-1 also leads to leukocyte penetration at the site of injury, which results stimulation of proliferation, migration and angiogenesis in endothelial cells.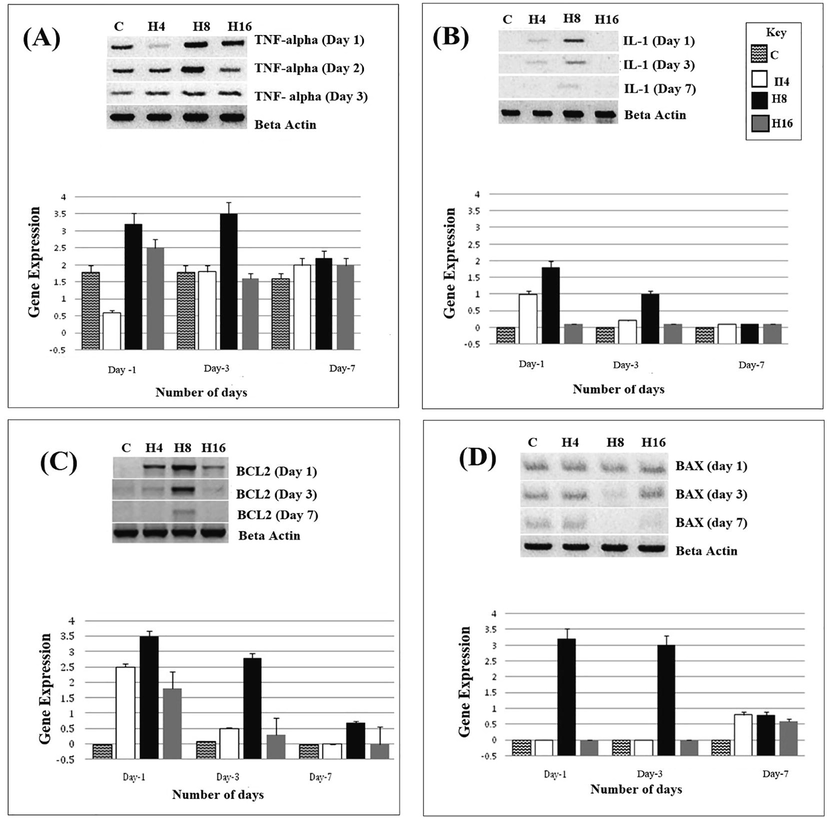
Shows the expression of TNF-alpha (3A), IL-1 (3B), BCL-2 (3C) and BAX (3D) in H4, H8 and H16 treated HUVECs, at day 1, day 3 and day 7.
4.7 H8 hydrogels mediate BCL2 expression with no effect on BAX gene
BCL-2 is a regulatory protein considered as an oncogene which regulates cell death (apoptosis) either by inducing or inhibiting apoptosis, proliferation and angiogenesis. Surprisingly, we observed no expression of BCL2 in the control, with a slight expression in H16. Maximum expression was observed in H8, followed by H4. Day 1 continued to affect the expression of BCL2, which again confirms our claim of high heparin release in first 24 h. In case of H16 hydrogel, BCL2 was initially expressed at day 1, its expression diminished at day 3 and day 7 (Fig. 2C).
BAX is another apoptosis regulator protein which is known as BCL2 like protein 4 and belongs to the BCL2 gene family. Control showed a moderate expression of BAX at day 1, day 3 and day 7. H4, H8 and H16 hydrogels also revealed considerable expression at day 1 which was decreased in case of H16 at day 3. At day 7, only H8 was able to induce expression. Overall, we observed a low expression of BAX in HUVECs (Fig. 2D).
5 Conclusion
We conclude that heparin loaded hydrogels are potent regulators of angiogenesis. Angiogenesis is apparently regulated by VEGFA, VEGFR, HIF-1, AP-1, involving various other downstream regulating genes and molecular pathways. H8 carries more heparin, and that is the only component that evidently mediates angiogenesis. Heparin release at day 1 is maximum, and that is why our results are significantly tending to affect gene regulations at day 1. This study reveals some of the important molecular mechanisms involved in the heparin/chitosan-induced angiogenesis.
Acknowledgment
The authors extend their appreciation to HEC NRPU 7780 and Project number RG-1441-356 at King Saud University for funding this study, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Molecular identification of Campanulotes bidentatus (Phthiraptera, Philopteridae) infecting the domestic pigeon Columba livia from Saudi Arabia. Saudi J. Biol. Sci. 2021
- [CrossRef] [Google Scholar]
- Arif, M., Atta, S., Bashir, M.A., Khan, M.I., Hussain, A., Shahjahan, M., et al., 2021. The impact of Fosetyl-Aluminium application timing on Karnal bunt suppression and economic returns of bread wheat (Triticum aestivum L.). PLoS ONE 16(1): e0244931. 10.1371/journal. pone.0244931
- Baffert, F., Le, T., Sennino, B., Thurston, G., Kuo, C. J., Hu-Lowe, D., McDonald, D.M., 2006. Cellular changes in normal blood capillaries undergoing regression after
- Angiogenesis, vasculogenesis, and induction of healing in chronic wounds. Vascular and Endovascular Surgery. 2005;39(4):293-306.
- [Google Scholar]
- Investigating the effect of VEGF glycosylation on glycosaminoglycan binding and protein unfolding. Biochem. Biophys. Res. Commun.. 2006;340(3):836-839.
- [Google Scholar]
- Angiogenesis and chronic inflammation: cause or consequence? Angiogenesis. 2007;10(3):149-166.
- [Google Scholar]
- Differential distribution and modulation of expression of alpha 1/beta 1 integrin on human endothelial cells. J. Cell Biol.. 1991;114(4):855-863.
- [Google Scholar]
- VEGF165 promotes survival of leukemic cells by Hsp90-mediated induction of Bcl-2 expression and apoptosis inhibition. Blood. 2002;99(7):2532-2540.
- [Google Scholar]
- Simple surface coating of electrospun poly-L-lactic acid scaffolds to induce angiogenesis. J. Biomater. Appl.. 2015;30(1):50-60.
- [Google Scholar]
- Harmey J.H., ed. VEGF and Cancer. Springer Science & Business Media; 2004.
- AP-1 subunits: quarrel and harmony among siblings. J. Cell Sci.. 2004;117(25):5965-5973.
- [Google Scholar]
- Expression of Bax and Bcl-2 in tumour cells and blood vessels of breast cancer and their association with angiogenesis and hormonal receptors. Asian Pac. J. Cancer Prev.. 2012;13(8):3857-3862.
- [Google Scholar]
- Long-term delivery enhances in vivo osteogenic efficacy of bone morphogenetic protein-2 compared to short-term delivery. Biochem. Biophys. Res. Commun.. 2008;369(2):774-780.
- [Google Scholar]
- The chemopreventive effect of tanacetum polycephalum against LA7-induced breast cancer in rats and the apoptotic effect of a cytotoxic sesquiterpene lactone in MCF7 cells: a bioassay-guided approach. Cell Physiol Biochem.. 2015;36(3):988-1003. Epub 2015 Jun 15
- [CrossRef] [Google Scholar]
- Karimian, H., Fadaeinasab, M., Zorofchian Moghadamtousi, S., Hajrezaei, M., Razavi, M., Safi, S.Z., Ameen Abdulla, M., Mohd Ali, H., Ibrahim Noordin, M., Chemopreventive Activity of Ferulago angulate against Breast Tumor in Rats and the Apoptotic Effect of Polycerasoidin in MCF7 Cells: A Bioassay-Guided Approach. PLoS One. 2015 May 21;10(5):e0127434. 10.1371/journal.pone.0127434. eCollection 2015.
- Cooperative effect of TNFalpha, bFGF, and VEGF on the formation of tubular structures of human microvascular endothelial cells in a fibrin matrix. Role of urokinase activity. J. Cell Biol.. 1996;132(6):1177-1188.
- [Google Scholar]
- Preparation of water-soluble chitosan/heparin complex and its application as wound healing accelerator. Biomaterials. 2003;24(9):1595-1601.
- [Google Scholar]
- A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv.. 2018;8(14):7533-7549.
- [Google Scholar]
- Interleukin-1 receptor type 2 acts with c-Fos to enhance the expression of interleukin-6 and vascular endothelial growth factor A in colon cancer cells and induce angiogenesis. J. Biol. Chem.. 2015;290(36):22212-22224.
- [Google Scholar]
- Prevalence rate and molecular characteristics of Oestrus ovis L. (Diptera, Oestridae) in sheep and goats from Riyadh, Saudi Arabia. Animals. 2021;11:689.
- [CrossRef] [Google Scholar]
- A physical map of the region encompassing the human interleukin-1 alpha, interleukin-1 beta, and interleukin-1 receptor antagonist genes. Genomics. 1994;19(2):382-384.
- [CrossRef] [Google Scholar]
- Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am. J. Pathol.. 1998;152(6):1445.
- [Google Scholar]
- Up-Regulation of Bcl-2 in microvascular endothelial cells enhances intratumoral angiogenesis and accelerates tumor growth. Cancer Res.. 2001;61(5):2183-2188.
- [Google Scholar]
- Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev.. 2002;16(20):2684-2698.
- [Google Scholar]
- Induction of vascular endothelial growth factor by tumor necrosis factor α in human glioma cells possible roles of SP-1. J. Biol. Chem.. 1996;271(45):28220-28228.
- [Google Scholar]
- Beta adrenergic receptors stimulation attenuates phosphorylation of NF-κB and IκBα in hyperglycemic endothelial cells. Cell Physiol. Biochem.. 2018;51(3):1429-1436.
- [Google Scholar]
- Glutamine treatment attenuates hyperglycemia-induced mitochondrial stress and apoptosis in umbilical vein endothelial cells. Clinics (Sao Paulo). 2015;70(8):569-576.
- [CrossRef] [Google Scholar]
- Molecular mechanisms of diabetic retinopathy, general preventive strategies, and novel therapeutic targets. Biomed Res Int.. 2014;2014:1-18.
- [CrossRef] [Google Scholar]
- Stimulation of β-adrenergic receptors plays a protective role via increased expression of RAF-1 and PDX-1 in hyperglycemic rat pancreatic islet (RIN-m5F) cells. Arch. Med. Sci.. 2017;13(2):470-480.
- [Google Scholar]
- Differential expression and role of hyperglycemia induced oxidative stress in epigenetic regulation of β1, β2 and β3-adrenergic receptors in retinal endothelial cells. BMC Med Genomics.. 2014;30(7):29.
- [CrossRef] [Google Scholar]
- Int J Diabetes Dev Ctries. 2016;36:407.
- [CrossRef]
- Sher Zaman Safi, Kalaivani Batumalaie, Rajes Qvist, Kamaruddin Mohd Yusof, and Ikram Shah Ismail, “Gelam Honey Attenuates the Oxidative Stress-Induced Inflammatory Pathways in Pancreatic Hamster Cells,” Evidence-Based Complementary and Alternative Medicine, vol. 2016, Article ID 5843615, 13 pages, 2016. 10.1155/2016/5843615.
- Mechanisms and regulation of endothelial VEGF receptor signalling. Nat. Rev. Mol. Cell Biol.. 2016;17(10):611-625.
- [Google Scholar]
- Stabilization of vascular endothelial growth factor mRNA by hypoxia and hypoglycemia and coregulation with other ischemia-induced genes. Mol. Cell. Biol.. 1995;15(10):5363-5368.
- [Google Scholar]
- Molecular dynamics-based model of VEGF-A and its heparin interactions. J. Mol. Graph. Model.. 2018;82:157-166.
- [Google Scholar]
- Yar, M., Gigliobianco, G., Shahzadi, L., Dew, L., Siddiqi, S. A., Khan, A. F., Chaudhry, A. A., Rehman, I. u., MacNeil, S., 2016. Production of chitosan PVA PCL hydrogels to bind heparin and induce angiogenesis. Int. J. Polymeric Mater. Polymeric Biomater., 65(9), 466-476.
- Involvement of interleukin-8, vascular endothelial growth factor, and basic fibroblast growth factor in tumor necrosis factor alpha-dependent angiogenesis. Mol. Cell. Biol.. 1997;17(7):4015-4023.
- [Google Scholar]
- Signaling transduction mechanisms mediating biological actions of the vascular endothelial growth factor family. Cardiovasc. Res.. 2001;49(3):568-581.
- [Google Scholar]
- Binding affinities of vascular endothelial growth factor (VEGF) for heparin-derived oligosaccharides. Biosci. Rep.. 2012;32(1):71-81.
- [Google Scholar]







