Translate this page into:
Evaluation of different high doses aqueous plant extracts for the sustainable control of Aedes aegypti mosquitoes under laboratory conditions
⁎Corresponding author. drnazeerento@gmail.com (Nazeer Ahmed)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Aedes aegypti mosquitoes represent a significant public health hazard as they serve as vectors for a variety of arboviral infections, including Dengue, Zika, and Chikungunya. This necessitates the development of efficacious vector control strategies. The current study conducted at the Medical Entomology laboratory of the Nuclear Institute for Food and Agriculture (NIFA) Peshawar, Pakistan, evaluated the potential use of locally sourced aqueous-based plant extracts against different life stages of Aedes aegypti mosquitoes. The utilization of plant extracts, including Parthenium hysterophorus, Nicotiana tabacum, Melia azedarach, and Fagonia indica, offers a potentially effective and ecologically sustainable strategy for pest control. The above excerpts present environmentally friendly alternatives that are biodegradable and have a little impact on the environment. These alternatives are in contrast to synthetic pesticides and contribute to the promotion of sustainable and ecologically responsible methods of pest control. The study tested various plant extracts, specifically those from Parthenium hysterophorus, Nicotiana tabacum, Melia azedarach, and Fagonia indica. These were examined at high concentrations to assess their toxicity to the mosquito species. The extract of P. hysterophorus demonstrated impressive efficacy, displaying 100 % effectiveness across all mosquito life stages. This was closely followed by N. tabacum, F. indica, and M. azedarach in decreasing order of efficacy. To better understand the potency of these plant extracts, their LC50 values were determined after 24, 48, and 72 h post-exposure. LC50 values, a common measure in toxicology, indicate the concentration at which 50 % of the test organisms are killed. Among the tested extracts, M. azedarach exhibited the highest LC50 values (1.703 %, 2.142 %, 2.640 %), implying lower toxicity, while P. hysterophorus showed the lowest LC50 values (0.120 %, 0.420 %, 0.975 %), indicating high toxicity to Aedes aegypti mosquito larvae. Based on the comparative analysis of toxicity, the extracts' efficiency order was established as follows: P. hysterophorus extracts > N. tabacum extracts > F. indica extracts > M. azedarach extracts. These findings suggest that P. hysterophorus and N. tabacum, particularly at a 3 % concentration, hold promising potential as components in eco-friendly integrated vector management (IVM) strategies. This approach would serve as an alternative to the traditional reliance on synthetic pesticides, which often pose environmental and health risks due to their residual toxicity.
Keywords
Aedes aegypti mosquitoes
Mosquito control
Aqueous plant extracts
Eco-friendly
Laboratory conditions
Larvicidal activity
Adulticidal activity
Sustainable management
1 Introduction
A. aegypti mosquitoes, transmitting diseases like dengue, Zika, and chikungunya, pose a global health threat, particularly in tropical regions. Given environmental and resistance concerns with chemical insecticides, the demand for alternative, eco-friendly mosquito control methods is increasing. (Ahmed et al., 2021; Iqbal et al., 2021).
The ecological consequences of chemical pesticides, in conjunction with the increasing issue of insecticide resistance, have prompted a need for alternate and environmentally sustainable approaches to mosquito control. In order to enhance the understanding of the subject matter, it is imperative to thoroughly examine the environmental and resistance issues associated with chemical pesticides. Various methods are used to control the different insect pest populations. These methods include chemical insecticides, biological control, synthetic insecticides, resistance varieties, and semi chemicals, which affect the growth of insects, development, and population (Ahmed et al., 2013; Ahmed et al., 2016; Fu et al., 2017; Ahmed et al., 2018; Ahmed et al., 2019). However, some studies also indicated entomopathogenic fungus and laboratory temperature to control their populations (Saif-Ur-Rehman et al., 2019a, 2019b; Mastoi et al., 2020). The best way of controlling insect pests at present is through synthetic insecticides that have traditionally been widely used (Ahmed et al., 2021; Iqbal et al., 2021; Ullah et al., 2018). Botanical insecticides gain extra attention and are considered environmentally friendly, biodegradable, and far safer than chemical insecticides (Cetin et al., 2004; Ahmed et al., 2016; Iqbal et al., 2021; Ahmed et al., 2021). Plant extracts, rich in bioactive compounds like alkaloids, phenols, terpenoids, and flavonoids, show promise as natural, insecticidal alternatives for combating mosquito vectors. (Ahmed et al., 2016; Iqbal et al., 2021; Ahmed et al., 2021).
Laboratory assessments of aqueous plant extracts offer environmentally benign strategies for Aedes aegypti control, reducing ecological damage and pesticide resistance. Such controlled conditions permit precise comparisons between extracts, revealing potential bioactive compounds with larvicidal or adulticidal effects. The established insecticidal activities of Artemisia annua and Ocimum sanctum underscore these prospects, bolstering the development of plant-based insecticides (Sharma et al., 2014; Fernandes et al., 2021; Xia et al., 2023). While these studies provide valuable insights, there is still a need for further research to explore the potential of other aqueous plant extracts and to compare their efficacy against A. aegyptimosquitoes. Therefore, the current study was planned to work out effective and environmentally safe plant extracts from the available indigenous stock for formulations against A. aegypti mosquitoes. This research will contribute to the development of eco-friendly strategies for managing A. aegypti mosquitoes.
2 Materials and methods
The in-vitro study was conducted for exploiting different plant extracts against Aedes aegypti Mosquitoes at the Medical Entomology Laboratory of Nuclear Institute for Food and Agriculture (NIFA) Peshawar.
2.1 Collection and preparation of plant extracts
The plants; Parthenium hysterophorus, Nicotiana tabacum, Fagonia indica and Melia azedarach were collected from the local area of Peshawar. The plants were washed and air-dried for 48 h at room temperature. The selection of Parthenium hysterophorus, Nicotiana tabacum, Fagonia indica, and Melia azedarach, obtained from local sources in Peshawar, due to their geographical accessibility and potential historical utilization as botanical assets. The chosen doses (0.5–0.9 % and 1–3 %) are likely indicative of an effort to examine a spectrum of dilutions in order to accurately evaluate the impacts of the treatment. The plant materials leaf, flower, and seed were taken and ground into small pieces with the help of an electric blender and filtered with stainless steel mesh (0.01 mm). Out of the selected plants, 100 gm of powder was mixed with 1 L of water to prepare a 10 % stock solution. The solution was then left for 48 h. The stock solution was filtered with muslin cloth and then was serially diluted in different concentrations (0.5–0.9 % and 1–3 %) to evaluate the Treatment effect. Serial dilution was done by using the following formula:
Required concentrations; 0.5 %, 0.6 %, 0.7 %, 0.8 % 0.9 %, and 1 %, 1.5 %, 2 %, 2.5 %, 3 %.
4.5 ml of the stock solution were taken and diluted in 90 ml of water to make a 0.5 % concentration.
Similarly, for 3 % concentration, 27 ml were taken from the stock solution and dissolved in 90 ml water.
2.2 Bioassay
Aqueous-based extracts of various plants were used against the various stages of Aedes aegypti Mosquito according to standard WHO (World Health Organization) procedures.
2.3 Ovicidal trails
Aedes aegypti mosquito eggs, collected from NIFA's Medical Entomology laboratory in Peshawar, were exposed to various plant extract concentrations in plastic cups, with about 100 eggs per cup. The control group was subjected to tap water. Each concentration was replicated thrice. Data on egg hatching was recorded, with hatched eggs separated using a plastic dropper. The toxicity of plant extracts was evaluated by counting hatched and unhatched eggs at 24, 48, and 72-hour post-exposure intervals. The following formula was used to calculate Percent egg hatching:
2.4 Larvicidal potential
For larval mortality hundred numbers of the third instar, uniform size larvae were taken from the Medical Entomology laboratory of NIFA, Peshawar. The Larvae were then placed in disposable plastic cups containing different concentrations of plants extracts. Each tested concentration was repeated three times and the control only with tap water. During data recording, the dead larvae were separated from alive larvae with the help of a small plastic dropper. The plant extracts toxicity effect was determined by carefully counting the number of dead and alive larvae after 24, 48, and 72-hours’ post-exposure time. Percent larval mortality was recorded from the average of three replications. The percent mortality was corrected using the following formula (Abbott, 1925).
Whereas P0 = Larval Mortality in treated Treatment PC = Larval Mortality in the control Treatment.
Abbott’s formula was used to correct the mortality data, and the lethal concentration values of LC50 were calculated by using a probit analysis program.
2.5 Pupicidal potential
In the case of the pupal trial hundred numbers of uniform size, pupae were taken from the Medical Entomology laboratory of NIFA, Peshawar. It was then placed in plastic cups containing varied concentrations of the extracts. Each tested concentration was repeated and undertaken three times and the control was treated only with tap water. The motionless pupae were considered dead and unable to emerge into adults. During data recording, the dead pupae were separated from alive pupae with the help of a dropper. The plant extracts toxicity effect was determined through carefully counting the number of emerging and dead pupae after 24, 48 and 72-hours’ post-exposure time. Percent adult emergence was recorded by using the formula:
2.6 Adult deterrence activity
The adult deterrence test was performed following Rahuman et al. (2009) procedure with slight modification. The experiment was performed in the Medical Entomology laboratory of NIFA, Peshawar. In the presence of various Treatments in varying concentrations of aqueous extracts, the adult's ovipositional deterrent effect and the number of eggs deposited were investigated. During the pupal stage, about 20 male pupae and 20 female pupae were separated and placed in rearing cages (24.5 × 24.5 × 24.5 cm) at a temperature of 27 ± 2 °C and relative humidity of 75 ± 5. In the cages, the pupae were let to develop into adults. Adult males were given a 10 % sugar solution in disposable cups with cotton wicks regularly, while females were fed with Bovine blood/Albino mice after the fifth day of their emergence. Five cups holding 30 ml of different extracts solution with the same concentration were placed in each cage in the various treatments test, along with a 9-cm piece of filter paper for egg ling. The sixth (06) cup, devoid of extracts, was used as a control. Three replications of various treatments with the same concentration for each bioassay were done in cages put side by side. Under a stereomicroscope, the data was counted based on the number of eggs laid in Treatment and control cups after 24, 48, and 72 h of exposure time. The following formula was used to calculate effective repellency for each Treatment and concentration:
Where the EF is the effective repellency, NC is the number of eggs in control, and NT is the number of eggs in Treatment.
2.7 Statistical analysis
The percentage of larval mortality was calculated by dividing the number of dead and inactive larvae by the total number of larvae that were tested. Microsoft Excel and IBM SPSS Statistics for Windows software were used for data analysis. The Pearson fit test was used to determine whether Probit’s model adequately fit the data provided by the experiment. The percentage of egg hatchability at each dose was calculated by dividing the number of eggs hatched by the total number of eggs inserted, multiplied by 100. The average hatchability at each dose was calculated, including the standard deviation. One-way analysis of variance was used to determine whether there was a significant difference in the mean hatchability between the different doses.
3 Results
In various treatments with different concentrations, (1 %, 2 %, 3 %, 4 %, 5) after 24 h of exposure, minimum hatching was noted in Parthenium (6 %), (4.6 %), (2.6 %), (1.3 %), (0.0 %), followed by Fagonia indica (15.3 %), (9.3 %), (6 %), (6 %), (4.3 %), Melia azedarach (13.6 %), (11.3 %), (10 %), (9.6 %), (8.6 %)), tobacco (17.3 %), (15 %), (11.6 %), (9 %), (5 %). The hatching was significantly higher in the natural control (80 %), (86.6 %), (87.6 %) (80 %), (86.6 %) while low hatching was recorded in standard Treatment (3.6 %), (3.3 %), (2.3 %), (0.0 %), (0.0 %).
After 48 h of exposure, the incubation order of various treatments with different concentrations is Parthenium (4.6 %), (3.3 %), (2.3 %), (0.6 %), (0.0 %) followed by Fagonia indica (11.6 %), (8 %), (5.3), (3 %), (1.6 %), Melia azedarach (10 %), (9 %), (6.6 %) (6.6 %), (3.3 %) Tobacco (14.3 %), (12.3 %), (6.3 %), (6 %), (3.3 %). High emergence showed the control treatment (86.6 %) (81.6 %), (86.6 %), (86.6 %), (81.6 %), and the standard treatment was significantly lower among the treatments (3 %), (3 %), (1.6 %), (1.6 %), (1 %).
After 72 h, the minimum hatching rates recorded by various treatment at high dose concentrations was Parthenium (2.3 %), (1.6 %), (1.3 %), (0.6 %), (0.0 %), followed by Fagonia indica (6 %), (5.3 %), (4.3 %), (3.3 %), (0.0 %), Melia azedarach (7 %), (5.6 %), (4.6 %), (3.6 %), (2.6 %), Tobacco (12.3 %) (10 %) (5.3 %) (1 %) (0.0 %). 100 % hatching noted in the control check (100 %), (100 %), (100 %), (100 %), (100 %) and no hatching was observed in standard check compared to the treatments in all the tested concentrations (0.0 %), (0.0 %), (0.0 %), (0.0 %), (0.0 %) (Fig. 1).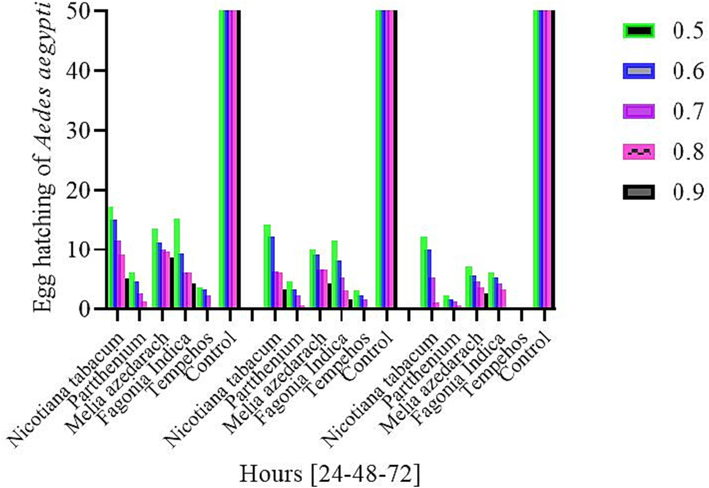
Percent egg hatching of Aedes aegypti mosquitoes in various extracts at high dose concentrations under different times (24, 48, and 72 h).
After 24 h’ time at 1 %, 2 %, 3 %, 4 % and 5 % dose concentration of various Treatments, the mortality was order is Parthenium (45.6 %), (48.3 %), (56 %), (83 %), (94 %), followed by tobacco (39.3 %), (39.6 %), (41 %), (42.6 %), (75.6 %), Fagonia indica (36.6 %) (40 %), (41.6 %), (44 %) (73.3 %), Melia azedarach (33.6 %), (41 %), (48.6 %), (52.3 %), (59 %). In simultaneous exposure to different treatments at various concentrations, the mortality was compared with the control group for Probit comparison and showed mortality of (1.2 %), (1.6 %), (1.3 %), (2.0 %), (2,0%). The standard check for comparison at the same time higher among the Treatments (%), (83 %), (86.6 %), (92.6 %), (93.6 %).
In the dose concentration 1 %, 1.5 %, 2 %, 2.5 % and 3 % after 48 h, the maximum mortality was observed in Parthenium (55 %), (57 %), (61.3 %), (90.6 %), (91 %), followed by tobacco (43.6 %), (46.3 %), (48 %), (49.3 %), (80 %), Fagonia indica (42 %), (45 %), (48 %), (51.6 %), (77.6 %), Melia azedarach (37 %), (42 %), (51 %), (55 %), (62.3 %). The control treatment showed low mortality in all (1.3 %), (1, 2 %). (3 %), (1.3 %), (1.3 %) while the standard checks at the same time was significantly high (81.3 %), (81.3 %), (92.6 %), (93.3 %), (96 %) in all treatments.
The dose concentration of different treatments after 72-hours exposure time in concentrations 1 %, 1.5 %, 2 %, 2.5 %, and 3 % Parthenium showed high efficacy against 3rd instar larvae of Aedes aegypti mosquitoes and resulted in high mortalities (80.6 %), (84.6 %), (91.6 %), (99.6 %), (100 %) followed by Tobacco (54.3), (60.6 %), (65 %), (75.3 %), (85 %), Fagonia indica (50.3 %), (55.6 %), (60 %), (68.3 %), (79.6 %), Melia azedarach (39 %), (48 %.), (55.6 %), (62 %), (69 %). The mortality was compared with natural control check for Probit comparison and showed less mortality (1 %), (1.6 %), (1 %), (1.6 %) (1 %). In all treatments the standard check was significantly high (95 %), (96 %), (100 %), (100 %), (100 %). After 24 to 72 h from 1 % to 3 % dose concentrations, the larval mortality parameter increased as the concentration and time increased. 80.6 %), (84.6 %), (91.6 %), (99.6 %), (100 %) (Fig. 2).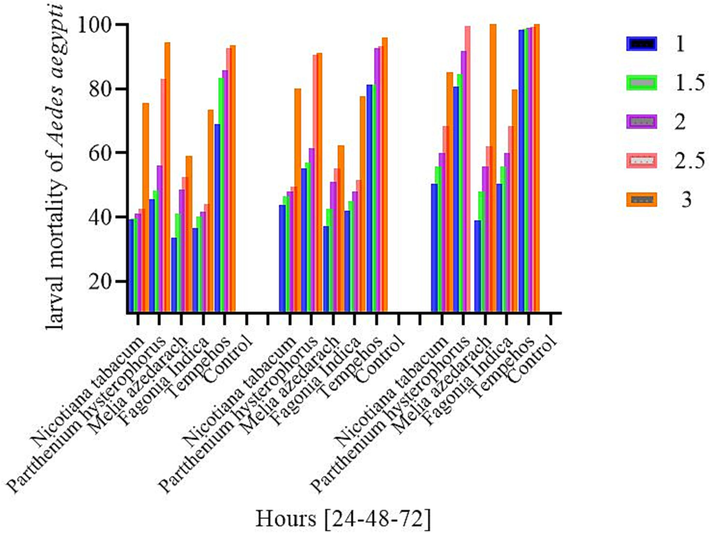
Percent larval mortality of Aedes aegypti mosquitoes in various plants extracts at high dose concentration in different time (24, 48, 72 h).
After 24 h the results showed that Parthenium has the lowest LC50 values (0.975 %) followed by Tobacco (1.573 %), Fagonia indica (1.931 %), and Melia azedarach (2.640 %) respectively. The standard temephos was found significantly low among all the treatments with (0.256) LC50 values.
Similarly, after 48 h the results revealed the lowest LC50 values was found in Parthenium (0.420 %), followed by Tobacco (1.103 %), Fagonia indica (1.412 %), and Melia azedarach (2.142 %). Overall the standard check temephos was found significantly low (0.104 %) among the botanical extracts. In case of 72 h of time the Parhnium extracts showed the lowest Lc50 values of (0.120 %) followed by Tobacco (0.876 %), Fagonia indica (1.104 %), and Melia azedarach (1.703. The standard check was found to be extremely low in all the treatments, with the lowest LC50 values of (0.003 %) (Table 1).
% Lethal Concentration
Time
24
48
72
Treatments
LC50
95 %CL
LC50
95 % CL
LC50
95 %CL
Nicotiana tabacum
1.573
1.03–2.56
1.103
0.83–1.458
0.876
0.370–1.203
Parthenium hysterophorus
0.975
0.41–1.28
0.420
0.21–1.057
0.120
0.010–1.00
Melia azedarach
1.931
1.50–2.77
1.412
1.00–1.205
1.104
0.622–1.05
Fagonia indica
2.640
1.98–2.41
2.142
1.42–2.016
1.703
1.336–2.591
Temephos
0.256
0.21–1.09
0.104
0.13–1.005
0.003
0.001–0.562
Among the dose concentrations, 1 %, 2,3%, 4 % and 5 % after 24 h was observed in Parthenium (7.3 %), (7 %), (6 %), (0.0 %), (0.0 %) followed by Tobacco (12 %), (9.6 %), (8.3 %), (5.6 %, (3.3 %), Fagonia indica (17.3 %), (13 %), (8.6 %), (8.6 %), (11 %). Melia azedarach (20.6 %), (13 %), (8.6 %), (8.6 %), (11 %), The emergence in the control check was significantly high (88 %), (86.6 %), (87.6 %) (80 %), (86.6 %) in all the treatments and significantly low emergence was recorded in the standard check (3.3 %), (3.0 %), (0.0 %), (0.0 %), (0.0 %).
In different concentration of various Treatments after 48 h of period the minimum emergence was observed in Parthenium (8.6 %), (5 %), (2.6 %), (0.0 %), (0.0 %) followed by Tobacco (14.3 %), (11.3 %), (7.3 %), (4.3 %), (2.0 %), Fagonia indica (9.3) (7) (6) (4.6) (2.6) Melia azedarach (20 %), (11 %), (10.6), (7.6 %), (5.0 %), The emergence was compared with control Treatment (86.6 %) (81.6 %), (86.6 %), (86.6 %), (92.3 %) while, the Standard check was significantly low among the treatments (4.3 %), (0.0 %), (0.0 %), (0.0 %), (0.0 %).
After 72 h’ period of exposure in different concentrations of various Treatments the minimum emergence was recorded in Parthenium (2.6 %), (2.3 %), (0.6 %), (0.0 %), (0.0 %) followed by Tobacco (8.6 %), (3.6 %), (1.6 %), (1.3 %), (0.0 %) Fagonia indica (7 %), (3.6 %), (3 %), (2.3 %), (0.0 %), Melia azedarach (13.3 %), (8.6 %), (3.6 %), (2 %), (1 %). For comparison the emergence rate was compare with standard check and control check was as, 100 % emergence was noted in control. (100 %), (100 %), (100 %), (100 %), (100 %) while, the standard check was significantly low than all the treatments (2 %), (2 %), (1.6 %), (1.6 %), (1 %) (Fig. 3).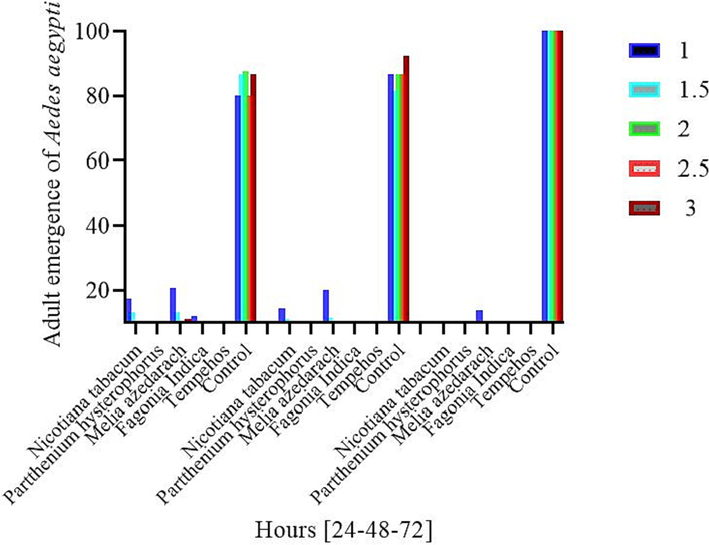
Percent adult emergence of Aedes aegypti mosquitoes in various plant extracts under different exposure times at a high dose concentration.
Among the various treatments of different concentrations after 24 h post exposure the low adult egg laying and high deterrence was observed in treatment Parthenium (10.6 %), (6.3 %), (5.6 %), (2.3 %), (0.0 %) followed by Tobacco (25 %), (22 %), (20 %), (16.6 %), (15 %), Fagonia indica (25.6 %), (24 %), (21 %), (19.6 %), (17.66 %), Melia azedarach (26 %), (25.6 %), (25.6 %), (22 %), (18.6 %), The treatments were compared with natural control check (54.3 %), (67.6 %), (74.6 %), (88 %), (90 %) showed high percent egg laying rate, while less egg were laid in the standard check (2 %), (0.6 %), (0.0 %), (0.0 %), (0.0 %).
Different concentrations of various treatments after 48 h post exposure the minimum percent adult eggs were observed in Parthenium (4.6 %), (3.3 %), (1.3 %), (0.6 %), (0.0 %) followed by Tobacco (23.3 %), (17.3 %), (15.3 %), (13.6 %), (12 %), Fagonia indica (22 %), (21 %), (19 %), (15.6 %), (14 %), Melia azedarach (25.3 %), (20 %), (18.3 %), (17 %), (15 %)), The natural control check showed high adult egg laying (74.6 %), (78.6 %), (81 %), (91.3 %), (93 %), and the standard check showed high adult deterrence potential with minimum egg laying (1.6 %), (0.3 %), (0.0 %), (0.0 %), (0.0 %).
After 72 h of exposure period, various Treatments of different concentrations (1 %, 1.5 %, 2 %, 2.5 %, 3 %) least adult egg laying were observed in the Parthenium (5.3 %), (3 %), (1.0 %), (0.0 %), (0.0 %) followed by Tobacco (22 %), (17.3 %), (13.6 %), (12.3 %), (11.3 %), Fagonia indica (21.6 %), (18.6 %), (16.3 %), (12.6 %), (11.7 %), Melia azedarach (23.6 %), (17 %), (16 %), (15 %), (13.3 %), The treatments were compared with natural control check with maximum egg laying (98.3 %), (98.6 %), (99 %), (99.3 %), (100 %), and the standard check was significantly low showing minimum adult egg laying (0.3 %), (0.0 %), (0.0 %), (0.0 %), (0.0 %) (Fig. 4).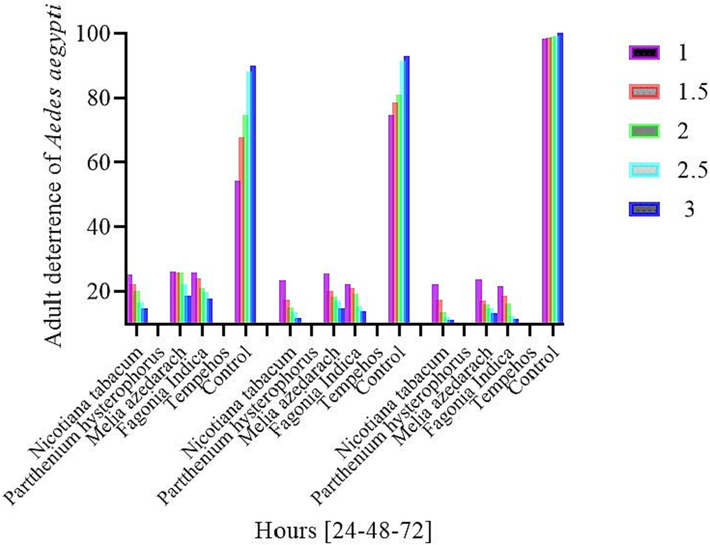
Percent adult deterrence of Aedes aegypti mosquitoes exposed to various treatments in high dose concentrations after 24, 48, and 72 h of time.
4 Discussion
4.1 Percent egg hatching of Aedes aegypti mosquitoes in various extracts at high dose concentrations under different times (24, 48, and 72 h)
Plant extracts have been extensively studied for their mosquito control potential. Early research of Qualls and Xue (2009) demonstrated how plant-derived compounds disrupt mosquito life cycles, leading to reduced egg hatching. More recently, Govindarajan and Benelli (2017) confirmed the ovicidal activity of Feronia elephantum extracts against Aedes aegypti, reducing egg hatching. Our study aligns with these findings, showing the effectiveness of high-dose plant extracts in decreasing egg hatching over time. However, the impact varies with plant type, extraction method, and concentration (Kumar et al., 2012; Li et al., 2022, Zhang et al., 2022). Specific extracts displayed stronger effects, highlighting the need for comprehensive exploration of plant species and concentrations.
Our study aligns with recent research Benelli (2020) that shows increased egg hatch deterrence over time following exposure to Ocimum canum extract. Extended exposure to high-dose botanical extracts disrupts the Aedes aegypti mosquito life cycle, holding promise for vector management amid mosquito resistance to synthetic insecticides (Liu, 2015). Plant extracts present eco-friendly, renewable, and biodegradable alternatives for population control by inhibiting egg hatching. However, identifying specific bioactive compounds responsible for ovicidal activity and assessing their safety for non-target organisms is crucial, as emphasized in previous studies (Pavela and Benelli, 2016; Korunić et al., 2016). Our findings support the documented potential of plant extracts in mosquito control, with larvicidal and ovicidal properties well-established (Ghosh et al., 2012; Pavela and Benelli, 2016).
4.2 Percent larval mortality of Aedes aegypti mosquitoes in various plants extracts at high dose concentration in different time (24, 48, 72 h)
Our study demonstrated a significant increase in larval mortality over time for all plant extracts. Parthenium showed the highest efficacy within 24 h, resulting in mortality rates between 45.6 % and 94 % at 1 %-3% concentrations. Tobacco, Fagonia indica, and Melia azedarach extracts also exhibited notable larval mortality, but lower than Parthenium, consistent with Ghosh et al.'s research (2012). At 48 h, the plant extracts maintained their lethal impact, with Parthenium showing persistent efficacy (55 %-91 %). This trend aligns with Yang et al.'s findings (2020), attributing prolonged impact to continuous larval exposure to plant compounds. After 72 h, Parthenium's efficacy increased further (100 % mortality at 3 %), while other extracts showed substantial larval mortality, similar to Saliva et al.'s report (2008).
Importantly, our research showed negligible larval mortality in the control group across all time frames, which underscores the substantial larvicidal efficacy of the plant extracts. The standard check concurrently displayed high larval mortality, affirming the accuracy of our testing protocol. The escalating larval mortality with increasing extract concentration and exposure duration substantiates the larvicidal potential of these botanicals. Our results, indicating a dose-dependent response in mosquito larvae to plant extracts, agree with Govindarajan et al.'s findings (2016). Additionally, we corroborate Kumar et al.'s research (2012) showcasing the effectiveness of Parthenium extract in causing Aedes aegypti larval mortality. Our results are consistent with Isman's 2020 review, suggesting that variations in the efficacy of plant extracts can be attributed to the intricate interactions of bioactive compounds present within each extract.
4.3 Percent lethal concentration of Aedes aegypti mosquitoes exposed to various plant extracts of high dose after 24, 48 and 72 h
As early as 24 h into the exposure period, Parthenium extract emerged as the most potent, with the lowest LC50 value (0.975 %), an indication of its higher toxicity towards Aedes aegypti larvae. This is consistent with the findings of Sharma et al. (2018), who observed a significant larvicidal effect of Parthenium hysterophorus against Aedes aegypti. Other plant extracts, Tobacco (1.573 %), Fagonia indica (1.931 %), and Melia azedarach (2.640 %), also displayed considerable larvicidal activities but at varying degrees, thereby illustrating their potential as botanical insecticides.
Upon extending the exposure to 48 h, there was a noticeable decrease in LC50 values for all plant extracts, with Parthenium maintaining its lead, showing an LC50 value of 0.420 %. Previous studies have also reported the enhanced larvicidal effects of plant extracts with prolonged exposure (Ghosh et al., 2012). In this context, our findings align with the existing literature, as increased exposure led to increased mortality in mosquito larvae.
At the final time point of 72 h, the trend continued, with Parthenium exhibiting an LC50 value as low as 0.120 %, further establishing its prominent larvicidal properties. Fagonia indica and Melia azedarach also demonstrated a substantial decrease in LC50 values to 1.104 % and 1.703 %, respectively, signifying their larvicidal potential.
Indeed, the lower LC50 values of temephos across all exposure times (0.256 %, 0.104 %, 0.003 % for 24, 48, and 72 h respectively) underscore its proven efficacy as a larvicide. However, concerns about the development of resistance among Aedes populations and the environmental implications of synthetic insecticides drive the need for alternative methods (Luz et al., 2019). Furthermore, as pointed out by Wei et al. (2015), extracts from Tobacco and Melia azedarach have shown promise against a range of insect pests.
4.4 Percent adult emergence of Aedes aegypti mosquitoes in various plant extracts under different exposure times at a high dose concentration
Findings from previous studies align with the trend seen in our investigation, which show a marked decrease in adult emergence of Aedes aegypti mosquitoes under increased concentrations of plant extracts. This suppression of mosquito metamorphosis aligns with the findings by Rajkumar and Jebanesan (2008), who illustrated a decrease in adult emergence following treatment with plant-derived Eucalyptus globulus extracts, marking a precedent for the results observed in our study.
Our study concurs with Pavela's 2016 review emphasizing the potential of plant extracts, rich in secondary metabolites, in hindering mosquito larval growth and development. The tested extracts, including Parthenium, Tobacco, Fagonia indica, and Melia azedarach, likely introduced growth-inhibiting secondary metabolites into the aquatic environment, suppressing Aedes aegypti adult emergence.
Our findings of reduced adult emergence with increased extract concentration align with Nagoor Meeran et al.'s 2020 study, which observed significant inhibition of Aedes aegypti adult emergence using 2 %-3% Tephrosia purpurea and Cleistanthus collinus extracts. This reaffirms that elevated plant extract concentrations can disrupt Aedes aegypti maturation.
In another related study, Amer and Mehlhorn (2006) examined the impact of various plant extracts on the life cycle of Aedes aegypti and found significant reductions in adult emergence. This validates our results and further establishes the potential of botanical extracts as biocontrol agents in managing mosquito populations. However, not all investigations have yielded identical findings. Benelli et al. (2013) highlighted that the effectiveness of plant extracts could be species-specific.
4.5 Percent adult deterrence of Aedes aegypti mosquitoes in various extracts under different time intervals at high dose concentrations
Parthenium extract displayed the most potent oviposition deterrent effect among the tested plant extracts. This potent oviposition deterrence aligns with the findings of a study by Amir et al., (2017), which established the ovicidal activity of Parthenium hysterophorus against Aedes aegypti. The present study, however, extended this understanding by demonstrating that this activity persists across different time intervals and concentration ranges.
The Tobacco extract's efficacy against adult Aedes aegypti aligns with previous studies highlighting the larvicidal and repellent properties of Tobacco-derived products (Panneerselvam and Murugan, 2013). Our study extends existing knowledge by showcasing oviposition deterrence in mosquitoes subject to high-dose Tobacco extracts. Comparable deterrent effects were found with Fagonia indica and Melia azedarach extracts, in line with previous research (Chellappandian et al., 2015; Sujitha et al., 2015). The study suggests that various plant extracts might serve as broad-spectrum deterrents, presenting a promising strategy for expanded vector control. Distinguished oviposition rates among treated and untreated mosquitoes, and a standard temephos-treated group underscore plant extracts' potential as oviposition deterrents. A dose-dependent decrease in oviposition, aligning with previous studies (Warikoo et al., 2011), implies a potential dose-dependent toxicity or deterrent mechanism. The research seems to only examine particular plant extracts obtained from a single geographical location, perhaps constraining the applicability of the results to different areas or mosquito species. Moreover, this study evaluates the impacts of plant extracts within relatively brief time periods, specifically 24, 48, and 72 h. This observation may fail to encompass the enduring consequences or fluctuations in the life cycles of mosquitoes. Nevertheless, the primary emphasis of the study is on plant extracts with high-dose concentrations. It could be advantageous to investigate a more extensive array of concentrations in order to enhance comprehension of dose–response connections. The experiments appear to have been conducted within a controlled laboratory environment. In a similar vein, while this is crucial for maintaining scientific rigor, it may not comprehensively depict the intricate and ever-changing habitats in which mosquitoes flourish in their natural settings. However, it is worth noting that the research lacks field testing, a crucial component for evaluating the actual effectiveness and feasibility of plant-based mosquito control techniques in real-world scenarios.
5 Conclusion
The study showed that concentration and exposure time influenced Aedes aegypti egg hatching. Parthenium extracts exhibited strong ovicidal activity, even at low concentrations and extended exposure. Fagonia indica and Melia azedarach also reduced egg hatching, but efficacy declined over time. Plant extracts hold potential as effective ovicides against Aedes aegypti, providing eco-friendly vector Moreover, our research highlights the considerable potential of plant-based extracts as environmentally benign options for mosquito control. The results of this study have important significance for public health, since they provide viable and environmentally friendly approaches to address the issue of mosquito-borne diseases. The integration of plant-based tactics into forthcoming mosquito control endeavors has the potential to enhance vector management in a more efficient and ecologically mindful manner. control. Field studies are needed to assess effectiveness, environmental safety, and non-target species impact.
Acknowledgement
Authors would like to acknowledge the support provided by Researchers Supporting Project number RSP2023R358, King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- A method of computing the effectiveness of an insecticide. J. Econ. Entomol.. 1925;18(2):265-267.
- [CrossRef] [Google Scholar]
- Effect of different plant extracts on termite species (Heterotermis indicola) J. Bioresour. Manage.. 2016;3(2)
- [CrossRef] [Google Scholar]
- Resistance of seven cabbage cultivars to green peach aphid (Hemiptera: Aphididae) J. Econ. Entomol.. 2018;111(2):909-916.
- [Google Scholar]
- Host selection behavior of the green peach aphid, Myzus persicae, in response to volatile organic compounds and nitrogen contents of cabbage cultivars. Front. Plant Sci.. 2019;10:79.
- [Google Scholar]
- Ahmed, N., Alam, M., Saeed, M., Ullah, H., Iqbal, T., Al-Mutairi, K.A., Salman, M., 2021. Botanical Insecticides Are a Non-Toxic Alternative to Conventional Pesticides in the Control of Insects and Pests. In: Global Decline of Insects. IntechOpen.
- Larvicidal effects of various essential oils against Aedes, Anopheles, and Culex larvae (Diptera, Culicidae) Parasitol. Res.. 2006;99(4):466-472.
- [Google Scholar]
- Evaluation of larvicidal activity of Parthenium hysterophorus against Aedes aegypti. Int. J. Mosq. Res. 2017;4(2):1-4.
- [Google Scholar]
- Plant-borne compounds and nanoparticles: challenges for medicine, parasitology and entomology. Environ. Sci. Pollut. Res.. 2020;25:10149-10150.
- [Google Scholar]
- Larvicidal activity of a botanical natural product, AkseBio2, against Culex pipiens. Fitoterapia. 2004;75(7–8):724-728.
- [Google Scholar]
- Ovicidal and oviposition deterrent activities of medicinal plant extracts against Aedes aegypti L. and Culex quinquefasciatus Say mosquitoes (Diptera: Culicidae) Osong public health and research perspectives. 2015;6(1):64-69.
- [Google Scholar]
- Ovicidal, pupicidal, adulticidal, and repellent activity of Helicteres velutina K. Schum against Aedes aegypti L.(Diptera: Culicidae) Brazilian J. Vet. Med.. 2021;43
- [Google Scholar]
- Intraguild predation on the aphid parasitoid Aphelinus asychis by the ladybird Harmonia axyridis. BioControl. 2017;62(1):61-70.
- [Google Scholar]
- Plant extracts as potential mosquito larvicides. Indian J. Med. Res.. 2012;135(5):581-598.
- [Google Scholar]
- A facile one-pot synthesis of eco-friendly nanoparticles using Carissa carandas: ovicidal and larvicidal potential on malaria, dengue and filariasis mosquito vectors. J. Clust. Sci.. 2017;28:15-36.
- [Google Scholar]
- Eugenol, α-pinene and β-caryophyllene from Plectranthus barbatus essential oil as eco-friendly larvicides against malaria, dengue and Japanese encephalitis mosquito vectors. Parasitol. Res.. 2016;115(2):807-815.
- [Google Scholar]
- Iqbal, T., Ahmed, N., Shahjeer, K., Ahmed, S., Al-Mutairi, K.A., Khater, H.F., Ali, R. F., 2021. Botanical Insecticides and their Potential as Anti-Insect/Pests: Are they Successful against Insects and Pests? In: Global Decline of Insects.
- Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol.. 2020;65:31-49.
- [Google Scholar]
- A review of natural insecticides based on diatomaceous earths. Poljoprivreda. 2016;22(1):10-18.
- [Google Scholar]
- Impact of Parthenium hysterophorus leaf extracts on the fecundity, fertility and behavioural response of Aedes aegypti L. Parasitol. Res.. 2012;108:853-859.
- [Google Scholar]
- Enhanced CO2 capture for photosynthetic lycopene production in engineered Rhodopseudomonas palustris, a purple nonsulfur bacterium. Green Chem.. 2022;24(19):7500-7518.
- [CrossRef] [Google Scholar]
- Insecticide resistance in mosquitoes: impact, mechanisms, and research directions. Annu. Rev. Entomol.. 2015;60:537-559.
- [Google Scholar]
- Dengue vector control strategies in an urban setting: an economic modelling assessment. Lancet. 2019;377(9776):1673-1680.
- [Google Scholar]
- Effect of heat stress on life history of pea aphid, Acyrthosiphon pisum (Harris)(Hemiptera: Aphididae) based on life-table. Pak. J. Agri. Sci. 2020;57(1):325-332.
- [Google Scholar]
- Pharmacological properties and molecular mechanisms of thymol: prospects for its therapeutic potential and pharmaceutical development. Front. Pharmacol.. 2020;8:380.
- [Google Scholar]
- Adulticidal, repellent, and ovicidal properties of indigenous plant extracts against the malarial vector, Anopheles stephensi (Diptera: Culicidae) Parasitol. Res.. 2013;112:679-692.
- [Google Scholar]
- Essential oils as eco-friendly biopesticides? Challenges and constraints. Trends Plant Sci.. 2016;21(11):1000-1007.
- [Google Scholar]
- Field evaluation of three botanical repellents against Psorophora ferox, Aedes atlanticus, and Aedes mitchellae. J. Am. Mosq. Control Assoc.. 2009;25(3):379-381.
- [Google Scholar]
- Efficacy of larvicidal botanical extracts against Culex quinquefasciatus Say (Diptera: Culicidae) Parasitol. Res.. 2009;104(6):1365-1372.
- [Google Scholar]
- Bioactivity of flavonoid compounds from Poncirus trifoliata L. Family: Rutaceae) against the dengue vector, Aedes aegypti L. (Diptera: Culicidae) Parasitol. Res.. 2008;104(1):19-25.
- [Google Scholar]
- Use of indigenous isolates of Metarhizium Isaria and Beauveria as potential Bio-Control agents against Sitophilus oryzae under Laboratory condtion. Pak. J. Agric. Sci.. 2019;56(4):421-429.
- [Google Scholar]
- Potential of four entomopathogenic fungi isolates as biological control agents against two aphid species under laboratory conditions. Pak. J. Agric. Sci.. 2019;56(2):421-429.
- [Google Scholar]
- Strong larvicidal potential of Artemisia annua leaf extract against malaria (Anopheles stephensi Liston) and dengue (Aedes aegypti L.) vectors and bioassay-driven isolation of the marker compounds. Parasitol. Res.. 2014;113:197-209.
- [Google Scholar]
- Effects of essential oils on Aedes aegypti larvae: alternatives to environmentally safe insecticides. Bioresour. Technol.. 2008;99(8):3251-3255.
- [Google Scholar]
- Green-synthesized silver nanoparticles as a novel control tool against dengue virus (DEN-2) and its primary vector Aedes aegypti. Parasitol. Res.. 2015;114:3315-3325.
- [Google Scholar]
- Larvicidal activity of medicinal plant extracts against Culex quinquefasciatus Say. Culicidae, Diptera) Int. J. Mosq. Res.. 2018;5(2):47-51.
- [Google Scholar]
- Larvicidal and irritant activities of hexane leaf extracts of Citrus sinensis against dengue vector Aedes aegypti L. Asian Pac. J. Trop. Med.. 2011;4(8):610-614.
- [Google Scholar]
- The toxicity and physiological effect of essential oil from Chenopodium ambrosioides against the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae) Crop Prot.. 2015;76:68-74.
- [Google Scholar]
- Synthesis and biological activities of oxazolidinone pleuromutilin derivatives as a potent anti-MRSA agent. ACS Infect. Dis. 2023
- [CrossRef] [Google Scholar]
- Chemical composition and larvicidal activity of essential oils from Peganum harmala, Nepeta cataria and Phellodendron amurense against Aedes aegypti (Diptera: Culicidae) Saudi Pharm. J.. 2020;28(5):560-564.
- [Google Scholar]
- Calcium homeostasis in Parkinson’s disease: from pathology to treatment. Neurosci. Bull.. 2022;38(10):1267-1270.
- [Google Scholar]







