Translate this page into:
Evaluation of calcium, phosphorus and soil organic matter effects on lead accumulation in Cenchrus ciliaris L. grown near a cement factory in Jazan region, Saudi Arabia
⁎Corresponding author. amasrahi@jazanu.edu.sa (Abdurrahman S. Masrahi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Heavy metals associated with cement production and their environmental impacts have received much attention recently. Lead (Pb) is the second most hazardous element after arsenic (As). However, information about its availability and uptake by plants in polluted soil as influenced by soil properties has shown some contradictions. We evaluated the influence of soil calcium (Ca), soil phosphorus (P), and soil organic matter (SOM) on Pb uptake by Cenchrus ciliaris L. based on distance from cement dust polluted soil near a cement factory in Jazan region, Saudi Arabia. We also evaluated the potential of Cenchrus ciliaris L. as a hyperaccumulator of Pb. Soil and plant samples were collected in triplicates from three zones based on distance from the fence of the factory: 10 – 100, 100 – 350, and 350 – 700 m. Concentrations of total Pb, total Ca, and total P in soil and plant tissues were detected using ICP-AES. SOM and other soil properties were also analyzed. Bioconcentration and translocation factors (BF&TF) of Pb were also calculated. Results showed that Pb concentration in Cenchrus ciliaris roots had a strong negative relationship with SOM (R2 = 0.93) and P concentration in soil (R2 = 0.97) but a moderate negative relationship with Ca concentration in soil (R2 = 0.69). The highest TF value was 1.87. It can be concluded that SOM, Ca, and P concentrations in soil negatively affected Pb availability and uptake by Cenchrus ciliaris. Also, based on the highest TF value, Cenchrus ciliaris could be used as a hyperaccumulator in Pb-contaminated soils.
Keywords
Heavy metals
Cenchrus ciliaris L.
Bioconcentration factor
Translocation factor
Lead
Cement polluted soil
1 Introduction
Cement production is a major anthropogenic source of particulate matter (PM), greenhouse CO2 emission, and heavy metals released into the environment. The majority of heavy metals in cement dust contain arsenic (As), lead (Pb), chromium (Cr), cadmium (Cd), copper (Cu), nickel (Ni), mercury (Hg), and zinc (Zn) (Addo et al., 2012; Dong et al., 2015). Heavy metal pollution is a significant global environmental risk to human health and food security due to its persistence in the environment and bioaccumulation in the food chain (Peralta-Videa et al., 2009; Wuana and Okieimen, 2011). Therefore, the environmental impacts of cement production, especially the heavy metal emissions with respect to their toxicity and persistence, have received more attention recently (Li et al., 2019; Olatunde et al., 2020). Among heavy metals, Pb is the second most hazardous element after As, according to the Agency for Toxic Substances and Disease Registry (Agency for Toxic Substances and Disease Registry, 2015). Since many Pb pollutants are becoming essential concerning modern human life, soil contamination with Pb is more likely to persist in the environment (Yang et al., 2000).
Although Pb is considered an unessential element for plants, it can easily get absorbed and accumulated in different plant organs. In soils, a significant increase of Pb content in the surface layer has been noticed near industries and roads (De Abreu et al., 1998; Jankowski et al., 2019). However, the bioavailable portion of Pb is challenging to measure reliably since it usually accumulates on the soil surface. Its availability varies in soil and is controlled by different soil properties, such as organic matter content, pH, texture, phosphorus (P) or carbonate precipitates, cation exchange capacity, surface-specific area of soil particles (Sharma and Dubey, 2005), and calcium (Ca) concentration (Simon, 1978). In the case of Ca, some studies evaluated its relationship to Pb availability and toxicity or Pb tolerance in plants using hydroponic cultures (Garland and Wilkins, 1981; Antosiewicz, 2005). These studies demonstrated that increased Ca concentration reduced either Pb uptake or Pb toxicity in plants.
On the other hand, studies on Pb plant uptake from soils (Simon, 1978) or the relationship between Pb and other soil properties (Blaylock et al., 1997; Lee, 1998; Sauve et al., 1998) have shown some contradictions. Simon (1978) found that the Pb2+/Ca2+ ratio strongly controlled Pb toxicity and tolerance to plants (with variations based on vegetation structure). However, no correlation was noticed between soil organic matter (SOM) and Pb. On the contrary, Lee et al. (1998) found that Pb adsorption into soil particles (less available to plants) increased with increasing SOM and pH between pH 3 and 8.5. Nevertheless, Sauve et al. (1998) reported that the solubility of Pb decreased from pH 3 to pH 6.5 and was independent of SOM. However, they later found that in the pH range of 6.5 to 8, the formation of Pb-organic complexes increased and that higher SOM in this pH range resulted in higher dissolved Pb in soil. Therefore, variations in the relationship between Ca and Pb availability in soil and its uptake by plants are poorly understood and are much controlled by a complex interaction between soil properties. In addition, variation in plant uptake and accumulation of Pb is species-specific (Pourrut et al., 2011).
Cenchrus ciliaris L. (also known as Buffel grass) is a vigorous C4 perennial grass common in tropical and subtropical arid rangelands due to its excellent drought tolerance (Marshall et al., 2012). Recently, Cenchrus ciliaris has been reported as a potential hyperaccumulator of heavy metals (Kumar and Fulekar, 2018). Therefore, the goals of this study were: 1) to evaluate bioaccumulation of Pb by Cenchrus ciliaris L. as influenced by soil Ca, soil P, and SOM along with other soil properties based on distance from cement dust-polluted soil in the Jazan region, south of Saudi Arabia. 2) to evaluate the potential of Cenchrus ciliaris L. as a hyperaccumulator of Pb in the cement-polluted area.
2 Material and methods
2.1 Site description
The study site is located in a vicinity of a cement factory built in the Jazan region, south of Saudi Arabia. The factory was built in 1981 in Ahd-El-Masarha, 70 km southwest of Jazan city (16o44′07.5″ N latitude and 43o03′03.7″ E longitude). The land is mostly plain with some hills. The cement factory had been built in the area due to its richness of limestone rocks. Thus, the dust exhausted from the factory is a complex mixture of heavy metals, including Pb.
The location of study area is close to the Red Sea shores (around 35 km). The region in the summer comes up with storms of dust that are followed by heavy rains. The average annual humidity is 68%, the average temperature is 30.4 °C, and the average rainfall is 139.7 mm (Dabbagh and Abderrahman, 1997; Masrahi et al., 2017; Tounekti et al., 2018). It is noteworthy that the land around and near the factory is a grazing plain where goats, camels, and cows feed on the grass, shrubs, and trees. The plain is rich with grasses in cold seasons, attracting livestock breeders' attention.
2.2 Experimental design
The study area was divided into three zones based on distance, starting from the factory's fence: 1st zone: 10 – 100 m, 2nd zone: 100 – 350 m, 3rd zone: 350 – 700 m. Samples from each zone were collected in 3 replicates. From each zone, sample replicates were collected in ascending order by distance, giving three sub-zones within each zone. The soil samples within each zone were collected randomly toward the western, southwestern, and northwest directions from the factory's fence. The prevailing wind direction in the region varies throughout the year but mainly toward the west and east directions (Youssef et al., 2012).
2.3 Soil and plant samples
Around 200 g of surface soil (0–20 cm) was collected from each zone as described above. For Cenchrus grass, entire plants (shoot and roots) were collected from each zone. To ensure capturing the whole root system, we sampled a core surrounding each bush with a depth of 10 cm. Plant and soil samples were placed in nylon bags and transformed into the lab. Shoot and roots were separated, washed with distilled water, and dried up at 40 °C until a stable weight was obtained. Similarly, soil samples were dried as described above. Shoot and root samples were ground into powder.
2.4 Soil physical and chemical properties
A portion of each soil sample was used for physical and chemical analysis. These analyses include soil particle sizes, pH (1:2.5 soil: water ratio), water holding capacity (WHC), soil organic matter (SOM), electrical conductivity (EC), and total dissolved solids (TDS) (Wilde et al., 1979).
2.5 ICP-AES analysis of Pb, Ca, and phosphorus
Concentrations of total Pb, total Ca, and total P were detected using an inductively coupled plasma atomic emission spectrometry (ICP-AES, ICAP-6300, Thermo Fisher Scientific). Briefly, one to two grams of plants and soils were digested in 2.5 ml/ conc. Nitric acid and 7.5 ml/conc. hydrochloric acid. Samples were filtered through Whatman filters (Ashless, Diameter 125 mm). Additional HCL (5 ml) and reagent water (20 ml) were added to the samples for washing the filters according to a standard method (METHOD 3050B) (EPA, 1996).
2.6 Bioconcentration and translocation factors
Bioconcentration and translocation factors (BF and TF, respectively) were calculated according to Yoon et al. (2006) and Singh et al. (2010). BF is a ratio that measures the ability of a plant to accumulate a particular metal concerning its concentration in soil (Kumar and Fulekar, 2018), which was calculated with the following formula:
Where C represented the heavy metal concentration. TF represented the relative translocation of metals from roots to leaves of a plant (Kumar and Fulekar, 2018) and was calculated using the following formula (where C = heavy metal concentration):
2.7 Statistics and Principal Component Analysis (PCA)
Linear regression was used to understand the relationship between each pair of variables. In addition, data were also visualized by Principal Component Analysis (PCA) to understand the relationship among multiple variables simultaneously. All data were analyzed using XLSTAT (2022).
3 Results
3.1 Soil physical and chemical properties
Soil's physical and chemical characteristics are listed in Table 1. The soil in the closest zone to the factory (10 – 100 m) had a high percentage of large soil particles (4000 µm) compared to the other two zones (100–350 and 350 – 700 m, respectively). Moreover, the middle zone (100–350 m) had the highest percentage of particles < 125 µm compared to the closest and farthest zones from the factory (10–100 and 350 – 700 m, respectively). Soil WHC matched with soil particle size percentage, as the highest WHC (45 %) was in the middle zone, with the highest percentage of particles < 125 µm compared to the other two zones. Soil pH, TDS, and EC decreased with increasing distances. However, SOM increased with increasing distances.
Zone
Soil particle size
pH
SOM (%)
TDS (ppm)
EC
(mScm−1)
WHC (%)
4000 µm
2000 µm
500 µm
250 µm
125 µm
>125 µm
(10–100 m)
28.6 ± 2.9
13.5 ± 0.9
18.6 ± 0.9
8.2 ± 0.5
12.7 ± 2.7
17.5 ± 3.0
6.1 ± 0.9
4.2 ± 0.8
322 ± 1.6
123.7 ± 4.1
18 ± 1.0
(100–350 m)
2.9 ± 0.9
10.5 ± 2.5
28.4 ± 1.1
9.5 ± 0.8
10.3 ± 0.5
35.2 ± 1.7
5.7 ± 1.0
7 ± 1
92.1 ± 0.0
4.9 ± 3.5
45 ± 0.8
(350–700 m)
6.0 ± 1.8
14.1 ± 0.8
23.1 ± 0.6
15 ± 2.8
18.5 ± 0.7
3.5 ± 0.3
5.8 ± 0.6
8.6 ± 3.3
4.6 ± 0.0
0.66 ± 0.12
31.5 ± 13.5
3.2 ICP-AES analysis of Pb, Ca, and P
Concentrations of Pb, Ca, and P in both soil and plant tissues are listed in Table 2. For soil Pb, the highest concentration was found in the closest zone to the factory, followed by the farthest and middle zones, respectively. The concentration of Pb in roots decreased with increasing distance. However, in shoots, Pb concentrations increased with increasing distance. Similarly, Ca concentrations in soil increased with increasing distance. In contrast, in the shoot, the highest Ca concentration was found in the closest zone, followed by the farthest and middle zones. P concentrations in both soil and shoot increased with increasing distance.
Zone
Pb in soil (ppm)
Pb in root (ppm)
Pb in shoot (ppm)
Ca in soil (ppm)
Ca in shoot (ppm)
P in soil (ppm)
P in shoot (ppm)
(10–100 m)
1.34 ± 0.02
1.32 ± 0.79
0.32 ± 0.1
115.70 ± 15.1
215.60 ± 81
0.39 ± 0.05
16.85 ± 5.8
(100–350 m)
1.05 ± 0.37
1.01 ± 0.84
0.40 ± 0.22
179.92 ± 143
158.77 ± 23.7
0.96 ± 0.5
23.94 ± 20.7
(350–700 m)
1.25 ± 0.55
0.56 ± 0.04
0.99 ± 0.93
183.70 ± 139
203.57 ± 83.8
1.42 ± 0.14
41.26 ± 7.3
3.3 Simple linear regression and principal component analysis (PCA)
The simple linear regression for the relationship between different variables is shown in Fig. 1. Ca concentration in soil and Pb concentration in root showed a moderate negative relationship (R2 = 0.69) (Fig. 1A). In contrast, a moderate positive relationship seems to exist between Ca concentration in soil and Pb concentration in the shoot (R2 = 0.4) (Fig. 1B). Soil pH seems to have a strong positive relationship with Pb concentration in soil (R2 = 0.78) (Fig. 1C), but a week relationship with Pb concentration in the shoot (R2 = 0.14) (Fig. 1D). However, Pb concentration in the shoot had a solid negative relationship with Pb concentration in root tissues (R2 = 0.91) (Fig. 1E). Moreover, a strong positive relationship existed between Pb/Ca soil ratio and soil pH (R2 = 0.99) (Fig. 1F). For SOM, it had a strong positive relationship with Pb concentration in the shoot (R2 = 0.71) (Fig. 1G), and a robust negative relationship with Pb concentration in the root (R2 = 0.93) (Fig. 1H). A strong negative relationship exists between Pb concentration in root and soil P concentration and soil TDS (R2 = 0.97 and R2 = 0.96, respectively) (Fig. 1I and Fig. 1J, respectively).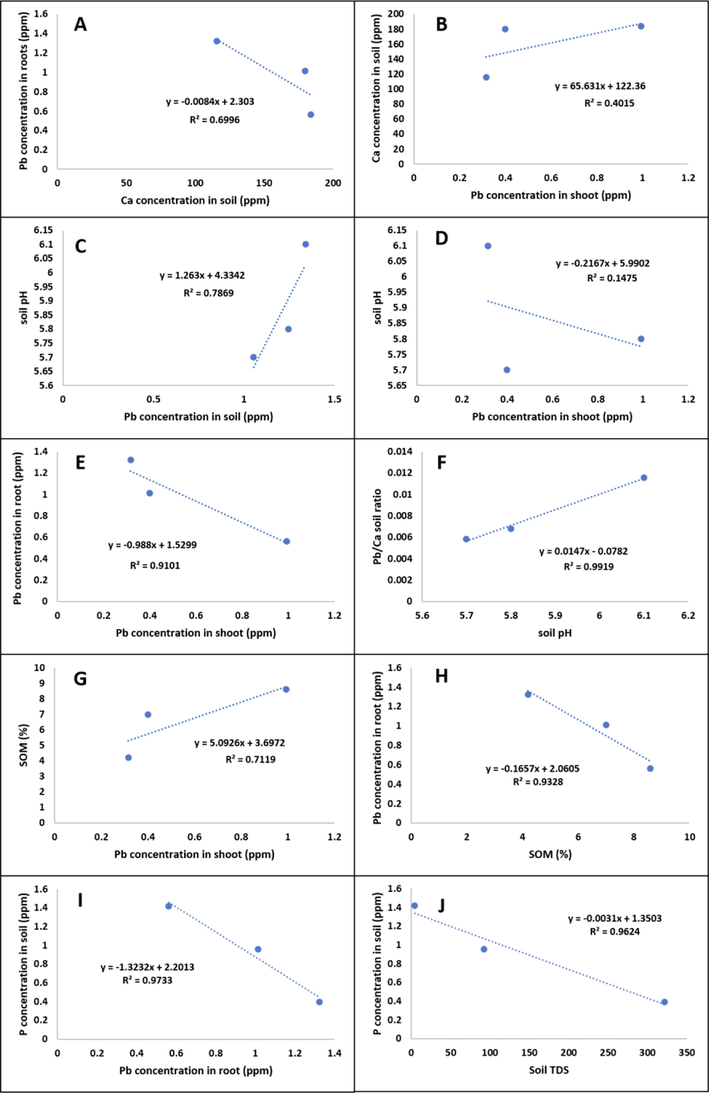
Simple linear regression showing relationships between pairs of different variables. A: Pb concentration in root vs Ca concentration in soil. B: Ca concentration in soil vs Pb concentration in shoot. C: soil pH vs Pb concentration in soil. D: soil pH vs Pb concentration in shoot. E: Pb concentration in soil vs Pb concentration in shoot. F: Pb/Ca soil ratio vs soil pH. G: SOM vs Pb concentration in shoot. H: SOM vs Pb concentration in root. I: P concentration in soil vs Pb concentration in root. J: P concentration in soil vs soil TDS.
For PCA (Fig. 2), visualization of all variables showed that the two principal components account for 75.97 % and 24.03 % of the variance, respectively (100 % in total). The PCA plot revealed an apparent clustering of SOM %, P concentration in soil and shoot, and Pb concentration in the shoot in the first quadrant (narrowed angles reflect a strong positive correlation). Ca concentration in soil and WHC were clustered in the second quadrant, while soil TDS and Pb concentration in the root were clustered in the third quadrant (negatively correlated with the first quadrant). Ca concentration in the shoot, Pb concentration, pH, and Pb/Ca soil ratio were all clustered in the fourth quadrant (negatively correlated with the second quadrant).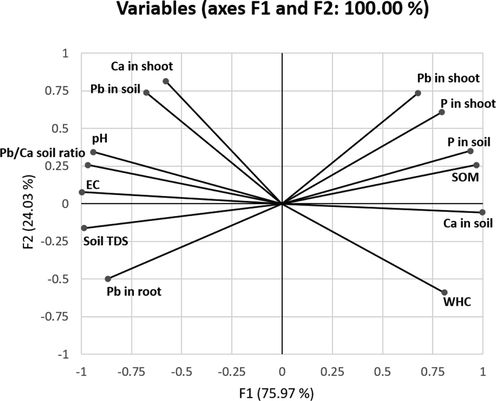
Principal component analysis (PCA) plot showing relationship among multiple variables simultaneously. Pb: Pb concentration. Ca: Ca concentration. P: P concentration. SOM: soil organic matter %. WHC: water holding capacity %. EC: electrical conductivity. TDS: total dissolved solids.
3.4 Bioconcentration and translocation factors
The results of BF and TF are listed in Table 3. The highest BF of Ca was found in the closest zone to the factory (BF = 1.8) but steady with the other two zones. However, the BF of Pb had a decreasing pattern with distance from the factory. On the contrary, TF of Pb showed an increasing pattern with distance from the factory.
Zone
BF of Ca
BF of Pb
TF of Pb
(10–100 m)
1.80 ± 0.5
0.97 ± 0.6
0.37 ± 0.3
(100–350 m)
1.20 ± 0.6
0.93 ± 0.5
0.67 ± 0.5
(350–700 m)
1.20 ± 1.6
0.53 ± 0.3
1.87 ± 1.7
4 Discussion
In this study, soil Pb concentration was positively correlated with Ca concentration in the shoot but had a moderate negative relationship with Ca concentration in soil. However, Pb concentration in Cenchrus ciliaris roots had a moderate negative relationship with Ca concentration in soil. Cenchrus ciliaris is a well-known drought-tolerant perennial grass that withstands heavy grazing and has a deep stabilizing root system (Marshall et al., 2012), which indicates its ability to tolerate nutrient deficiency. Our results support Antosiewicz (2005) findings that the correlation between Pb and Ca uptake and transport depends on the Ca deficiency tolerance of the plant. Their hydroponic study indicated that Pb uptake and transport to shooting in Ca deficiency tolerant plants did not depend on external Ca concentrations in the medium. However, in Ca deficiency non-tolerant plants, Pb concentrations in root and shoot increased with decreased external Ca content. Few studies have illustrated that the main pathway through which Pb enters Vicia faba and Zea mays L. roots are Ca2+ permeable channels (Wang et al., 2007; Pourrut et al., 2008).
Moreover, Garland and Wilkins (1981) and Kim et al. (2002) have demonstrated that the uptake of Pb can be inhibited by Ca in Hordeum vulgare L., Festuca ovina L., and rice roots. Thus, this evidence may explain our study's negative relationship between Ca concentration in soil and Pb concentration in Cenchrus ciliaris roots. However, in the soil environment, factors other than Ca have substantial roles in determining Pb uptake and accumulation by plants.
Our findings showed that the Pb/Ca soil ratio strongly positively correlated with soil pH (in the range of ∼ pH 5.7 – 6). In contrast, Pb root concentration strongly negatively correlated with SOM and P concentration in soil. These findings are controversial with other studies. For example, Simon (1978) reported that the Pb2+/Ca2+ ratio strongly controlled Pb toxicity, but no correlation was noticed with SOM. Lee et al. (1998) demonstrated that Pb availability decreased with increasing both SOM and pH (in the range between pH 3 and 8.5). Sauve et al. (1998) reported that the solubility of Pb decreased from pH 3 to pH 6.5 and was independent of SOM. However, from pH 6.5 to 8, there was a positive relationship between SOM and P solubility. However, these studies did not report P concentration in their soils or indicate its correlation with Pb. It is well-documented that P can significantly reduce Pb solubility by forming Pb-phosphate complexes such as pyromorphites, which are the most insoluble form of Pb in soils (Blaylock et al., 1997; Hettiarachchi et al., 2001). Therefore, it is possible in our study that pH directly impacted P solubility in soil and that with increased P concentration in soil, Pb became less available to plants (due to the formation of P-Pb complexes). This can also be noted from the positive relationship between Pb concentration in roots and soil TDS found in our study. In addition, the negative relationship between Pb concentration in root and SOM in our study could be related to the formation of insoluble Pb-organic complexes, which have been shown to decrease Pb desorption from SOM since Pb can compete with most other metals for the adsorption sites on SOM (Kerndorff and Schnitzer, 1980; Jin et al., 1996; Strawn and Sparks, 2000) and soil minerals (Li et al., 2013; Li et al., 2014).
In the current study, Pb and Ca BFs followed a decreasing pattern with increased distance from the cement factory. On the other hand, the TF of Pb increased with distance. Moreover, our results suggest that as Pb concentration increased in root tissues, less Pb was translocated to shoot tissues. Generally, plant species with TF > 1 can be regarded as hyperaccumulators (Zhao et al., 2003; Masarovičová et al., 2010; Rezvani and Zaefarian, 2011). In our study, the highest TF value (TF = 1.87) was found in the farthest zone from the factory. Considering the relatively low Pb concentrations in the factory soil, these results suggest that Cenchrus ciliaris is a potential hyperaccumulator and bioremediatory plant. It is well known that the uptake of Pb in plants and its translocation to aerial parts differs from one species to others, as some plants tend to accumulate Pb in shoot tissues, whereas others block its translocation to aerial parts (Pourrut et al., 2011). Nazir et al. (2011) reported that Cenchrus spp. grown in contaminated industrial areas significantly accumulated different heavy metals in root tissues. Several studies have also reported that Cenchrus ciliaris is a hyper-root-accumulator, which can be used for phytoremediation purposes (Keeling and Werren, 2005; Ines et al., 2016; Kumar and Fulekar 2018). Nevertheless, it has been illustrated that Pb enters plants mainly through apoplast pathways or Ca2+ channels and in small quantities through leaves. However, once in roots, Pb tends to be sequestered in root cells due to the blockage by Casparian strips within the endodermis (Pourrut et al., 2011). It is worth mentioning here that concentrations of heavy metals may vary in the study area throughout the year due to the uneven distribution of dust. In a recent risk-assessment study conducted by Javed et al. (2019), the concentration of Pb in surface soil collected from Ahd-El-Masarha (where the cement factory exists) had an average of 2.95 ppm, which is higher than the highest soil Pb concentration found in the current study (1.34 ppm).
5 Conclusion
It can be concluded from this study that SOM, Ca, and P concentrations in soil negatively affected Pb availability and uptake by Cenchrus ciliaris. Our results also suggest that Pb concentrations in soil positively correlate with soil pH and Pb/Ca ratio. In addition, based on TF value, Cenchrus ciliaris showed a promising tool to be used as a hyperaccumulator in Pb-contaminated soils. However, due to our results' contradictions with previous studies, more research on Pb availability and uptake by Cenchrus ciliaris in contaminated soils is needed to better understand its fate and relationship with different soil properties.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Evaluation of heavy metals contamination of soil and vegetation in the vicinity of a cement factory in the volta region. Ghana.Int. J. Sci. Technol.. 2012;2(1):40-50.
- [Google Scholar]
- Study of calcium-dependent lead-tolerance on plants differing in their level of ca-deficiency tolerance. Environ. Pollut. 2005
- [Google Scholar]
- Enhanced accumulation of pb in indian mustard by soil-applied chelating agents. Environ. Sci. Technol. 1997
- [Google Scholar]
- Management of groundwater resources under various irrigation water use scenarios in saudi arabia. Arab. J. Sci. Eng. 1997
- [Google Scholar]
- Distribution of lead in the soil profile evaluated by dtpa and mehlich-3 solutions. Bragantia 1998
- [Google Scholar]
- Assessing metal exposures in a community near a cement plant in the northeast us. Int. J. Environ. Res. Public Health. 2015
- [Google Scholar]
- Agency for Toxic Substances and Disease Registry, 2015. Priority list of hazardous substances. https://www.atsdr.cdc.gov/spl/resources/2015_atsdr_substance_priority_list.html (accessed November 2022)
- Method 3050b: Acid digestion of sediments, sludges, and soils. Environ. Prot.. 1996;2:3-5.
- [Google Scholar]
- Effect of calcium on the uptake and toxicity of lead in hordeum vulgare l. And festuca ovina l. New Phytol. 1981
- [Google Scholar]
- Effects of irrigations with treated municipal wastewater on phenological parameters of tetraploid cenchrus ciliaris l. J. Food Process Technol. 2016
- [Google Scholar]
- Lead and cadmium content in grass growing near an expressway. Arch. Environ. Contam. Toxicol. 2019
- [Google Scholar]
- Risk-based exposure assessment for multiple toxic elements encountered by children in school playgrounds and parks in the southwest region of saudi arabia. Environ. Monit. Assess. 2019
- [Google Scholar]
- Kinetics of single and multiple metal ion sorption processes on humic substances. Soil Sci 1996
- [Google Scholar]
- Phytoremediation: The uptake of metals and metalloids by rhodes grass grown on metal-contaminated soil. Remediation Journal: The Journal of Environmental Cleanup Costs, Technologies & Techniques. 2005
- [Google Scholar]
- Research article rhizosphere bioremediation of heavy metals (copper and lead) by cenchrus ciliaris. J. Environ. Sci. 2018
- [Google Scholar]
- Atmospheric mercury emissions from two pre-calciner cement plants in southwest china. Atmos. Environ. 2019
- [Google Scholar]
- Lead retention in a calcareous soil influenced by calcium and phosphate amendments. J. Hazard. Mater. 2013
- [Google Scholar]
- Immobilization of lead in soil influenced by soluble phosphate and calcium: Lead speciation evidence. J. Environ. Qual. 2014
- [Google Scholar]
- Buffel grass (cenchrus ciliaris) as an invader and threat to biodiversity in arid environments: A review. J. Arid Environ. 2012
- [Google Scholar]
- Principles of classification of medicinal plants as hyperaccumulators or excluders. Acta Physiol. Plant. 2010
- [Google Scholar]
- Hyperaccumulators of heavy metals of industrial areas of islamabad and rawalpindi. Pak. J. Bot. 2011
- [Google Scholar]
- Distribution and ecological risk assessment of heavy metals in soils around a major cement factory, ibese, nigeria. Sci. Afr. 2020
- [Google Scholar]
- The biochemistry of environmental heavy metal uptake by plants: Implications for the food chain. Int. J. Biochem. Cell Biol. 2009
- [Google Scholar]
- Potential role of nadph-oxidase in early steps of lead-induced oxidative burst in vicia faba roots. J. Plant Physiol. 2008
- [Google Scholar]
- Lead uptake, toxicity, and detoxification in plants. Rev. Environ. Contam. Toxicol.. 2011;213
- [Google Scholar]
- Bioaccumulation and translocation factors of cadmium and lead in'aeluropus littoralis' Aust. J. Agric. Res. 2011
- [Google Scholar]
- Soil solution speciation of lead (ii): Effects of organic matter and ph. Soil Sci. Soc. Am. J. 1998
- [Google Scholar]
- Heavy metals in soils, vegetation development and heavy metal tolerance in plant populations from metalliferous areas. New Phytol 1978
- [Google Scholar]
- Accumulation and translocation of heavy metals in soil and plants from fly ash contaminated area. J. Environ. Biol. 2010
- [Google Scholar]
- Effects of soil organic matter on the kinetics and mechanisms of pb (ii) sorption and desorption in soil. Soil Sci. Soc. Am. J. 2000
- [Google Scholar]
- Physiological responses of the halophyte salvadora persica to the combined effect of salinity and flooding. Int. J. Agric. Biol. 2018
- [Google Scholar]
- Effect of indole-3-acetic acid on lead accumulation in maize (zea mays l.) seedlings and the relevant antioxidant response. Environ. Exp. Bot. 2007
- [Google Scholar]
- Determination of major nutrient element in plant sample by wet ashing method. Soil and Plant Analysis for Tree Culture (fifth ed.). Oxford & IBH Publishing Co.; 1979.
- Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. Int. Sch. Res. Notices. 2011
- [Google Scholar]
- Identification of rice varieties with high tolerance or sensitivity to lead and characterization of the mechanism of tolerance. Plant Physiol 2000
- [Google Scholar]
- Accumulation of pb, cu, and zn in native plants growing on a contaminated florida site. Sci. Total Environ. 2006
- [Google Scholar]
- Coupling of remote sensing data aided with field investigations for geological hazards assessment in jazan area, kingdom of saudi arabia. Environ. Earth Sci. 2012
- [Google Scholar]
- Assessing the potential for zinc and cadmium phytoremediation with the hyperaccumulator thlaspi caerulescens. Plant Soil 2003
- [Google Scholar]







