Translate this page into:
Evaluation of Cuscuta reflexa seed essential oil on TPA-induced inflammation in mice
⁎Corresponding authors. khan.zulfanooreen7860@gmail.com (Zulfa Nooreen), nsiddiqui@ksu.edu.sa (Nasir A. Siddiqui),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Objectives
Cuscuta reflexa is parasitic plant of subtropical and temperate areas, commonly named as amarbel or akasvel in India. Present study is to investigate the In-vivoanti-inflammatory potential of C. reflexa seed essential oil.
Materials and methods
In this study the essential oil was hydro-distilled from dried seeds and analyzed for chemical profiling and anti-inflammatory activity. In –vivo activity was performed on mice inducing TPA as disease causing agent. Study of biochemical parameter and oxidative stress was performed by taking homogenate of ear pinna of mice.
Results
Chemical analysis is done by GC and GC-MS study, we found β-Bisabolene and Hexahydro farnesyl acetone as the major component as 13.2 and 14.2% respectively along with cis-chrysanthenyl acetate, caryophyllene oxide, carotol, caryophyllene oxide. In-vitro activity of cell viability was assessed using RAW 264.7 macrophages, showed no reduction in viable cell in the processed culture. In-vivo it significantly reduces the inflammation by inhibiting pro-inflammatory cytokines (IL-1β, IL-6 and TNF-α).
Conclusions
Cuscuta reflexa seed essential oil was found effective in the management of inflammation by encountering the inflammatory mediators (cytokines).
Keywords
Cuscuta reflexa
Convolvulaceae
Gas chromatography
Anti-inflammatory activity
1 Introduction
Akashbela, devil’s hair or Cuscuta reflexa Roxb belong to Convolvulaceae family. It is rootless climbs towards sky. This parasitic plant is commonly located at Ceylon upto an altitude of 2346 m, India, Pakistan and many other countries. Traditionally the plant has reported for many medicinal properties. Many pharmacological activities have been reported at extract level only. (Khan et al., 2018). The essential oil of C. reflexa contain mainly cis-3-butyl-4-vinylcyclopentane (26.4%) along with limonene (5.1%) and (E)-nerolidol (9.5%) (Paudel et al., 2014). On non-volatile components, many chemical compounds have been isolated by researcher’s time to time but some are commonly available as lycopene, kaemferol, cuscutalin, palmitic acid, oleic acid. The major bioactive compound in the plant are bergenin, hyperoside and amarbellin (Saini et al., 2015) and alkaloid, saponins and flavonoids in the plantwhich attribute role in biological activities.
Seed of C. reflexa has been studied for bioactivities like antispasmodic, antiviral anti-inflammatory, antibacterial antifungal, antidiabetic and antitumor (Costea and François, 2005) anthelmintic, treatment of bilious and carminative (Khan et al., 2010), diuretic activity (Sharma et al., 2009). The extract of C. reflexa has been reported for muscle relaxant, antioxidant, cardiotonic, hair growth promoter, anticonvulsant and hemodynamic properties (Seru et al., 2013). According to tribal information this plant has been used to cure inflammation and cancer (Knolle and Greken, 2000). C. reflexa showed moderate anti-proliferative activities in HCT116 colorectal cell lines against while its component 1-O-p-hydroxycinnamoyldlucose showed promising activity (Riaz et al., 2017). Water extract of the plant was able to block the binding of NF-κB to its DNA binding motifs, making it evident that the extract down regulates TNF-α and COX-2 via NF-κB inhibition thus it has anti-inflammatory property (Suresh et al., 2011). Lots of essential oils are used from long time to reduce inflammation. Angelica sinensis volatile oil showed marked reduction in the inhibition of pro-inflammatory cytokines (Li et al., 2016).
Exhaustive work has been done to investigate new drug molecule, extract and oil used for relief from pain and inflammation from decades. Inflammation is a complex biological response to various stimuli hence activation of inflammatory mediators which cause emergence of pro-inflammatory cytokines. This causes damage in cells, tissue and organs. If it remains persistent may become life threatening disease like rheumatoid arthritis and cancer.
Many non-steroidal anti-inflammatory medicines have been investigated but almost all synthetic compound showed side effect. The presence of bioactive compound in plant has attracted many researchers to work. Nowadays phytochemicals are gaining attention for treatment of disease over synthetic molecule. Present work is chemical investigation and anti-inflammatory profile of C. reflexa essential oil followed by chemical characterization.
2 Materials and methods
2.1 Chemicals and reagents
Acetone, dimethyl sulfoxide (DMSO), Griess reagent kit, malondialdehyde, thiobarbituric acid (TBA), trichloroacetic acid (TCA), 12-O-tetradecanoylphorbol-13-acetate (TPA), and indomethacin were purchased from Sigma-Aldrich (USA), phosphate buffer saline tablet (PBS) was taken from Himedia, Mumbai and from Thermo Scientific (USA) TNF-α, IL-6 and IL-1β biochemical kit. Every material used in present work was of analytical grade.
2.2 Plant material
The seed of C. reflexa were purchased in August 2018 from the local market in Lucknow, India and identified by the Scientist, Botany and Pharmacognosy, Department, CSIR-CIMAP, Lucknow. Voucher no. 774 has been deposited with Institute’s herbarium.
2.3 Isolation of essential oil
The essential oil of crushed seed of C. reflexa (500 g) was obtained by hydro-distillation in a Clevenger-type apparatus. The yield of the essential oil was 0.05% (w/w) on a dry weight basis. The oil was dried over anhydrous sodium sulfate and stored in a sealed glass vial at low temperature until further analysis.
2.4 Gas chromatography (GC) and gas chromatography-mass spectrometry (GC-MS) analysis
GC analysis of the samples was done with a Agilent gas chromatograph (7890 B), equipped with a HP-5 fused silica capillary column (5% phenyl)-polymethyl siloxane stationary phase; 30 m × 0.32 mm internal diameter; film thickness 0.25 µm) and a flame ionization detector (FID). Hydrogen was the carrier gas at 1.7 mL min−1. Oven temperature was programmed from 60 °C to 240 °C with increase of 3 °C min−1 and final hold time of 2 min. Injector and detector temperatures were set at 280 °C and 290 °C, respectively. The samples (0.01 µl, neat for essential oil and 0.1 µl, diluted in hexane for absolute) were injected in the spit mode (1:100).
Gas chromatography-Mass spectroscopy of C. reflexa essential oil was performed on a Clarus 680 GC interfaced with a Clarus SQ 8C Quadrupole mass spectrometer of Perkin Elmer fitted with Elite-5 MS fused-silica capillary column (30 m × 0.25 mm i.d., film thickness 0.25 µm). Temperature of oven was planned from 60 °C to 240 °C, at 3 °C min-1to 270 °C at 5 °C min−1; Temperature of injector was 250 °C; transfer line and source temperatures were 220 °C; 0.03 µl neat of injection size; 1:50 split ratio; Helium as a carrier gas at 1.0 mL min−1;70 eV ionization energy and 40–450 amu mass range scan.
The constituents of the essential oil were identified on the basis of retention index, determined using a homologous series of n-alkanes (C7-C30 hydrocarbons, Supelco Bellefonte, PA USA), To compare data of sample and literature data from MS Library search (NIST and WILEY). The relative quantity of individual chemical compounds was determined by optimizing the peak area of GC (FID response) without employing correction factor (Adams, 2007).
2.5 Experimental animals
Present study was conducted on female Swiss albino mice (18–22 g). PSIT Kanpur has Animal House Facility to bred and maintain. The animals were fed with standard rodent pellet diet and water was given to the animals. Permission and approval for animal studies was obtained from CPCSEA (Reg. No. 1273/PO/RE/S/09/CPCSEA), Government of India through the Institutional Animal Ethics Committee.
2.6 Cell viability assay
Cell viability was determined by using MTT assay. RAW 264.7 were washed by buffer (PBS), decant the solution and seeded the cell in DMEM in 96-well microplates for 24 hrs. at 8X 104 densities per cell. Test samples of C. reflexa essential oil (CREO) 1,2 and5% were dissolved in DMSO. After incubation of 48 h 10 µl of MTT mg/mL in a PBS buffer were added again plates were incubated at 37 ͦC for 4 h., removed the medium and dissolved formazan crystals in 100 mL DMSO. ELISA plate reader from BioRad was used to determine the optical density at 540 nm. Cell viability was calculated by following equation from six replicate well.
2.7 In-vivo study
Swiss albino female mice (7–8 week; 25 ± 2 g) were acclimatized in controlled environmental conditions (55 ± 10% RH and 12 h day/ night cycle), 7 days before commencement of experiment. During experiment mice were divided into six group (n = 6). Right ear of the mouse was used for testing. Sample was applied topically followed by TPA after 30 min (Yadav et al., 2009). Experiment were performed under the guidelines of Institutional Animal Ethics Committee approved by CPCSEA, Government of India.
2.8 Acute dermal irritation test
Determination of acute dermal irritation study was performed on the swiss albino mice. 1-inch square area was shaved on (n = 6). 5% sample was applied on the shaved skin while acetone as the vehicle. Assessment of dermal vulnerability, itchiness, redness, skin irritation and skin reactions were observed at 0, 4, 24, 48, 72 h. Skin oedema and erythema were recorded on the basis of arbitrary grading scale of 1–5. For ear erythema the score was taken as 1erythema formation, 2 very slight erythema (barely perceptible), 3 average erythema, 4 well-developed erythema, 5serious erythema (meat redness). Similarly, weight of both the ears (left & right) were taken and calculate the difference. On last day 6 hr after treatment the animals were sacrificed by cervical dislocation method and ears of each mice were collected. With the help of 8 mm biopsy punch ears were excised, weighed and calculate the difference. Right ear part of every ear was retaining in PBS buffer (PH 7.4) till further experiment. For ear oedema,1 no oedema, 2 very slight oedema, 3 well-defined oedema, 4 moderate oedema, 5severe oedema (beef raise than 1 mm). Similarly, primary irritation score was assessed for all animals of the study by cumulative irritation index (Aroonrerk and Kamkaen, 2009).
2.9 TPA induced skin inflammation in mice
Present study was done as described in Kumar et al., 2018. TPA (disease control) was applied to both the ears (inner and outer side) as 2.5/ear solubilize in 20 µl acetone. Test sample of C. reflexa essential oil (CREO) along with positive control was applied on the ear after 30 min of TPA application. All the test samples and positive control sample should be properly dissolved in acetone. In vehicle control group only, acetone was applied whereas in toxin group TPA was introduced only and maximum inflammation was observed.
2.10 Tissue weight measurement
Thickness of ear was measured by digital micrometer before and after induction of inflammation. After sacrificing the mice 6.5 mm diameter disc was used to remove ear by help of metal punch and weighed ear. Tissue (ear) homogenates (10% PBS) were prepared collect the supernatant and proceed for further investigations for the effect of CREO for pro-inflammatory cytokines.
2.11 Evaluation of pro-inflammatory cytokines
Previously collected mice ear was homogenized by using tissue homogenizer (Pro 200; Pro Scientific, USA) with 10% PBS and placed in cold ice and centrifuged for 15 min at 4 °C and 10,000 rpm. Supernatant fluid was collected for further experiments.
Estimation of cytokines TNF- α, IL-6 and IL-1β were done as prescribed in the ELISA kits (Thermo Scientific, USA) (Deepika et al., 2014).
2.12 Measurement of malondialdehyde (MDA) and nitric oxide (NO) production
-
Lipid peroxidation assay: 200 μl of w/v was mixed with 200 μl of ear supernatant followed by mixing. Prepared solution was centrifuged for 5 min at 5000 rpm 200 μl aliquot solution was taken and 0.67% TBA inequal amount was added. Boil the solution on water bath for 10 min. Absorbance was measured at 600 and 535 nm including blank with TCA and TBA
-
75 μl of Griess reagent taken with equal amount of ear supernatant in a micro titer plate and mix well followed by incubation for 10 min at 37 °C. At 540 nm absorbance was taken and nitrite concentration was determined by standard curve plotted by sodium nitrite serial dilution method
2.13 Histopathology
Ear of animals was kept in 10% formalin solution. Each sample was treating with xylene and then fixed with paraffin. Cut into section of 5 µm and stained with hematoxylin-eosin and examined the image under light microscope at magnification of 10X and 40X for finding the inflammatory cellular response.
2.14 Statistical analysis
Experimental data was recorded as mean ± standard error. One-way analysis of variance (ANOVA) was used to summarize the results. GraphPad PRISM® version 5.01(GraphPad Software, Inc., USA). p < 0.05 was considered indicative of significance.
3 Results
3.1 GC and GCMS analysis
A total of 29 chemical constituents were identified in the seed essential oil by retention index and mass spectral data (Fig. 1). The concentrations of the volatile components are presented in Table 1, according to their elution order on an HP-5 fused silica capillary column. Components of the seed essential oil were Hexahydro farnesyl acetone (14.2%), β-Bisabolene (13.7%), and Carotol (9.8%), cis-Chrysanthenyl acetate (4.7%), Caryophyllene oxide (4.9%), α-Asarone (3.0%), trans-α-Bergamotene (3.2%), (E)-caryophyllene (3.1%).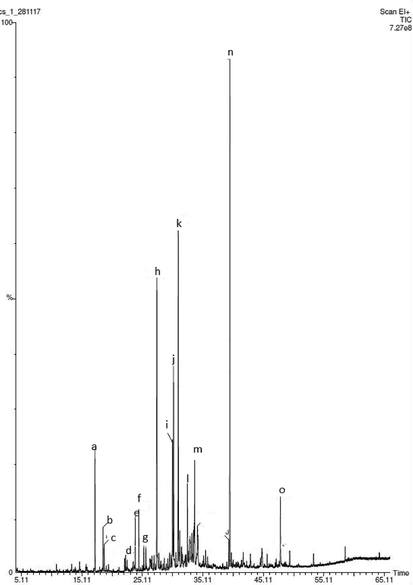
Gas chromatographic profile (TIC) of C. reflexa essential oil as a) Cis-chrysanththenol acetate, b) trans-sabinyl acetate, c) carvacrol, d) (E)-β-Damascenone, e) (E)-caryophyllene, f) α-trans-bergamotene, g) (E)-β-farnesene, h) β-Bisabolene, i) spathnlenol, j) caryophyllene oxide, k) carotol, l) Daucol, m) α-asarone, n) Hexahydrofarnesyl acetone, o) (E)-phytol.
S.no.
Compound
RIa
RIb
Content
(%)
1.
Linalool
1100
1095
0.3
2.
cis-Chrysanthenol
1164
1160
0.2
3.
α-Terpineol
1193
1186
0.2
4.
cis-Chrysanthenyl acetate
1264
1261
4.7
5.
trans-Sabinyl acetate
1294
1289
2.1
6.
Carvacrol
1300
1298
0.4
7.
Isoledene
1383
1374
0.7
8.
(E)-β-Damascenone
1388
1383
0.8
9.
β-Cubebene
1392
1387
0.2
10.
(E)-caryophyllene
1426
1417
3.1
11.
trans-α-Bergamotene
1439
1432
3.2
12.
(E)-β-Farnesene
1459
1454
0.9
13.
Germacrene D
1489
1480
1.5
14.
(E)-Methyl iso-eugenol
1495
1491
0.7
15.
β-Bisabolene
1512
1505
13.2
16.
δ-Amorphene
1518
1511
0.7
17.
Spathulenol
1583
1577
3.2
18.
Caryophyllene oxide
1590
1582
4.9
19.
Salvial-4(14)-en-1-one
1600
1594
0.3
20.
Carotol
1605
1594
9.8
21.
Daucol
1650
1641
2.0
22.
14-Hydroxy-9-epi-(E)-caryophyllene
1671
1668
0.4
23.
α-Asarone
1681
1675
3.0
24.
α-Bisabolol
1695
1685
0.6
25.
Hexahydrofarnesyl acetone
1846
1847
14.2
26.
(5E,9E)-Farnesyl acetone
1905
1913
0.7
27.
(E)-Phytol
2117
2112
2.0
28.
n-Tricosane
–
2300
0.9
29.
n-Pentacosane
–
2500
0.9
3.2 Cytotoxicity assessment
The cell viability of RAW 264.7 macrophages was evaluated. Fig. 2 shows that CREO at concentrations of 1%, 2% and 5% did not affect cell viability. There is no significant change in live cell population when compared with normal control.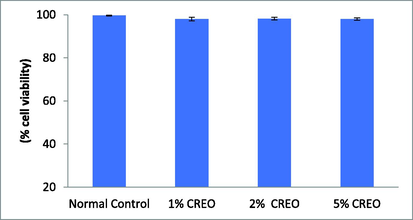
Cell viability of CREO using MTT assay in RAW 264.7 Data are mean ± SEM; n = 3 normal versus test.
3.3 In-vivo anti-inflammatory
3.3.1 Acute dermal irritation
Impact of CREO on TPA-induced odeomatogenic response was noticed in TPA induced ears of mice after 6 hrs. In present study reduction in skin inflammation is shown in time dependentmanner reported as 38.51, 43.64, 52.32% decreased in weight of ear was recorded. 5% sample showed marked decreased in edema as compared to the toxin control group (TPA).
3.3.2 TPA induced skin inflammation
Initially no erythema on the ear of animals was observed. After 4, 24, 48, and 72 hrs of treatment significant increase in auricle blood flow was recorded as compared to the vehicle control as 0.33 and 0.5 respectively. Ear thickness was recorded before and after application of TPA at the duration of 4 and 24 h with acetone. The vehicle and positive control group did not showed edema when compared to toxin group. Ear weight 6.5 mm discs biopsy punch are used to calculate the weight of each ear and found that there is tremendous increased in weight in TPA induced group while reduction in positive control or also showed dose dependent decrease in the ear weight (Fig. 3).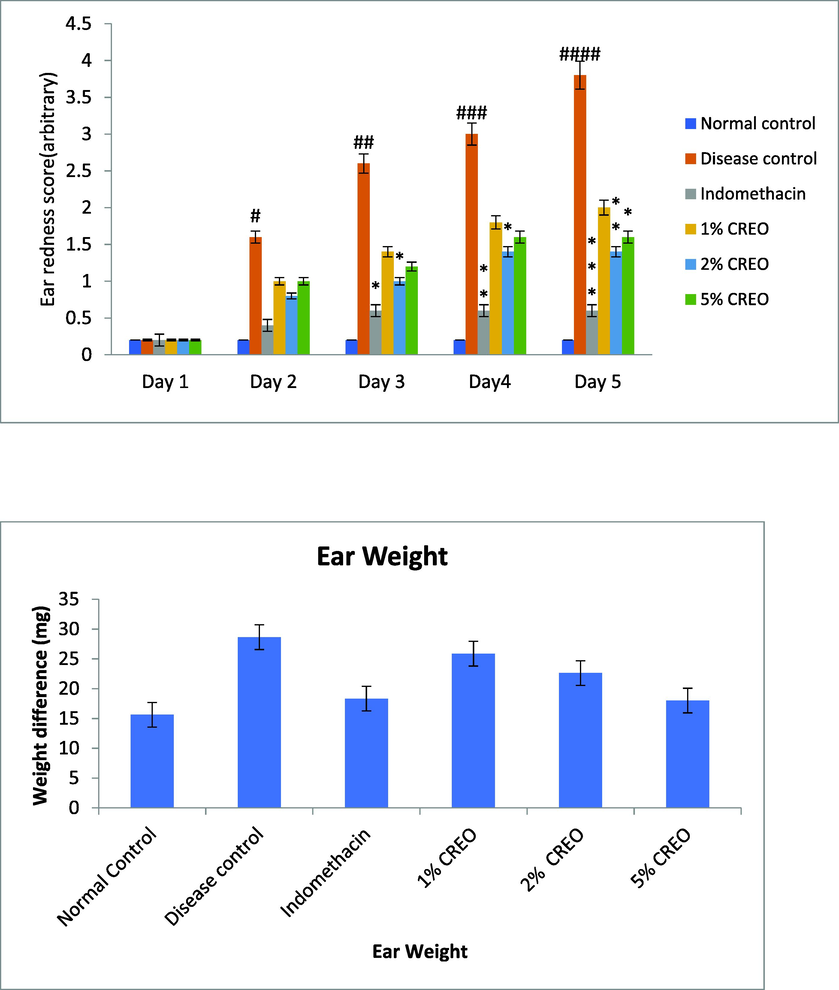
Effect of CREO on TPA-induced ear inflammation. Data are mean ± SEM; n = 5. (a) Ear redness, (b) Ear weight, ANOVA; Tukey test p < 0.05.
3.3.3 Measurement of oxidative stress markers
Lipid per oxidation and nitric oxide oxidative parameters are performed. The treatment of CREO showed dose dependent reduction in the level. Both the oxidative markers have significantly decrease the TPA induced activity.
Table 2. Data are mean ± SEM; N = 5. #Normal versus toxin, * Toxin versus treatment (ANOVA; Tukey test), p < 0.05.
Parameter
Normal control
Disease control
Indomethacin
1% CREO
2% CREO
5% CREO
LPO (μm/ml)
2.80 ± 0.50
20.85 ± 1.40
6.81 ± 0.60
9.81 ± 1.64
8.45 ± 1.63
6.17 ± 0.59
NO (μm/ml)
5.39 ± 0.85
32.18 ± 2.08
9.14 ± 0.84
20.64 ± 1.56
14.62 ± 1.04
7.56 ± 2.03
3.3.4 Measurement of IL-1β, IL-6, TNF-α level in serum
After the completion of experiment the serum was collected and measure IL-6, IL-1β, and TNF-α in mice serum by ELISA kits according to the instructions (Fig. 4). This experiment TPA induced group clearly showed increased in cytokines level whereas the concentration of 5% showed significantly reduced the in production of IL-6, TNF-α and IL1-β and these results were compared to TPA (negative) control and positive control groups (Table 3). Data are mean ± SEM; N = 5. #Normal versus toxin, * Toxin versus treatment (ANOVA; Tukey test), p < 0.05.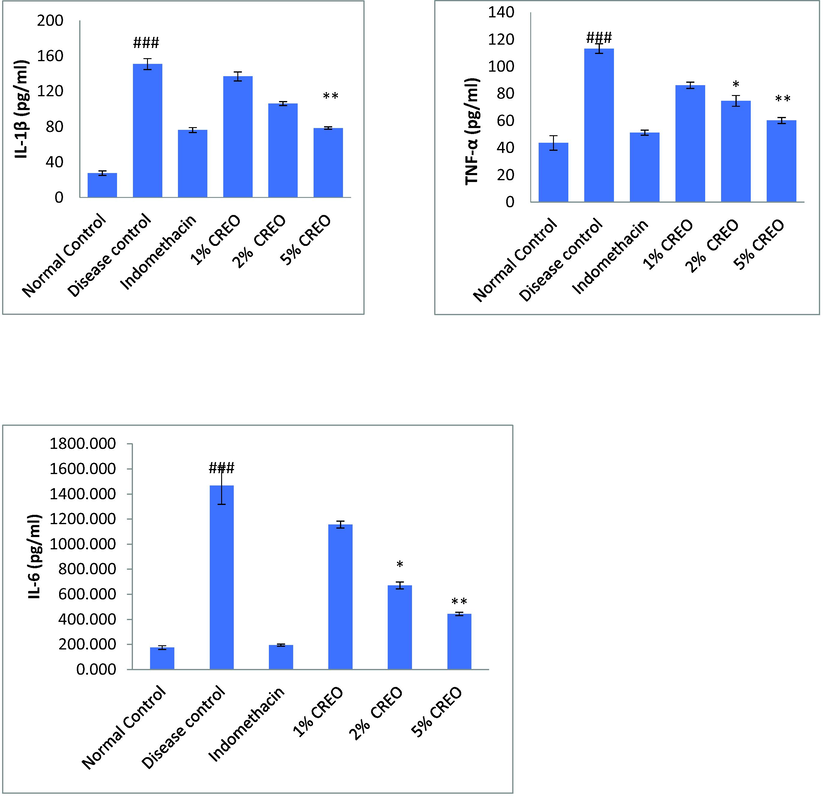
Effect of CREO on inflammatory mediators in the ears tissue isolated from TPA induced inflammation. Data are mean ± SEM; n = 5 #Normal versus disease control, *Disease control versus treated, ANOVA; Tukey test p < 0.05.
Parameter
Normal control
Disease control
Indomethacin
1% CREO
2% CREO
5% CREO
TNF- α
43.64 ± 5
113.34 ± 3
51.28 ± 1
86.18 ± 2
74.7 ± 3*
60.2 ± 2**
IL-1 β
27.65 ± 2
150.76 ± 6
51.27 ± 2
136.81 ± 5
106.24 ± 3
78.43 ± 1**
IL-6
175.37 ± 14
1466.08 ± 147
195.40 ± 8
1156.18 ± 27
671.18 ± 27*
443.07 ± 13**
3.4 Histopathology
In present study the vehicle used during the experiment showed no effect in the cutaneous morphology of the ear of mice, while the TPA control group showed severe increase in the diameter of ear. Leucocyte infiltration and ear edema are observed vigorously but after the treatment with 5% CREO there is significantly reduce in the elevated interleukins Fig. 5.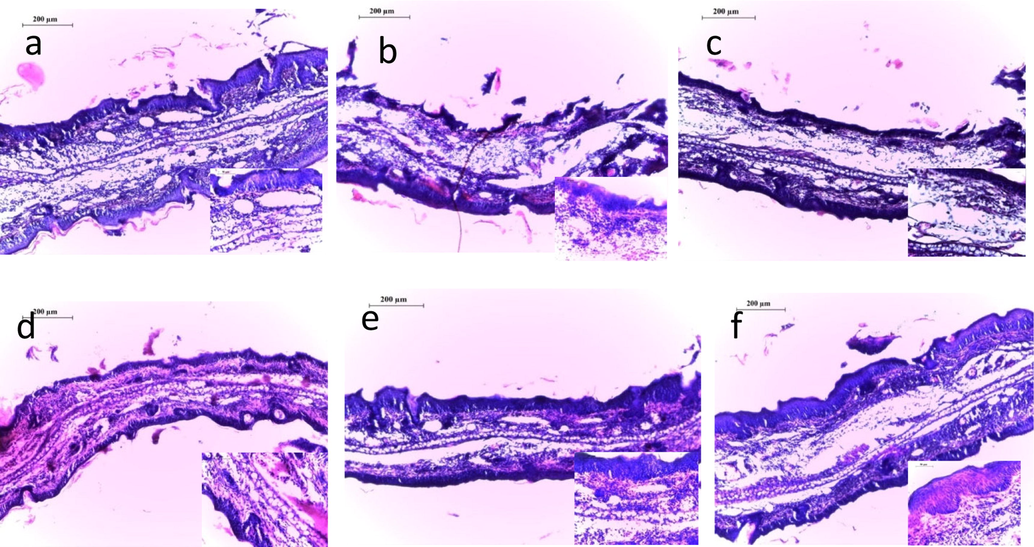
Histopathological profile of ear tissues under microscopy (a) Normal control, (b) Disease control, (c) Indomethacin, (d) 1%CREO, (e) 2%CREO (f) 5%CREO.
4 Discussion
C. reflexa have many medicinal properties as reported in the ancient literatures its time of theflowering time is from March to April. Here we isolate the seed part and investigate for anti-inflammatory activity. (Muhammad et al., 2021). The plant seed has ability to survive in dormant condition upto20 years and regrow in favorable condition. They have no roots but its seed grows into host plant, after sometimes it destroys the host plant fully and covers large area. According to literature itdestroys many fields of farmers. It is a global problem in agriculture. But it has many benefits as claimed by traditional literatures like anti-inflammatory agent used by Unani physician since long time. Traditionally this whole plant is used in muscular pain, liver diseases, depression, fever, urination disorders, mental disorders, skin diseases in Ayurvedic system of medicine (Ansari et al., 2020). One of the researchers investigated for analgesic and anti-inflammatory activities of the plant and exhibited significant anti-inflammatory effect against carrageenan induced paw edema in methanolic and aqueous fraction (Sahaet al., 2016). In another study a compound terrestrosin D showed marked reduction in inflammation and fibrosis in the lungs of mice when exposed to bleomycin (Qiu et al., 2019). Methanolic and aqueous extract of C. reflexa was determined for anti-inflammatory activity by carrageenan and histamine induced paw edema models and found effective at the dose of 300 mg/Kg p.o (Suresh et al., 2011).
CREO at various concentrations are tested for anti-inflammatory activity. Inflammation, basic response of any type of injury is to protect the body. Excretion of inflammatory mediators like IL-1β, IL-6 and TNF-α inhibits pro-inflammatory mediator synthesis and elevates anti-inflammatory cytokines. In order to promote cascade, TPA is applied to the mouse ear which increases vascular permeability, mast cell infiltration and edema. Histamine and serotonin released by mast cells which further elevate neutrophil influx and vascular permeability. Arachidonic acid released from phospholipid is caused by using TPA which further release inflammatory mediators. These inhibitors are active against edema and leukocyte infiltration in present TPA model of mouse ear inflammation. CREO contain linalool, carvacrol, β-bisabolene, spathulenol, phytol and many more compounds which shows antioxidant and anticancer activities.
In present study we found CREO possess the ability to reduce inflammation by inhibiting IL-1β, IL-6 and TNF-α inhibit by stimulated monosaccharide with lipo-polysaccharide and nitric oxide. Increase in lipo-polysaccharide and involvement in redox-sensitive steps enhance nuclear factor ĸβ activation that is why CREO shows anti-inflammatory action against TPA induce ear mouse model. Thus, we can say that C. reflexa essential oil significantly reduces inflammation by reduction in inflammatory responses against TPA in skin. By using these results one can further use the oil for preparation of pharmaceutical product for anti-inflammatory purpose.
5 Conclusion
The present study significantly demonstrates the chemical profiling of volatile oil of C. reflexa seed and also first time for anti-inflammatory activity and found that the essential oil of this plant is helpful in the reduction of inflammation at the concentration of 5%. This may be an important finding for patient and farmers both. As this plant is known as weed so, from this result we can say that it can be used in formulation in future.
Acknowledgements
This work was funded by Researcher Supporting Project number RSP2023R26, King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Adams, R.P., 2007. Allured Publishing Corp Carol Stream, Illinois, USA https://www.researchgate.net/publication/283650275.
- Andriamaharavo, N.R., 2014. Retention Data. NIST Mass Spectrometry Data Center. NIST Mass Spectrometry Data Center.
- Aftimoon (Cuscuta reflexa Roxb.): A Parasitic Plant with Therapeutic Potentials, Acta Scientific. Pharmaceut. Sci.. 2020;4:90-97.
- [CrossRef] [Google Scholar]
- Anti-Inflammatory Activity of Quercus infectoria Glycyrrhiza uralensis, Kaempferiagalanga and Coptischinensis, the Main Components of Thai Herbal Remedies for Aphthous Ulcer. J. Health Res.. 2009;23:17-22.
- [Google Scholar]
- The biology of canadian weeds. 133. Cuscuta campestrisYuncker, C. Gronovii Willd. Ex Schult., C. Umbrosa Beyr. C. epithymum (L.) and C. epilinum Weihe. Canadian Journal Plant Science 2005:293-316. doi/pdf/10.4141/P04-077
- [Google Scholar]
- Exploration of anti-inflammatory activity of turmeric and onion combination on phorbol ester induced ear inflammation in mice. Ann. Phytomed.. 2014;3(2):50-54.
- [Google Scholar]
- Development of RAPD markers for authentication of medicinal plant Cuscuta reflexa, Eurasia. J. Biosci.. 2010;4:1-7.
- [CrossRef] [Google Scholar]
- Traditional uses, Chemistry and Pharmacological activities of Cuscuta reflexaRoxb: A Compendious Review. Int. J. Sci. Res. Rev.. 2018;7:685-693.
- [Google Scholar]
- Local control of the immune response in the liver. Immunol. Rev.. 2000;174:21-34.
- [CrossRef] [Google Scholar]
- Essential oil from waste leaves of Curcuma longa L. alleviates skin inflammation. Inflammopharmacology. 2018;26:1245-1255.
- [CrossRef] [Google Scholar]
- Effects of volatile oils of Angelica sinensis on an acute inflammation rat model. Pharm. Biol.. 2016;54:1881-1890.
- [CrossRef] [Google Scholar]
- Evaluation of the anti-diarrheal effects of the whole plant extracts of Cuscuta reflexa Roxb in pigeons. Toxicol. Rep.. 2021;8:395-404.
- [CrossRef] [Google Scholar]
- Volatile analysis and antimicrobial screening of the parasitic plant Cuscuta reflexa Roxb from Nepal. Nat. Prod. Res.. 2014;28:106-110.
- [CrossRef] [Google Scholar]
- Terrestrosin D from Tribulus terrestris attenuates bleomycin-induced inflammation and suppresses fibrotic changes in the lungs of mice. Pharm Biol. 2019;57:694-700.
- [CrossRef] [Google Scholar]
- Natural products from Cuscuta reflexa Roxb. with antiproliferation activities in HCT116 colorectal cell Lines. Nat. Product Res.. 2017;31:538-587.
- [CrossRef] [Google Scholar]
- Evaluation of analgesic and anti-inflammatory activity of Cuscuta reflexa extracts on animal models. Indian Drugs. 2016;53:54-62.
- [CrossRef] [Google Scholar]
- A parasitic medicinal plant Cuscutareflexa: An overview. Int. J. Sci. Eng. Res.. 2015;69:951-959.
- [Google Scholar]
- Ethnobotanical literature survey of three Indian medicinal plants for hepatoprotective activity. Int. J. Ayurveda Pharm. 2013;4:378-381.
- [CrossRef] [Google Scholar]
- Comparative study of Cuscuta reflexa and Cassytha filiformis for diuretic activity. Pharmacognosy Res.. 2009;1:327-330.
- [Google Scholar]
- In vitro anti-inflammatory and anti-cancer activities of Cuscuta reflexa Roxb. J. Ethnopharmacol.. 2011;134:872-877.
- [CrossRef] [Google Scholar]
- Topical antiinflammatory effects of Ocimum basilicum leaf extract in the phorbol-12,13-dibutyrate model of mouse ear inflammation. Planta. Med.. 2009;75:923.
- [CrossRef] [Google Scholar]
Further reading
- Anti-inflammatory effect of Leucas cephalotes Spreng in 12-O-tetradecanoylphorbol-13-acetate-induced inflammation in mice. Annals of Phytomedicine. 2012;1:62-66.
- [Google Scholar]







