Translate this page into:
Evaluation of in vivo sub-chronic and heavy metal toxicity of under-exploited seaweeds for food application
⁎Corresponding authors. Abirami.ganesan@fnu.ac.fj (Abirami R. Ganesan), bala.m.k@sejong.ac.kr (Balamuralikrishnan Balasubramanian)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Seaweeds are known to be rich source of micronutrients and bioactive compounds. The main objective of this study was to find the toxicological evaluation and heavy metal accumulation of five under-exploited edible seaweeds in animal model followed by dosage determination for regular consumption as a food by humans and food application. Some under-exploited seaweeds like Acanthophora spicifera, Gracilaria edulis, Padina gymnospora, Ulva fasciata and Enteromorphoa flexuosa were selected forthis study. The ED50 study was conducted in Wistar strain rats for 90 days with single dose administration of seaweed extract of 2000 mg/kg/BW. At the end of 90th day rats were euthanized, serum of the rats examined for biochemical, haematological, liver enzymes, and vital organs were dissected out for heavy metal analysis and urine samples collected intermediary to analyse electrolyte minerals. Result showed that no-observed adverse effect level (NOEL) on five seaweeds, did not cause any death and no significant variation in biochemical and haematological parameters, the values were found within standard values. Locomotor activity suggests normal action, organ necropsy showed no histopathology lesions, regular cell alignment in the tissue cross section. Heavy metals like arsenic, lead were found in trace amount and no mercury accumulation found in kidney, liver and brain of rats. Therefore, these five seaweeds were safe for human consumption and also for food product developement.
Keywords
Seaweed
Sub-chronic toxicity
Acanthophora spicifera
Gracilaria edulis
Padina gymnospora
Ulva fasciata
Enteromorphoa flexuosa
1 Introduction
Toxicology is the study of finding adverse effect of chemicals on living organisms (Neuwinger, 1996). This determines detailed description of symptoms, bio-mechanisms, treatment and detection of poison; especially the poisoning effects during consumption (Ahmed, 1991). It is also essential to know the concentration of dosage of any plant fresh/by-products for humans through animal models. For instance, human clinical trial using 3 g of Undaria seaweed extract with 75% fucoidan daily does not show any toxicity (Irhimeh et al., 2007). Another research on Ulva lactuca (Delgado-Roche et al., 2019), Kappaphycus based carrageenan softgel (Sutrisni et al., 2019) in rat model shows non-toxic effect even indose-dependent manner. Some researchers examined the effect of seaweed bioactive compounds such as fucoidan, carrageenan, and ulvan polysaccharide did not show any toxicity at various concentrations (600 mg/kg b.w 1200 mg/kg b.w). Additionally, seaweeds tend to accumulate heavy metal since marine ecosystem consists of varied level of mineral concentration and also heavy metals could accumulate in organs in long term consumption (Abirami and Kowsalya, 2012a, 2012c). Marine algal toxins are responsible for illness in human which associates with other seafood consumption. Approximately 20% of all foodborne disease outbreaks in US results in consumption of seafood. Furthermore, long term studies would prove the actual figure of bioactive/chemical interaction in body and body metabolites; thus, subchronic toxicity for 90 days would prove toxicity level and intensity of accumulation in the body of animal model (Li et al., 2005). Due to increasing industrial activities benthic algae was accumulated with trace mineral such as Cd, Pb, Cr and Cu (Almela et al., 2006; Rose et al., 2007).
Many researchers proved seaweeds nutrient composition and bioactive compounds (Abirami and Kowsalya, 2012b; Bhuvaneswari et al., 2013; Seedevi et al., 2015; Abirami and Kowsalya, 2017) but scarce of data available in toxicological evaluation of seaweed from Indian coast and their heavy metal accumulation in body metabolites. In our earlier study, the safety evaluation and consumption pattern of K. alvarezii and U. lactuca (Kowsalya and Abirami, 2011) were reported and some underexploited edible seaweed existing in the coastal region were identified through survey questionnaire. From this data, someseaweed found to be edible in nature and not utilized for food application, thus it is necessary to evaluate the toxicity of wholesome seaweed rather than its bioactive compounds to develop futuristic food products from whole seaweed.
The coastal region of Tamilnadu, South India covering the Gulf of Mannar and Palk Bay coast supports an ironic flora of marine algae ecosystem. Amongst red algae Acanthophora spicifera, and Gracilaria edulis, brown algae Padina gymnospora, green algae namely Ulva fasciata and Enteromorpha flexuosa are grown excessively and not utilised as wholesome food. Therefore, these seaweeds need to examined for toxicological evaluation for regular consumption in human diet. Thus, the primary objective of this study was to evaluate toxicity evaluation in animal models, followed by heavy metal accumulation in vital organs. Then, the dosage determination of seaweed for human consumption on regular basis was evaluated. These seaweeds are rich in essential amino acids and essential fatty acids that could aid to eradicate nutritional deficiency, food security challenges and nutraceutical products to meet future food industrial needs.
2 Materials and methods
2.1 Seaweed extracts preparation
Fresh seaweeds were washed thoroughly in distilled water and shade dried. Then, they were coarsely powdered for further extraction. Around 250 g of five seaweeds were powdered separately and packed in a soxhlet extractor. 20 vol of distilled water was added into the flask and heated at 60 °C temperature (Abirami and Kowsalya, 2012b). The soluble active ingredients of extract remained in the flask and repeated extraction process was carried out to elute all bioactive compounds. Consequently, the mixture was concentrated by rotary evaporator to obtain maximum effectiveness. The final mixture was lyophilized and stored at 4 °C in a refrigerator to carry out the toxicological study. Furthermore, freshly prepared water extract of seaweeds was used in this study to avoid any fungal contamination for 90 days of toxicity evaluation.
2.2 Subchronic toxicity of underexploited seaweeds in albino rats
2.2.1 Dosage determination
LD50 is the dose that is lethal to 50 per cent of population. LD50 of the extract was determined as per OECD guidelines. Young healthy female Wistar rats were used and test item was administered after 18-hour starvation. Test item was given orally to one rat at a dose of 5000 mg/kg/BW. Since the animal died within an hour after dosing, a lower dose of 2000 mg/kg was tested in another group of three rats to find ED50. Since no mortality was observed in the animals treated at a dose of 2000 mg/kg/BW (Abirami and Kowsalya, 2012a), results were confirmed in another group of three animals. Clinical signs of toxicity and mortality were observed up to 6 h after dosing and then noted daily up to 90 days on which animals were euthanized. This protocol followed as per Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) and approved by animal ethical committee (A47/05/2010/PhD) of K.M. Pharmacy College, Madurai, Tamilnadu, India.
2.2.2 Preparation of dosage
The crude extract was then prepared into a suspension form and used as drug, extract I was prepared from Acanthophora spicifera given to group I rats, extract II prepared from Gracilaria edulis given to group II rats, extract III prepared from Padina gymnospora given to group III rats, extract IV- Ulva fasciata given to group IV rats and extract V- Enteromorphoa flexuosa given to group V rats. 40 mg of aqueous extract of seaweed was dissolved in 2 ml of distilled water and used as a dosing regimen as described in our earlier study (Abirami and Kowsalya, 2012a).
2.2.3 Grouping and housing of experimental animals
Young, healthy Wistar strain albino rats of six months old, weighing 200–250 g and eighty young healthy male and female rats were selected. The rats were not used previously for any other experiments, and healthy individual rats were subjected to the research. Furthermore, they were acclimatized in laboratory conditions for a week before start of experiment. After acclimatization, rats were grouped into six groups namely control, group I, II, III, IV and V. Six rats in each group were housed separately in stainless steel cages with 28 ± 2 °C and following ethical guidelines 12 h light and dark cycle was maintained. Regular rat pellet supplied by Pranav Auro Industries Ltd, Sangli, India was used as standard diet for all rats. The food consumption of albino rats was recorded everyday by weighing the leftover food. Food and water given ad libitum.
2.2.4 Assessment of body weight of experimental animals
Individual bodyweight was evaluated by using a digital weighing balance before and after seaweed extracts administration (Abirami and Kowsalya, 2013). Bodyweight was recorded weekly for interpretation with food consumption during the period of dosing and on the day of sacrifice.
2.2.5 Observation of clinical signs and collection of blood samples
Clinical signs and symptoms such as mortality pattern, abnormalities or distress were monitored once before dosing, immediately after dosing and up to four hours of after dosing. Blood samples were collected by retro-orbital puncture technique to all the rats in control and experimental groups just before sacrificing. Biochemical parameters were evaluated in serum samples which were collected in plain tubes without any anticoagulant, whereas blood samples for haematological examination were collected in heparinized tubes. Analytical methods carried out in blood and urine have been shown in Table 1.
Parameters
Method
Reference
Hematological
Haemoglobin
RBC count
WBC count
Packed Cell Volume (PCV)
Mean Corpuscular Volume (MCV)
ABX pentra-120 Automated Haematology Analyzer
Raghuramulu et al., 2003
Mean Corpuscular Hemoglobin (MCH)
Mean Corpuscular
Hemoglobin Concentration (MCHC)
Biochemical
Glucose
Hexokinase method
Shaw and Wolfe, 1989
Total protein
Biuret method
Shaw and Wolfe, 1989
Albumin
BCG Dye binding method
Bush and Reed, 1987
Calcium
Arenazo III method
Weissmann et al., 1980
Inorganic phosphorus
Phosphomolydate method
Daly and Ertingshausen, 1972
Electrolytes (Sodium, Potassium, Chloride)
ISE method
Tietz, 1987
Serum cholesterol
CHOD PAP method
Allain et al., 1974
ALT (SGOT)
colorimetric method
Reitman and Frankel, 1957
AST (SGPT)
modified IFCC/UV kinetic method
Reitman and Frankel, 1957
Alkaline phosphatase
kinetic method
Reitman and Frankel, 1957
Blood urea nitrogen
Urease GLDH/UV kinetic method
Tietz, 1987
Creatinine
Jaffe/kinetic method
Raghuramulu et al., 2003
Urine analysis (urine volume, pH and sodium, potassium, chloride)
Flame photometer
Schachter et al., 1980
Histopathology (liver and kidney)
conventional method- Microscope
Raghuramulu et al., 2003
2.2.6 Assessment of heavy metal ions in organ samples
At the end of the research, vital organs such as liver, kidney and brain samples from each albino rat were taken, rinsed in saline and weighed individually. To prevent contamination samples were kept in dry ice and stored until samples were analyzed for heavy metals by Atomic Absorption Spectrophotometer (AAS). Digestion of sample was carried out using 2 ml of an acid mixture containing concentrated nitric,sulfuric, and perchloric acids in 5:1:1 respectively. Triple acid digested tissues were further heated at 60 °C in a water bath for overnight. Finally, the tissue sample was allowed to cool and further dilution was done with 10 ml of distilled water (Kenawy et al., 2000).
2.2.7 Histopathological examination
At the end of dosage period, all the animals were euthanized and sacrificed, three animals organ samples were assessed for pathological changes and lesions as per our previous method (Raghuramulu et al., 2003). Vital organs like liver and kidney weighed immediately without drying and then 10% formalin added. Full histopathology changes were examined under a microscope in comparison with control rats without administration of seaweed water extract.
2.2.8 Statistical analysis
The data obtained in the biochemical and haematological parameters were analysed using ANOVA Dunnett’s Multiple Comparison Test, and the values were expressed in mean and standard deviation. The control and experimental group were values were compared and significant difference between the groups was accepted at p < 0.05, p < 0.01. All the analysis were performed in GraphPad Prism software.
3 Results and discussion
3.1 Effect of seaweed extracts on the bodyweight of rats
Initial body weight of albino rats ranges between 235.32 g and 240.47 g and its mean weight was similar among all groups which in Fig. 1. The final weight of rats was high in all groups with a mean weight gain of 54% in group IV supplemented with Ulva fasciata when compared to control group. Besides, groups Ito V were also increased in body weight by 39%, 40%, 42% and 46.09% respectively. Summary of weight gain of seaweed administrated rats and control group was 348.12 g (43%), whereas group I was 328.89 g, group II was 331 g, group III was 342.01 g, group IV was 394.27 g and group V was 356.32 g. Furthermore, Ulva fasciata supplemented rats showed weight more and this may be due to high protein content (22.7%) as reported in our earlier study (Abirami and Kowsalya, 2012c), or enhanced protein digestibility and high biological value. It was interesting to note that increase in weight gain of seaweed extract-treated group also coincides with increased food intake.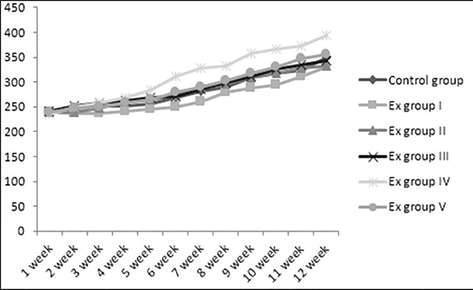
Body weight (g) of experimental rats in weekly basis.
Hence, regular consumption of unprocessed/ dried seaweeds may contain health benefits; moreover, some physical sicknesses can also be treated with seaweed on a daily basis deprived of irrespective diets. Many research studies available on the nutritional aspect of freshwater algae like spirulina ((Salazar et al., 1998)), chlorella vulgaris, Dunella (Rangsayatorn et al., 2002; Kottuparambil et al., 2019) but edible seaweeds are yet to be propagated and promoted in many developing nations. Furthermore, even small amount of seaweeds added in the regular diet can act as a powerhouse of nutrition.
3.2 Effect of seaweed extract on haematological parameters
Haematological parameters measured such as haemoglobin (Hb), red blood cells (RBC), white blood cells (WBC), platelet, packed cell volume (PCV), mean corpuscular volume (MCV) and mean corpuscular haemoglobin concentration (MCHC) persisted within reference value which is presented in Table 2. However, significant difference was observed between PCV and MCV values of experimental group when compared to control group; although these differences were found to be within the normal range. Moreover, no significant difference was observed between experimental groups treated with aqueous extract of Acanthophora spicifera, Gracilaria edulis, Padina gymnospora, Ulva fasciata and Enteromorpha flexuosa and control group. NS-Not significant, *p < 0.05, Dunnett’s Multiple Comparison Test, all values are expressed as mean ± SD for 6 albino rats in each group.
Parameters
Reference value
Control group
Group I
Group II
Group III
Group IV
Group V
f value
Haemoglobin (g/dl)
11–19
16.34 ± 3.42
16.10 ± 1.65
15.80 ± 1.41
15.40 ± 1.69
15.23 ± 1.89
15.2 ± 2.76
0.27NS
RBC (×106 cells/mm3)
5.4–8.5
8.76 ± 0.32
8.90 ± 0.85
7.59 ± 1.22
8.70 ± 1.37
7.88 ± 1.21
8.60 ± 0.88
1. 6NS
WBC (×103 cells/mm3)
3.7–4.9
7.32 ± 0.34
6.90 ± 1.16
6.12 ± 3.12
6.10 ± 1.17
7.12 ± 1.76
7.30 ± 2.31
0.54NS
PCV(%)
37.6–50.6
45.89 ± 0.12
45.43 ± 3.09
48.64 ± 2.05
45.81 ± 2.87
49.20 ± 2.99
43.20 ± 3.35
4.2*
(p < 0.05)
MCV (cumm)
57.3–66
62.70 ± 0.08
65.20 ± 0.76
62.00 ± 0.62
61.08 ± 4.32
60.14 ± 0.07
65.80 ± 0.76
9.1*
(p < 0.05)
MCH (pg)
11–15
17.54 ± 0.99
18.20 ± 0.73
17.82 ± 0.39
17.60 ± 1.23
17.98 ± 1.33
17.50 ± 0.98
0.47NS
MCHC (%)
30–35
32.90 ± 5.71
33.70 ± 3.98
33.32 ± 4.31
32.50 ± 2.76
33.65 ± 5.74
31.20 ± 2.76
0.28NS
In general, all these values are indicating changes in the metabolism of rats soon after new compound administration as a part ofthe daily diet; thus, all five-seaweed showed non-toxic effect on chronic treatment. Examination of serum and plasma parameters were correlated with risk evaluation; hematological system has a higher indication of toxicity in humans (91%) in early-stage when assays involve rodents than non-rodents (Féres et al., 2006).
3.3 Effect of seaweed extract on biochemical parameters
The biochemical parameters were assessed against the standard reference values. All experimental group rats showed a significant difference in cholesterol level, but these values seem to be reasonableare on par with standard value (refer Table 3). There was a significant increase in potassium level of group III rats supplemented by Padina gymnospora and decreased level in group V when compared to the control group rats. In general, macro-algae are known to be high in calcium, magnesium, phosphorus, potassium, sodium and iron compared to higher plants. As inferring to this statement, elevation of potassium is correlated in the present study. Similarly, high potassium concentration found in red seaweed like Kappaphycus alvarezii (Ganesan et al., 2019), Acanthophora species (Ganesan et al., 2018) and other marine-derived polysaccharide like Ulvan and carrageenan (Mohan et al., 2018). Furthermore, appreciable intake of calcium, potassium and sodium are always associated with lower systolic pressure and lower risk for hypertension (Kumar et al., 2008). Values are significantly different from the control group at *p < 0.05 Dunnett’s Multiple Comparison Test, all values are expressed as mean ± SD for 6 albino rats in each group.
Parameters
Standard Value
Control group
Group I
Group II
Group III
Group IV
Group V
f value
Glucose (mg/dl)
85–132
89.32 ± 5.08
83.51 ± 9.08
80.28 ± 13.69
83.43 ± 9.18
88 ± 12.39
90.15 ± 3.47
1.0NS
Cholesterol (mg/dl)
46–92
51.60 ± 1.80
74.01 ± 3.77
62.00 ± 0.89
70.00 ± 4.33
63.00 ± 15.14
67.00 ± 2.10
8.0*
(P < 0.05)
Blood urea nitrogen (mg/dl)
15–21
15.86 ± 1.56
16.10 ± 0.90
18.70 ± 2.63
19.25 ± 3.39
17.80 ± 2.76
16.60 ± 7.26
0.88NS
Total protein (g/dl)
6.3–8.7
7.20 ± 1.56
6.60 ± 0.93
6.90 ± 0.63
6.60 ± 1.54
6.50 ± 0.91
6.50 ± 1.01
0.35NS
Albumin (g/dl)
3.3–4.9
4.38 ± 0.36
4.70 ± 1.0
4.38 ± 0.63
4.70 ± 0.52
4.30 ± 0.96
4.70 ± 1.19
0.31 NS
SGOT (AST) (U/L)
37–92
79.89 ± 0.26
90.12 ± 0.72
84 ± 2.34
89.78 ± 3.26
89.56 ± 0.76
90.12 ± 0.36
38*
(p < 0.05)
SGPT (ALT) (U/L)
17–50
48.03 ± 5.60
51.75 ± 13.31
47.66 ± 11.07
48.36 ± 6.27
42.00 ± 7.84
47.00 ± 9.03
0.70NS
Sodium (mEq/L)
140–150
143 ± 2.47
138.0 ± 8.13
139.0 ± 7.70
144.0 ± 13.44
138.0 ± 6.15
141.0 ± 10.25
0.53NS
Potassium (mEq/L)
5.2–7.8
6.39 ± 0.43
6.9 ± 0.75
5.6 ± 0.62
7.4 ± 1.19
6.7 ± 1.22
5.4 ± 0.91
4.41*
(p < 0.05)
Calcium (mg/dl)
10.7–13.7
10.92 ± 0.64
11.80 ± 2.18
11.9 ± 3.45
12.6 ± 4.25
11.8 ± 1.88
11.9 ± 1.17
0.25 NS
Creatinine (mg/dl)
0.9–1.5
1.57 ± 0.146
1.49 ± 0.103
1.50 ± 0.10
1.37 ± 0.05
1.45 ± 0.23
1.78 ± 0.12
0.13 NS
Alkaline Phosphatase (U/L)
39–216
120.72 ± 2.98
132.06 ± 6.71
131.00 ± 6.00
116.16 ± 5.30
126.36 ± 22.65
115.95 ± 10.58
2.48 NS
However, no major impact on usual markers of liver toxicity (plasma levels of liver enzymes- SGOT, SGPT and ALP) were seen but then the significant difference in SGOT level of group I when compared to control group rats nevertheless these levels were found to be in normal range. Hence, all these five seaweeds did not induce any significant damage to organs, as compared to control group rats. In an additional note, an increase in transaminases (SGOT, SGPT) was one of the indicators of liver damage (Kumar et al., 2019). Additionally, AST is released in many metabolisms such as heart, lung, skeletal muscle and kidney; whereas ALT is only derived from liver and considered highly sensitive indicator for hepato-toxicity (Wali et al., 2019). Meanwhile, no variation of SGOT and SGPT was found showing non-hepatic-toxic nature of seaweeds.
3.4 Clinical signs and mortality pattern of rats
No mortality was observed among the rats. Initially, some rats showed hyperactivity and sneezing, but no adversative clinical expressions such as diarrhoea, haematuria and diurea were observed in all groups of treated rats during the experimental period. Locomotor activity such as distance travelled, sinuosity and movement also noticed which were normal and found similar action to control group rats fed with standard diet; similar results also found in other studies on locomotor activity of natural drugs (Liaquat et al., 2019). Other biological substance like stool, urine has also shown no changes throughout dosage. Eyeball colour of all albino rats looked similar all through the study. Therefore, all rats fed with seaweed extract indicated well-tolerated dosage level.
3.5 Effect of seaweed extract on organ weight of rats
The mean value of vital organs weight of treated groups and control group, as expressed in grams are given in Table 4. It was evident that no significant changes found in the weight of internal vital organs of experimental groups as compared to the control group. Furthermore, all vital organs like brain, kidney, liver, spleen, heart were similar among all experimental groups fed by five seaweed extract and this proves that weight gain achieved by rats were depended on muscular mass and not because of organ weight gain. NS-not significant, Dunnett’s multiple comparison test, all values are expressed as mean ± SD for 6 albino rats in each group.
Organs
Control group
Group I
Group II
Group III
Group IV
Group V
f value
Liver
3.21 ± 0.07
3.18 ± 1.07
3.34 ± 3.03
3.48 ± 2.04
3.21 ± 1.89
3.27 ± 2.23
0.019NS
Spleen
0.34 ± 2.83
0.28 ± 1.24
0.37 ± 8.07
0.34 ± 3.24
0.28 ± 4.13
0.26 ± 1.25
0.067NS
Heart
0.4 ± 3.16
0.34 ± 1.18
0.37 ± 2.12
0.42 ± 1.06
0.44 ± 3.04
0.41 ± 4.06
0.011NS
Kidney
1.46 ± 2.14
1.29 ± 1.03
1.24 ± 3.12
1.98 ± 1.26
1.92 ± 2.31
1.31 ± 1.98
0.15NS
Brain
0.48 ± 1.91
0.63 ± 2.01
0.59 ± 3.03
0.32 ± 2.26
0.49 ± 4.42
0.45 ± 1.76
0.097NS
3.6 Effect of seaweed extracts on histopathology of vital organs
At the end of study histopathological examination was done in organs such as liver and kidney, given in Figs. 1 and 2. None of the experimental group rats showed abnormalities and was similar to the control group. While correlating the results of biochemical parameters with pathology it was clearly evident that no toxicity sedimentation was found in organs after long term administration of seaweed. Organic toxicity or metal toxicity firstly affects vital organs especially liver but none of the organs was affected in our research, this result is on par with other seaweed like Sargassum liebmannii safety evaluation (Tapia-Martinez et al., 2019). Furthermore, no toxicological lesions proved to be non-toxic when compared to the observations in control group. Consequently, thisregular histological section of liver shows well-arranged cells with bright central vein (Lee et al., 2019). Moreover, kidney section showed well-arranged cells with proper glomerular basement membrane, looked compact which was correlated with our previous research on Kappaphycus alvarezii (Abirami and Kowsalya, 2012a) (see Fig. 3).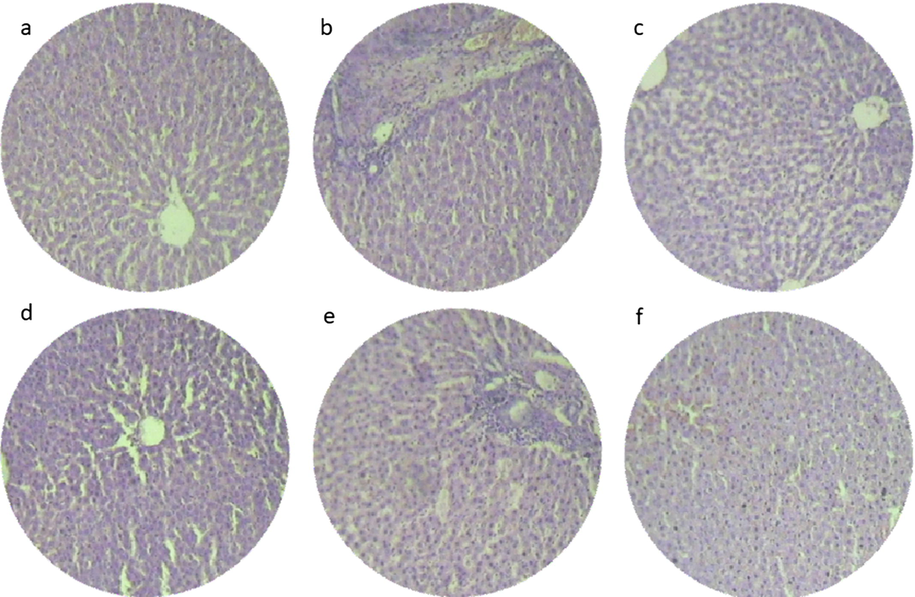
Histopathology section on liver region of experimental rats (a) Control group- standard diet, (b) group I- Acanthophora spicifera administrated rats, (c) group II- Gracilaria edulis administrated rats, (d) group III- Padina gymnospora administrated rats, (e) group IV- Ulva fasciata administrated rats, (f) group V- Enteromorphoa flexuosa administrated rats.
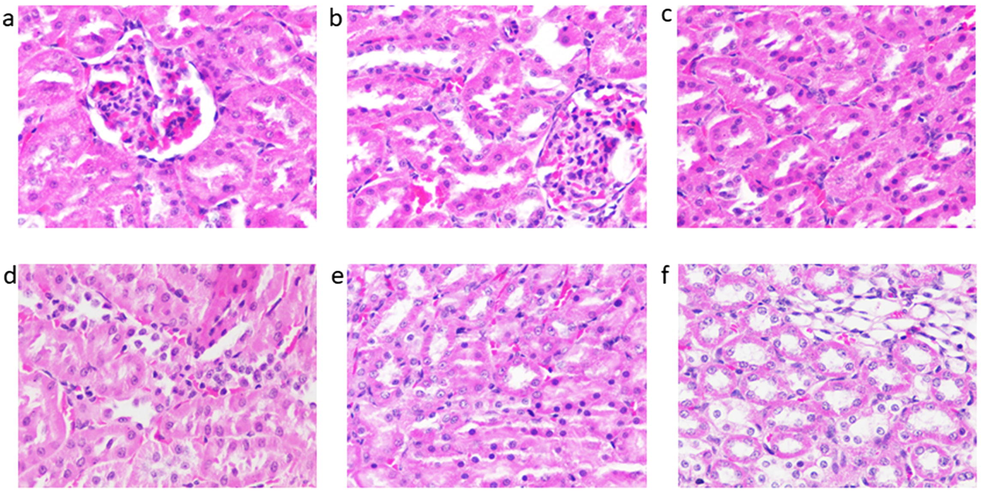
Histopathology section on kidney region of experimental rats (a) Control group- standard diet, (b) group I- Acanthophora spicifera administrated rats, (c) group II- Gracilaria edulis administrated rats, (d) group III- Padina gymnospora administrated rats, (e) group IV- Ulva fasciata administrated rats, (f) group V- Enteromorphoa flexuosa administrated rats.
3.7 Effect of seaweed extracts electrolyte levels on the urine sample
The urine electrolytes of seaweed extracts treated groups are given in Table 5. The urine pH ranged from 7.54 to 8.1 and Ulva fasciata supplemented rat had a higher pH of 8.1, probably due to the alkaline nature of seaweeds, this might be due to decreased inthe acidity of animal body but these changes were not statistically significant. Calcium level of the group III seemed to be high when compared to the control group, and this increment may be due to the high level of calcium content in the seaweed P. gymnospora. All the electrolytes values are within the reference level as specified for rodents (Raghuramulu et al., 2003). NS-not significant, Dunnett’s multiple comparisons Test, all values are expressed as mean ± SD, n = 6 in each group.
Parameter
Control Group
Group I
Group II
Group III
Group IV
Group V
f value
pH
7.87 ± 1.14
7.56 ± 2.23
7.54 ± 1.18
7.9 ± 2.45
8.1 ± 1.32
7.86 ± 2.12
0.084NS
Na (mEq/l)
140.2 ± 1.23
138 ± 2.34
140 ± 2.78
136 ± 1.29
142 ± 3.29
140 ± 3.2
1.9NS
K (mEq/l)
2.8 ± 2.42
2.5 ± 1.21
3.17 ± 1.78
3.02 ± 1.28
3.12 ± 1.67
2.98 ± 2.48
0.10NS
Cl (mEq/l)
170 ± 1.34
168 ± 2.02
173 ± 1.98
180 ± 1.71
174 ± 1.05
176 ± 2.56
32** (P < 0.01)
3.8 Heavy metal ions in vital organs
Heavy metal toxicity is one of the major concerns in the environment, and it is directly related to accumulation on the food system. This may lead to hazardous effect on livestock and human health. Additionally, seaweed tends to accumulate heavy metal in marine ecosystem and it may reflect in organs because organs are primary source of elimination of metal ions (see Table 6). It was surprising to note that mercury was not accumulated in any organs of tested rats which closely related to the result of Minamia et al. (2009). In general, metal accumulation not only depends on the amount present in raw seaweed or in the ecosystem but also depends on dietary composition, absorption in body system, internal regulator of the body. nd- not deducted.
Control
Group I
Group II
Group III
Group IV
Group V
As
Cd
Pb
As
Cd
Pb
As
Cd
Pb
As
Cd
Pb
As
Cd
Pb
As
Cd
Pb
Kidney
0.0028
0.024
0.17
0.21
0.012
0.06
0.79
0.04
0.6
0.72
0.031
0.8
0.78
0.04
0.079
0.98
0.012
0.12
Liver
0.20
0.018
0.437
0.09
0.039
0.08
0.51
0.037
0.29
0.236
0.012
0.57
0.156
0.025
0.12
0.76
0.04
0.08
Brain
0.17
nd
0.057
0.28
0.002
0.10
0.491
nd
0.08
0.396
nd
0.27
0.011
nd
0.03
0.05
nd
nd
In kidney samples, arsenic content was in the range of 0.21 ppm to 0.98 ppm, which was found to be high in group V (0.98 ppm) treated with E. flexuosa and low in group I (0.21 ppm) treated with A. spicifera. In liver samples, arsenic content was in the range of 0.09–0.510 ppm, the level was found to be low in group I (0.09 ppm) treated with A. spicifera and high in group II (0.51 ppm) treated with G. edulis. In brain samples, the arsenic content was in the range of 0.05–0.491 ppm, which was high in group II (0.491 ppm), supplemented with G. edulis and low in group V (0.05 ppm) treated with E. flexuosa.
In kidney samples, cadmium content was in the range of 0.012 to 0.04 ppm, which was low in group V (0.012 ppm) and high in group II and IV (0.04 ppm). In liver samples, it was in the range of 0.037–0.04 ppm which was found to be low in group II (0.037 ppm) and high in the group V (0.04 ppm) E. flexuosa treated rats. The cadmium content of brain samples was 0.002 ppm in group I fed with A. spicifera; all other groups did not contain cadmium. The cadmium content of liver, kidney and brain of the wild rodents were 102.88 ppb, 309.28 ppb and 255.6 ppb (Minamia et al., 2009)
In kidney samples, lead content was in the range of 0.06–0.802 ppm, which was high in group III (0.802 ppm) and low in group I (0.06 ppm). In liver samples, it was in the range of 0.08–0.57 ppm which was found to be high in group III and low in group I. In brain sample, lead content was in the range of 0.03–0.27 ppm, which was found to be low in group IV (0.03 ppm) U. fasciata treated rats and high in group III (0.27 ppm) P. gymnospora treated rats. Furthermore, heavy metal contents in organs were in negligible amount, and it was not because of seaweed it may be due to other metabolism involved. Accordingly, this accumulation of heavy metals is not an issue of concern in organs on seaweeds treated rats.
4 Conclusion
This study concluded that the selected seaweeds are articulated with non-toxic compounds whichclearly evident in the following seaweeds such as red algae Acanthophora spicifera, and Gracilaria edulis, brown algae Padina gymnospora, green algae namely Ulva fasciata and Enteromorpha flexuosa. Moreover, this seaweed is in abundance in marine coast of Gulf of Mannar as well as another coast of the world such as Fiji, Taiwan, Japan and Korean coast. Therefore, this study helps future researchers to develop nutrient-rich food products. These five underexploited seaweeds selected in this study were found to possess high nutritional value and nutraceutical potentials. Further, seaweeds belong to marine ecosystem might accumulate heavy metals due to pollution in the marine ecosystem. Hence, conduct of sub-chronic toxicity study is essential to ensure the normal haematology and biochemistry and other abnormalities in terms of histopathology. Heavy metal toxicity studies also showed that the metal ions did not accumulate in organs of the rats. The metal ion values were also found to be within tolerable value prescribed as safe levels. As a consequence, underexploited seaweeds studied were found to be safe for human consumption. Further, the use of 2 g upto 5 g on regular basis will not cause any toxic effect on humans was evident from our study.
Acknowledgement
Authors were gratefully acknowledged for facilities provided in K.M. College of Pharmacy, Madurai, Tamilnadu- India, and analytical facilities provided in Madurai Kamaraj University- India, for undertaking this study. The authors were grateful to the authorities of Fiji National University (FNU) and Sejong University, Republic of Korea for their support and offered other technical facilities. The authors MVA, NAA-D and VD would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through the Research Group Project No. RG-1435-071.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Sub–chronic toxicity and heavy metal toxicity study on Kappaphycusalvarezii in albino rats. Asian Pac. J. Trop. Biomed.. 2012;2(3):S1372-S1376.
- [Google Scholar]
- Phytochemical screening, microbial load and antimicrobial activity of underexploited seaweeds. Int. Res. J. Microbiol.. 2012;3(10):328-332.
- [Google Scholar]
- Nutritional and safety evaluation of underexploited seaweeds and nutraceutical potentials of Ulva fasciata. (unpublished doctoral dissertation). Coimbatore, India: Avinashilingam University for Women; 2012.
- Antidiabetic activity of Ulva fasciata and its impact on carbohydrate metabol-ism enzymes in alloxan induced diabetic rats. Int. J. Res. Phytochem. Pharmacol.. 2013;3(3):136-141.
- [Google Scholar]
- Quantification and correlation study on derived phenols and antioxidant activity of seaweeds from gulf of mannar. J. Herbs. Spices. Med. Plants. 2017;23(1):9-17.
- [Google Scholar]
- Seafood Safety. Washington DC: National Academy Press; 1991. p. :432.
- Enzymatic determination of total serum cholesterol. Clin. Chem.. 1974;20(4):470-475.
- [Google Scholar]
- Total arsenic, inorganic arsenic, lead and cadmium contents in edible seaweed sold in Spain. Food Chem. Toxicol.. 2006;44(11):1901-1908.
- [Google Scholar]
- In vitro antioxidant activity of marine red algae Chondrococcushornemanni and Spyridia fusiformis. J. Chem. Pharm. Res.. 2013;5(3):82-85.
- [Google Scholar]
- Bromcresol purple dye-binding methods underestimate albumin that is carrying covalently bound bilirubin. Clin. Chem.. 1987;33(6):821-823.
- [Google Scholar]
- Direct method for determining inorganic phosphate in serum with the “CentrifiChem”. Clin. Chem.. 1972;18(3):263-265.
- [Google Scholar]
- Chemoprotective effects of Ulva lactuca (green seaweed) aqueous-ethanolic extract against subchronic exposure to benzo (a) pyrene by CYP1A1 inhibition in mice. Phytother. Res.. 2019;33(4):958-967.
- [Google Scholar]
- Acute and chronic toxicological studies of dimorphandramollis in experimental animals. J. Ethnopharmacol.. 2006;108(3):450-456.
- [Google Scholar]
- Development of edible film from Acanthophoraspicifera: structural, rheological and functional properties. Food Biosci.. 2018;23:121-128.
- [Google Scholar]
- Quality enhancement of chicken sausage by semi-refined carrageenan. J. Food Process Preserv. 2019e13988
- [Google Scholar]
- Fucoidan ingestion increases the expression of CXCR4 on human CD34+ cells. Exp. Hematol.. 2007;35(6):989-994.
- [Google Scholar]
- Determination by AAS of some trace heavy metal ions in some natural and biological samples after their preconcentration using newly chemically modified chloromethylated polystyrene-PAN ion-exchanger. Anal. Sci.. 2000;16(5):493-500.
- [Google Scholar]
- Euglena as a potential natural source of value-added metabolites. A review. Algal Res.. 2019;37:154-159.
- [Google Scholar]
- Safety evaluation of seaweeds. Lap Lambert Acaedmic Publishing Company; 2011. p. :34. ISBN 978-3-8443-9182-4
- Seaweeds as a source of nutritionally beneficial compounds-a review. J. Food Sci. Technol.. 2008;45(1):1.
- [Google Scholar]
- Protective role of Carica papaya and Ficusbengalensis latex against CCl4 induced liver toxicity in experimental rats. J. Drug Del. Therap.. 2019;9(3):465-469.
- [Google Scholar]
- Toxicity testing of cosmetic ingredients using gametophyte beads of the brown alga Undariapinnatifida (Laminariales, Phaeophyta) J. App. Phycol.. 2019;31(3):2011-2023.
- [Google Scholar]
- Toxicological evaluation of fucoidan extracted from Laminaria japonica in Wistar rats. Food Chem. Toxicol.. 2005;43(3):421-426.
- [Google Scholar]
- Acute aluminum chloride toxicity revisited: Study on DNA damage and histopathological, biochemical and neurochemical alterations in rat brain. Life Sci.. 2019;217:202-211.
- [Google Scholar]
- Accumulation of heavy metals in the organs of wild rodents. Ann. Rep. by Research Institute for Science and Technology. 2009;1(21):11-17.
- [Google Scholar]
- Application of marine-derived polysaccharides as immunostimulants in aquaculture: a review of current knowledge and further perspectives. Fish Shellfish Immun 2018
- [Google Scholar]
- African Ethnobotany: Poisons and Drugs: Chemistry, Pharmacology, Toxicology. CRC Press; 1996.
- A Manual of Laboratory Techniques. Hyderabad, India: National Institute of Nutrition. Indian Council of Medical Research; 2003. p. :56-58.
- Phytoremediation potential of Spirulina (Arthrospira) platensis: biosorption and toxicity studies of cadmium. Environ. Pol.. 2002;119(1):45-53.
- [Google Scholar]
- A colorimetric methods for estimating SGOT and SGPT. Am. J. Clin. Pathol.. 1957;28:56-63.
- [Google Scholar]
- Arsenic in seaweed—forms, concentration and dietary exposure. Food Chem. Toxicol.. 2007;45(7):1263-1267.
- [Google Scholar]
- Subchronic toxicity study in mice fed Spirulina maxima. J. Ethnopharmacol.. 1998;62(3):235-241.
- [Google Scholar]
- Comparison of sodium and potassium intake with excretion. Hypertension.. 1980;2(5):695-699.
- [Google Scholar]
- Structural characterization and bioactivities of sulfated polysaccharide from Monostromaoxyspermum. Int. J. Biol. Macromol.. 2015;72:1459-1465.
- [Google Scholar]
- An integrated analysis of glucose, fat, and protein metabolism in severely traumatized patients. Studies in the basal state and the response to total parenteral nutrition. Ann. Surg.. 1989;209(1):63.
- [Google Scholar]
- Acute and subchronic (28-day) oral toxicity studies on the film formulation of k-carrageenan and konjac glucomannan for soft capsule application. Sci. Pharm.. 2019;87(2):9.
- [Google Scholar]
- Safety evaluation and antiobesogenic effect of Sargassum liebmannii J. Agardh (Fucales: Phaeophyceae) in rodents. J. Appl. Phycol. 2019:1-11.
- [Google Scholar]
- Probable source of discrepancies in alkaline phosphate assaysClin. Chem.. 1987;33(4):625.
- Amelioration of rifampicin and isoniazid induced liver oxidative damage and inflammation response by propolis extracts in rodent model. J. Biol. Active Prod. Nat.. 2019;9(1):57-66.
- [Google Scholar]
- A general method, employing arsenazo III in liposomes, for study of calcium ionophores: results with A23187 and prostaglandins. Proc. Natl. Acad. Sci.. 1980;77(3):1506-1510.
- [Google Scholar]







