Translate this page into:
Evaluation of botanicals for the management of Meloidogyne incognita infecting carrot and volatile nematicidal metabolite profiling
⁎Corresponding authors at: Section of Plant Pathology and Nematology, Department of Botany, Aligarh Muslim University, Aligarh, India (Mohd Ikram) and School of pharmacy, KPJ Healthcare University, Nilai, Malaysia (Ching Sian Tan). ikram.virologist@gmail.com (Mohd Ikram), tcsiang@kpjuc.edu.my (Ching Siang Tan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Plant-parasitic nematodes infect and cause substantial yield losses in numerous crops. Growing concern over chemical nematicides has attracted attention to safe alternatives. Meloidogyne incognita is one of the plant parasitic nematode species in the tropics and subtropics, which have a drastic economic effect on crops. This nematode is polyphagous, attacking both monocotyledons and dicotyledons crops. This study aimed to investigate the management of M. incognita using six botanical viz., Commelina benghalensis, Evolvulus nummularius, Gomphrena celosioides, Lindenbergia indica, Scoparia dulcis, and Vernonia cinerea under the pots condition. It was found that the pots treated with the amendment of fresh leaves (60 g) of selected botanicals with dried powder 10 g of L. indica efficiently reduced the infestation caused by M. incognita on carrots along with significantly increased growth and biochemical attributes. Out of the six botanicals, GC–MS analysis was performed with the two most effective plants, namely, L. indica and S. dulcis. Results from GC–MS analysis showed various volatile compounds in leaves extract of both plants, out of which phytol was a major compound. Our study concluded that various compounds, along with phytol shown by GC–MS analysis, suppress the infestation of M. incognita and increase the yield of carrots.
Keywords
Meloidogyne incognita
Plant-parasitic nematodes
Botanicals, GC–MS analysis
Sustainable managment
1 Introduction
Carrot (Daucus carota L.) is one of the economically significant vegetables grown worldwide due to its abundant fibers, vitamins, and minerals. However, carrots are well known for their high levels of β- carotene and α-carotene. It also incorporates falcarinol, a phytonutrient that has been reported to have chemopreventive potential against various forms of cancer (Dalgliesh et al., 2010).
PPNs are one of the most detrimental parasites that severely reduce the productivity of horticultural crops (Atkinson et al., 2012). PPNs feed on the cytoplasm of live plant cells. One of the most prevalent plants parasitic nematodes, Meloidogyne spp., affects plants worldwide. They infect more than 3000 species of plants, including a large number of cultivated plants (Abad et al., 2003), causing a projected yield loss of 12.3% (157 billion dollars) worldwide, of which $40.3 million reported from India (Singh et al., 2015). Meloidogyne incognita is the primary pathogen that affects carrot plants. The root-knot nematodes cause symptoms including galling, forking, stubbing, and fasciculation of the roots, which can impair the commercial value of carrots for fresh vegetable markets (Bridge and Starr 2007). Discovering economic controls that might decrease the pathogenic effects of Meloidogyne infection is crucial because the invasion of Meloidogyne spp. in carrot cultivation is one of the primary reasons for a decline in carrot production in India. Various methods applied for the management of nematodes include chemical, physical, biological, and cultural land management practices. The most efficient way for managing plant-parasitic nematodes is chemical treatment. Applications of nematode-preventative chemicals are very hazardous, accelerate biodegradation, and lead to environmental contamination. These chemicals have a long-term negative impact on the environment, which is a major concern (Ebone et al., 2019). Hence an eco-friendly strategy to combat the pathogenic effect of plant pathogenic nematodes is the best possible solution, providing an alternative to the chemically used pesticides. The nematode-invaded fields can be managed by nematicides, biocontrol agents, soil amendment (Jabeen et al., 2021), the use of antagonistic plants, and crop rotation (Kayani et al., 2012). The application of plant-derived products is an effective eco-friendly method for mitigating nematode infestations in various crops.
L. indica (L.) is a perennial plant of the Orobanchaceae family. It thrives on bare rocks and brick walls in moist, gloomy ravine areas. Several parts of this plant have Glycosides, such as saponins, oleanolic acid, and long-chain hydrocarbons. L. indica shows antibacterial activity against pathogenic bacteria (Walia and Singh 2014). S. dulcis (L.) known as sweet broom weed, is a perennial plant that is extensively spread in tropical and subtropical countries. The presence of bioactive phytochemicals is responsible for the pharmacological activities of S. dulcis, as shown by phytochemical screening. It has been reported to have antibacterial and antifungal activity (Latha et al., 2006). Different techniques have been utilized in the literature to identify the presence of phytochemicals, of which Gas chromatography-mass spectrometry (GC–MS) technique is the most popular method for plant metabolomics, particularly for making it easier to identify and measure the metabolites involved in the main pathways of primary metabolisms, such as organic acids, amino acids, sugars, sugar alcohols, and polyamines (Singh and Sharma 2022).
This study was designed to evaluate the nematicidal efficacy of the selected botanicals for managing the M.incognita, infecting carrot plants to provide an alternative to the chemical pesticides after considering the advantages of plant extract in managing nematode population as reported in our earlier studies (Ikram et al., 2022).
2 Material and methods
2.1 Pots experiment
2.1.1 Collection and maintenance of inoculum
Infected roots of eggplant were collected from the crop fields of Village Panjipur, Aligarh, India. The affected roots were cut off, and the detached egg masses were placed in a petri plate with purified water. Meloidogyne spp. was determined based on the perineal patterns reported by Eisenback. (Eisenback et al., 1980). Following the completion of the identifying process, a single eggmass was cultured and maintained in the greenhouse facility of the university campus. Eggmasses were extracted by hand using sterilized forceps from the roots of eggplant that were heavily infected. After hatching the eggs, the second-stage juveniles (J2s) were developed by incubating egg masses in distilled water (sterile) at a temperature of 27 ± 2 °C. Every twenty-four hours, the newly hatched juveniles were collected. Freshly hatched second-stage juveniles were standardized by concentration.
The clay pots (25 cm in diameter) contained two kg of sterile soil, a 3:1 mixture of sandy loam, and farmyard manure. The soil was mixed with 60 g of recently collected fresh leaves from the selected plant viz., C. benghalensis, E. nummularius, G. celosioides, S. dulcis, and V. cinerea. The pots were periodically watered to decompose leaves. Before sowing the seeds, each pot's soil was additionally supplemented with 10 g leaf powder of L. indica. The seeds of the carrot cultivar Red-Beauty were purchased from the local seed market of Aligarh. After being soaked in 0.01% HgCl2 for two minutes to perform surface sterilization, the seeds were rinsed thrice with distilled water. In the pots, these seeds were planted. Each pot was inoculated with 3000 J2 of M. incognita 15 days following seed germination. Each treatment and control group included five repetitions in a completely randomized design (CRD) order. Both untreated inoculated plants and plants that had not been inoculated were used as a control. During the experiment, the needed quantity of water was consistently added to the pots.
2.1.2 Observation and data collection
After ninety days, the carrot plants were pulled out from their pots, and the roots were separated from the plants. In order to ensure the eggmasses remained undamaged, the roots of each plant were washed in a tub of water. The data of growth, biochemical, and pathological parameters were collected. Cobb's sieving and decanting methods were used to estimate the nematode population accurately (Cobb 1918).
2.1.3 Estimation of chlorophyll and carotenoids content
Fresh leaves' chlorophyll and carotenoid content were measured using Mackinney's technique(Mackinney 1941). Briefly, 1 gm of freshly plucked leaves were crushed and centrifuged at 5000x g. The supernatant collected was washed with acetone to remove any residues. Absorbance was recorded at 645 & 663 nm for chlorophyll and 480 & 510 nm for carotenoids using a suitable blank.
2.1.4 Estimation of nitrate reductase activity (NRA)
NRA was estimated by the method described earlier (Jaworski 1971). To summarise, 200 mg of chopped leaves were placed into vials to which 2.5 ml of phosphate buffer, 0.5 ml of potassium nitrate, and 2.5 ml of isopropanol were added. The vials were kept in a BOD incubator for 2 h at 28 ± 2 °C in dark followed by adding sulphanilamide solution and NED HCl. The absorbance of the sample was then read at 540 nm using a suitable blank. The activity of nitrate reductase was then calculated and expressed as μ mole g−1h−1.
2.1.5 Estimation of the total phenolic content
The total phenolic content in the leaves was the estimated method adopted by ((Shahidi and Naczk 1995). Briefly diluted ethanolic extract was mixed with FC reagent and aqueous sodium carbonate, followed by heating the mixture at 45 °C. Total phenols were determined calorimetrically at 765 nm using a spectrophotometer. Total phenol concentrations quantified as gallic acid equivalent (a standard reference compound).
2.1.6 Estimation of whole metabolites using GC–MS
Fresh leaves of the plant L. indica and S. dulcis were collected from the botanical garden of AMU, India. At room temperature, the leaves dried. Dried leaves were then crushed with the help of a grinder-mixer. Solvent extracts were prepared by weighing the leaf powder (0.5 g) in 20 ml of 80% methanol–water mix. The mixture was vortexed and placed in an ultrasonicator bath for 15 min. After centrifuging at 5000 rpm for 15 min, the supernatant was filtered using Whatman filter paper no1.
2.1.7 GC–MS analysis
Samples for GC–MS were prepared as described previously with minor modifications (Lisec et al., 2006). Briefly, 250 µl of sample volume was transferred into GC glass vials & 1 µl of the sample was injected into the column with a split ratio of 10:1. At various temperature gradients, a distinct peak of metabolites was detected. The data were analyzed using Lab Solutions software. The identified metabolites were confirmed by comparing the peak spectra with standard mass spectra from available databases. The compounds were normalized against internal standards.
2.1.8 Identification of metabolites
The compounds from L. indica L. and S. dulcis L. were identified by comparing the peak retention time with the available compounds present in National Institute of Standards and Technology Libraries (NIST 14), NIST 14 s, and Willey 8. After comparing the constituents to those in the standard library (NIST and Willey) attached to the GC–MS instrument, the peak percentage area with a retention time of different compounds was obtained. The constituents of the plant extract were identified by name, molecular weight, structure and the relative percentage area of each compound (Sowmya et al., 2015). The GC–MS chromatogram of L. indica and S. dulcis with different retention times is shown in Fig. 1.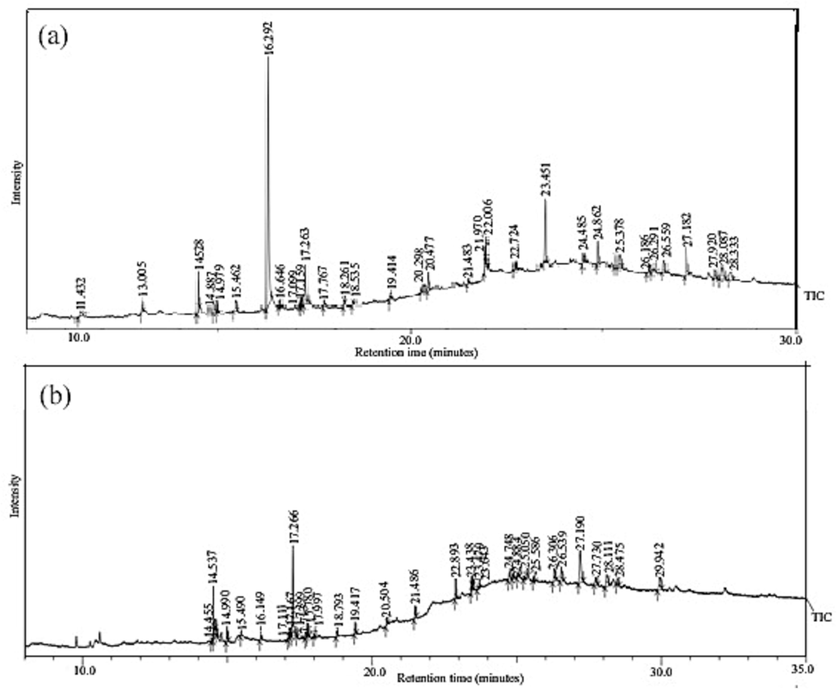
GC–MS chromatogram showing the presence of metabolites in (a) Lindenbergia indica L. and (b) Scoparia dulcis L.
2.2 Statistical analysis
The experimental data from the pots experiment was analysed using SPSS-17.0′s built-in one-way analysis of variance (ANOVA) program (SPSS Inc., Chicago, IL, USA). Duncan's Multiple Range Test was used to analyse the statistical significance of the treatment differences. P less than 0.05 was used to determine the significance level. When P was less than 0.05, the means' values were considered significant.
3 Results
3.1 Impact of fresh leaves on growth parameters
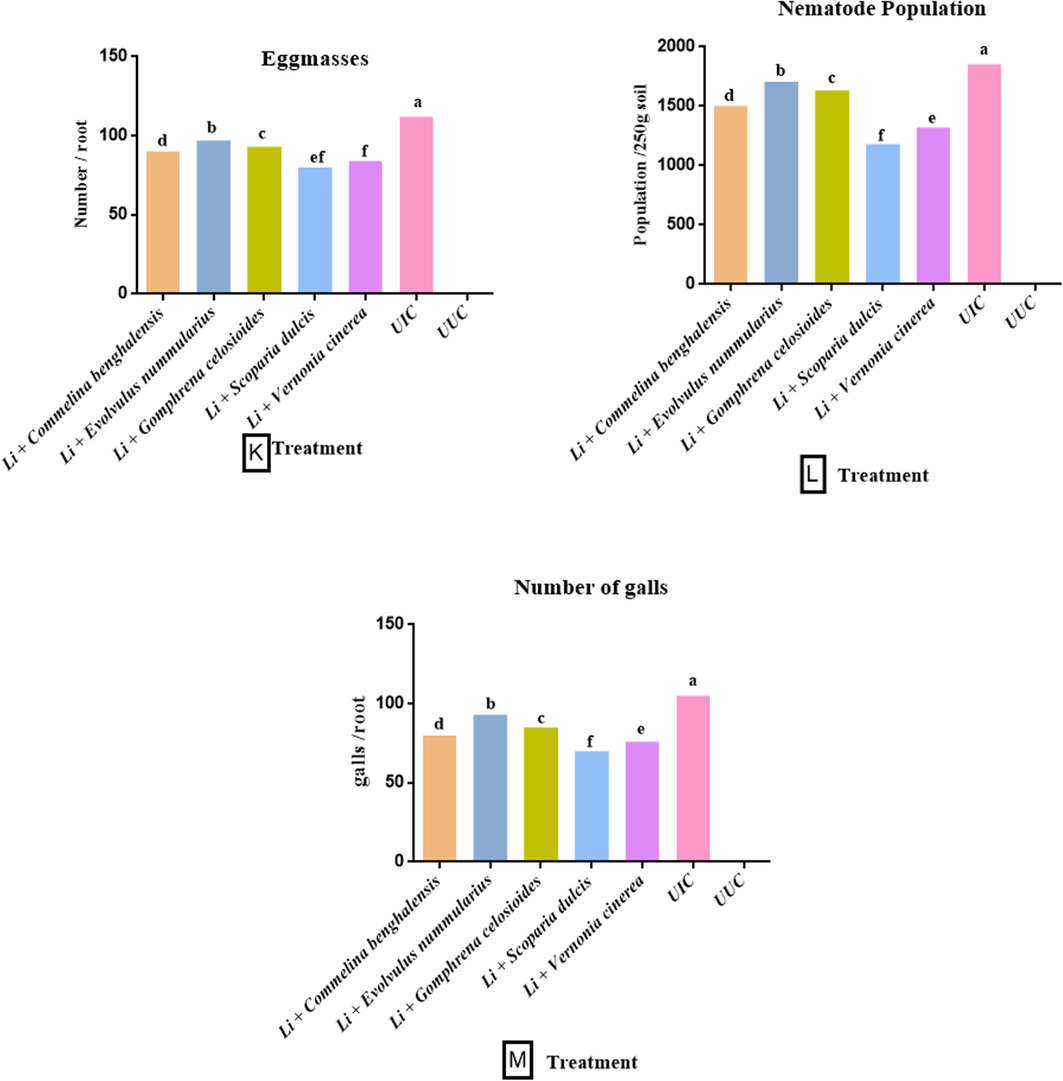
The soil amendment with various botanicals and powder of L. indica into the pot significantly reduced the growth of M. incognita as well as eggmasses/root, galls, and nematodes density. The plant's growth parameters were enhanced by using fresh leaves with Li. as a soil amendment. The powder of L. indica (10 g) was common with all treatments. The carrot plant treated with the addition of the fresh leaves of S. dulcis along with powder of L. indica was found to have maximum increase in the growth parameters, whereas treatment of E. nummularius with powder of L. indica showed the least effect in the growth parameters Fig. 3. The maximum shoot and root length (42.6 cm, 12.2 cm) were found in plants treated with the leaves of S. dulcis and the powder of Li.. It was followed by V. cinerea + Li. (41.5 cm, 11.8 cm), C. benghalensis + Li. (40.8 cm, 11.3 cm) and G. celosioides + Li. (39.4 cm, 10.7 cm). The leaves of E. nummularius + Li. (38.2 cm, 10.2 cm) was found to have the least effect on plant length (Fig. 2 A-B).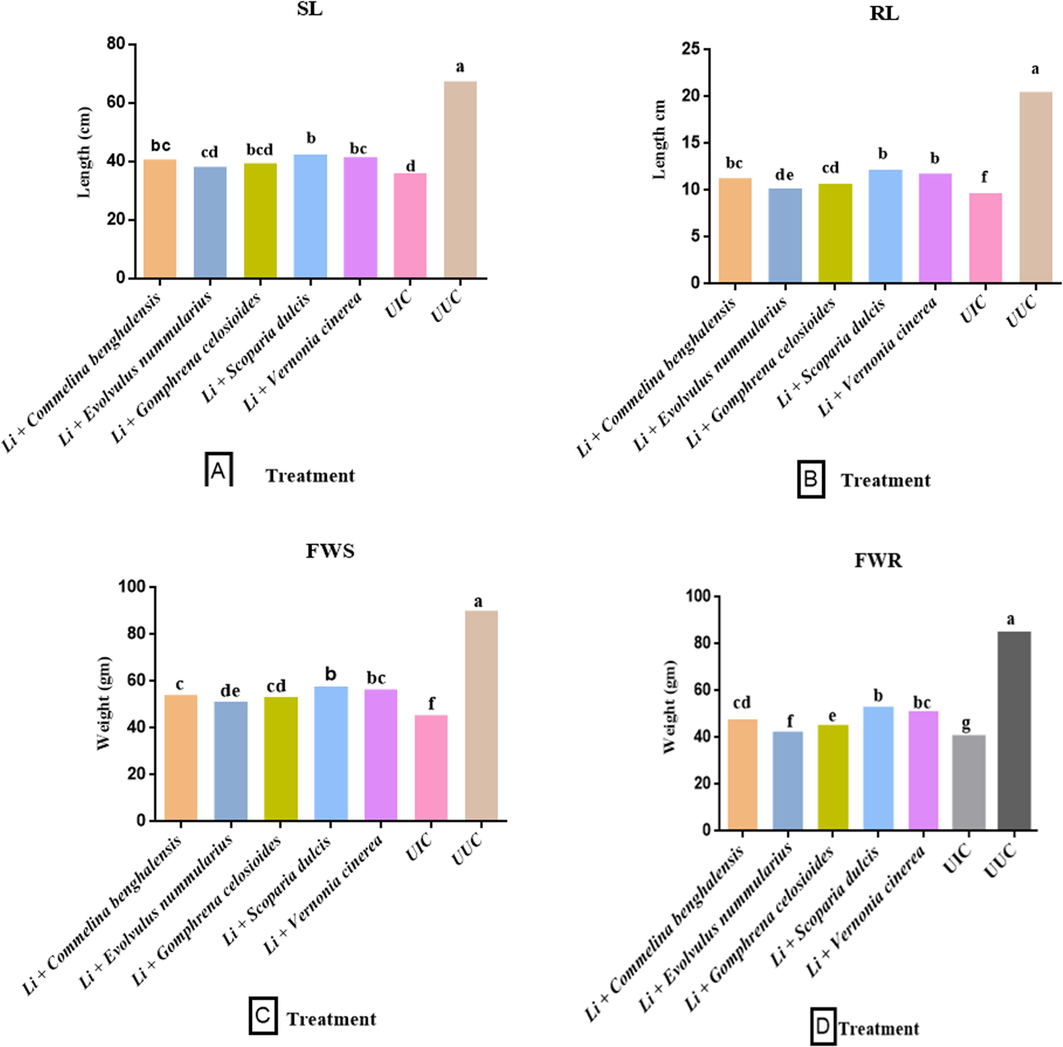
Effect of L. indica with some selected botanicals on the growth parameters A-F, biochemical parameters G-J, and pathological parameters K-M, of carrot. (UUC- Untreated uninoculated control; UIC- Untreated inoculated control; SL- Shoot length; RL- Root length; FWS- Fresh weight shoot; FWR- Fresh weight root; DWS- Dry weight shoot; DWR- dry weight root; NRA-Nitrate reductase activity). Data presented as means. According to Duncan’s multiple range test same letters are not significantly different.
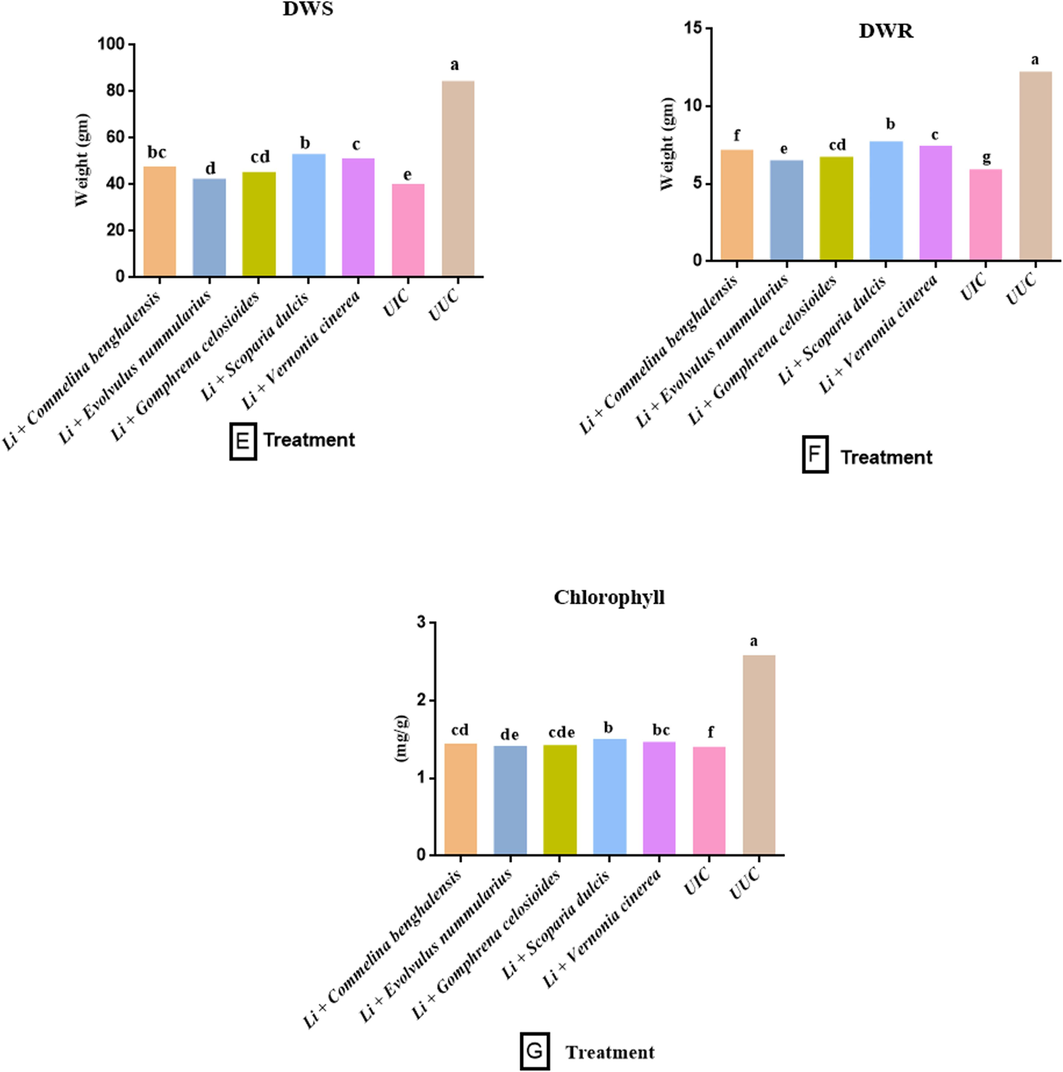
Effect of L. indica with some selected botanicals on the growth parameters A-F, biochemical parameters G-J, and pathological parameters K-M, of carrot. (UUC- Untreated uninoculated control; UIC- Untreated inoculated control; SL- Shoot length; RL- Root length; FWS- Fresh weight shoot; FWR- Fresh weight root; DWS- Dry weight shoot; DWR- dry weight root; NRA-Nitrate reductase activity). Data presented as means. According to Duncan’s multiple range test same letters are not significantly different.
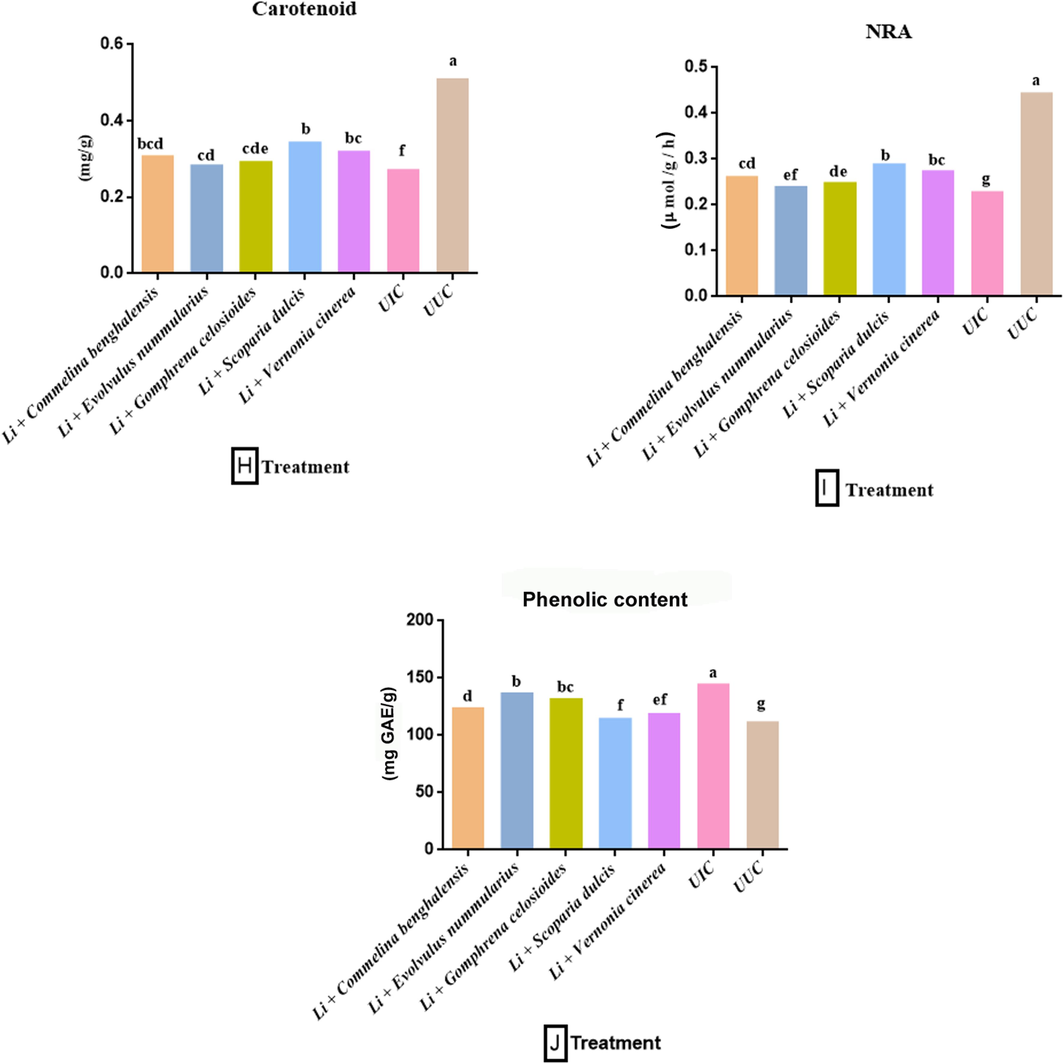
Effect of L. indica with some selected botanicals on the growth parameters A-F, biochemical parameters G-J, and pathological parameters K-M, of carrot. (UUC- Untreated uninoculated control; UIC- Untreated inoculated control; SL- Shoot length; RL- Root length; FWS- Fresh weight shoot; FWR- Fresh weight root; DWS- Dry weight shoot; DWR- dry weight root; NRA-Nitrate reductase activity). Data presented as means. According to Duncan’s multiple range test same letters are not significantly different.
Effect of L. indica with some selected botanicals on the growth parameters A-F, biochemical parameters G-J, and pathological parameters K-M, of carrot. (UUC- Untreated uninoculated control; UIC- Untreated inoculated control; SL- Shoot length; RL- Root length; FWS- Fresh weight shoot; FWR- Fresh weight root; DWS- Dry weight shoot; DWR- dry weight root; NRA-Nitrate reductase activity). Data presented as means. According to Duncan’s multiple range test same letters are not significantly different.
Similar results were seen for the shoot and root fresh weights. The treatment of S. dulcis + Li. show a maximum increment of fresh weight (57.88 g, 53.14 g), followed by V. cinerea + Li. (56.52 g, 51.25 g), C. benghalensis + Li. (54.23 g, 47.83 g), G. celosioides + Li. (53.47 g, 45.48 g) and E. nummularius + Li. (51.32 g, 42.54 g). Likewise, a similar effect was observed in the dry weight of the shoot and root. The treatment of S. dulcis + Li. show a maximum increment in shoot and root dry weight (8.56 g, 7.74 g) followed by V. cinerea + Li. (8.35 g, 7.48 g), C. benghalensis + Li. (8.21 g, 7.21 g), G. celosioides + Li. (7.95 g, 6.75 g) and E. nummularius + Li. (7.62 g, 6.53 g) (Fig. 2 C-F).
3.2 Impact of fresh chopped leaves on biochemical parameters
This was observed that nematode infection lowered the levels of chlorophyll, carotenoids, and nitrate reductase activity in the plant. The treatment of fresh chopped leaves of botanicals with powder of L. indica, increased the levels of these parameters. Results from our study revealed that the treatment of S. dulcis + Li. increased maximum levels of chlorophyll (1.512 mg/g), carotenoids (0.346 mg/g), and NRA (0.291 μmol g−1h−1), followed by V. cinerea + Li. (1.473 mg/g, 0.322 mg/g and 0.276 μmol g−1h−1), C. benghalensis + Li. (1.455 mg/g, 0.310 mg/g and 0.263 μmol g−1h−1) and G. celosioides + Li. (1.432 mg/g, 0.295 mg/g and 0.250 μmol g−1h−1). Whereas the lowest enhancement (1.420, 0.286, and 0.242 μmol g−1h−1) was observed in plant treated with E. nummularius + Li. in the comparison of UIC (1.410 mg/g, 0.274 mg/g and 0.230 μmol g−1h−1) (Fig. 2 G-I). This was also found that total phenolics content significantly increased in the nematode-infected plant compared to the (UUC) control (110.3 mg GAE/g). A maximum increase in total phenol content (145.4 mg GAE/g) was found in the plant treated with E. nummularius + Li. and a minimum in the plant treated with S. dulcis + Li. (115.2 mg GAE/g) (Fig. 2J).
3.3 Impact of fresh chopped leaves on pathological parameters
The result indicates that amendment of soil with chopped leaves of S. dulcis + Li. was found to have more influence in suppressing the pathogenic effect of M. incognita among all chosen botanicals. The addition of S. dulcis + Li. revealed the lowest number of galls in the root (70), followed by V. cinerea + Li. (76), C. benghalensis + Li. (80) and G. celosioides (85). In contrast, E. nummularius was found to least influential against the nematodes with the highest number of galls (93) compared to the untreated inoculated control (105) (Fig. 2M). The treatment of S. dulcis + Li. show the lowest number of egg masses (80), followed by V. cinerea + Li. (84), C. benghalensis + Li. (90), G. celosioides + Li. (93) and E. nummularius + Li. (97) was found to have the maximum number of egg masses on the roots of the plant compared to the UIC (112) (Fig. 2K).
The leaves of S. dulcis + Li. were found to have the maximum effect among all the botanicals to suppress in nematode population (1180), followed by V. cinerea + Li. (1320), C. benghalensis + Li. (1502) and G. celosioides + Li. (1633). While the least reduction of nematode population was observed in the plant treated with E. nummularius + Li. (1705) in comparison to the untreated inoculated control (1852) (Fig. 2L).
3.4 Metabolomic profiling of the most effective plant extract
The experiment was carried out on six plant species in which only two of the most prominent replicates (L. indica L. and S. dulcis L.) were chosen for GC–MS analysis. The GC–MS analysis of the two most effective plant extracts, L. indica and S. dulcis reveals a difference in the % area of metabolites responsible for nematode mortality. L. indica GC–MS revealed 29 metabolites, while S. dulcis revealed 30 metabolites. The GC–MS profile of L. indica reveals 94.5% of metabolites responsible for the declining nematode population, while in comparison, S. dulcis revealed only 80.75%. As a primary bioactive compound in the prevention of root-knot nematode, the concentration of phytol (Retention time: 16.29) was much higher in L. indica than in S. dulcis. Many other chemicals synergistically affect nematode population management, but phytol is primarily responsible for suppressing the population of M. incognita.
Effect of L.indica powder in combination with fresh chopped leaves of some botanicals on the shoot and root of carrot, T1-T5 Treatment, UUC-Untreated Uninoculated Control, UIC-Untreated Inoculated Control.
Table 1 and Fig. 1 shows the active principles from the chromatogram of the methanolic extract of the plant, together with their retention time and percentage composition. L. indica had a higher total % area of metabolites, indicating that this species could be further studied for bioprospecting cheap, safe, and affordable treatments for decreasing the population of root-knot nematode and disease-free yield. ND represents undetected metabolite.
S. No.
Metabolite
Retention
Time
Lindenbergia indica L. (Area%)
Scoparia dulcis L. (Area %)
1.
Methyl 5-(4- biphenylyloxymethyl)-2-furoate
11.432
1.12
ND
2.
Geranyllinalool
13.005
2.22
ND
3.
Neophytadiene
14.528
3.77
7.8
4.
Gernayl linalool isomer B
14.887
1.32
ND
5.
3,7,11,15-Tetramethylhexadec-2-en-1-ol
14.979
1.08
ND
6.
Eicosanoic acid, Methyl Ester
15.462
2.41
ND
7.
Phytol
16.292
37.8
19.55
8.
Oxacycloheptadec-8-en-2-one, (8Z)-
16.646
0.88
ND
9.
9,12-Octadecadienoic acid, methyl ester
17.099
0.53
ND
10.
9-Octadecenoic acid (Z)-, methyl ester
17.159
0.94
ND
11.
Phytol
17.263
5.15
ND
12.
Ethyl (9Z,12Z)-9,12-octadecadienoate
17.767
0.62
ND
13.
Octadecane
18.535
0.38
ND
14.
2,5-Di(trifluoromethyl)benzoic acid, 5-dodecyl ester
19.414
0.5
ND
15.
Heneicosane
20.298
0.83
ND
16.
1,2-Benzenedicarboxlic acid
20.477
2.54
ND
17.
1-Ethyl-1-decyloxy-1-silacyclopentane
21.483
0.7
ND
18.
Docosane
22.006
1.93
ND
19.
2-Octyl-1-dodecanol
22.724
0.82
ND
20.
Celidoniol, deoxy-
23.451
8.23
ND
21.
Octadecanal
24.485
1.46
ND
22.
Tetracontane
24.862
3.26
ND
23.
Propane, 1,2-dimethoxy-3-[(2-methoxyhexadecyl)oxy]-
25.378
2.4
2.29
24.
Tricosanal
26.186
0.83
ND
25.
Ergost-5-en-3-ol
26.291
0.81
ND
26.
16-Hentriacontanone
26.559
2.28
ND
27.
γ-Sitosterol
27.182
6.86
ND
28.
Lup-20 (29)-en-3-one
27.92
1.55
ND
29.
24-Norursa-3,12-diene
28.333
2.35
ND
30.
(2E)-3,7,11,15-Tetramethyl-2-hexadecene
14.45
ND
0.62
31.
2-Pentadecanone, 6,10,14-trimethyl-
14.633
ND
2.62
32.
3,7,11,15-Tetramethyl-2-hexadecen-1-ol
14.99
ND
2.8
33.
Hexadecanoic acid, methyl ester
15.49
ND
0.92
34.
Ethyl pentadecanoate
16.149
ND
0.77
35.
9,12-Octadecadienoic acid (Z,Z)-
17.11
ND
1.01
36.
7-Hexadecenoic acid, methyl ester, (Z)-
17.16
ND
1.54
37.
Tetracosanoic acid, methyl ester
17.39
ND
0.45
38.
9,12,15-Octadecatrienoic acid, ethyl ester, (Z,Z,Z)-
17.71
ND
0.9
39.
Octadecanoic acid, ethyl ester
17.99
ND
0.49
40.
3-Cyclopentylpropionic acid, 2-dimethylaminoethyl ester
18.79
ND
1.18
41.
2,5-Di(trifluoromethyl)benzoic acid, 3-hexadecyl ester
19.41
ND
2.11
42.
(E,E,E)-3,7,11,15-Tetramethylhexadeca-1,3,6,10,14-pentae
20.5
ND
1.58
43.
Squalene
22.89
ND
3.44
44.
2-Undecenoic acid, TMS derivative
23.43
ND
3.44
45.
Tetratetracontane
23.47
ND
1.21
46.
(2Z,6E,10E)-3,7,11,15-Tetramethyl-2,6,10,14-hexadecatetraen-1-ol
23.64
ND
0.67
47.
Androst-5-en-17-one, 3-(acetyloxy)-19-hydroxy-, (3.beta.)-
24.748
ND
2.32
48.
Octadecane, 1-iodo-
24.88
ND
1.29
49.
Stigmasta-3,5-diene
25.05
ND
0.97
50.
6-Methoxy-2,7,8-trimethyl-2-(4,8,12-trimethyltridecyl) chro
25.25
ND
0.79
51.
Heptadecanoic acid, ethyl ester
25.58
ND
1.03
52.
Ergost-5-en-3-ol, (3.beta.)
26.3
ND
4.33
53.
Stigmasta-5,22-dien3-ol, (3. beta.,22E)
26.53
ND
4.63
54.
Olean-12-en-3-one
27.73
ND
2.64
55.
Simiarenol
28.47
ND
2.2
56.
Phytyl decanoate
29.94
ND
5.16
Total metabolites
95.57
80.75
4 Discussion
Meloidogyne, a genus of polyphagous plant parasites, are known as root-knot nematodes. These microscopic worms induce root deformations known as galls or root knots, which reduce agricultural yields and result in substantial economic losses (Abad et al., 2008). Plants contain a lot of bioactive secondary metabolites, such as alkaloids, saponins, tannins, flavonoids, and glycosides, which play an important role in pathogen defense. The botanicals, upon decomposition, release multiple secondary metabolites and several other organic acids into the soil (Ntalli and Caboni 2017). These metabolites may be toxic, hinder the normal physiological process of nematodes, and causes their death. Most often, these chemical compounds inhibit the hatching of eggs and enhance juvenile mortality.
Our findings suggest using the selected botanicals as a soil amendment, releasing phytochemicals upon decomposition into the soil. These phytochemicals of selected plant leaves are responsible for nematicidal ability. The secondary metabolites can manage many plant-parasitic nematodes, including Meloidogyne incognita (Chitwood 2002). The outcomes mentioned above are consistent with (Hussain et al., 2011), who managed the nematodes into pots using Azadirachta indica, Calotropis procera, Datura stramonium, and Tagetes erecta. There are many types of plants that act as antagonists. However, the most well-known ones for their effectiveness against major nematodes include the Tagetes spp., Azadirachta indica, Brassica spp., and Crotalaria spp. (Grubišić et al., 2018). (Oka 2010), observed that using plant materials could change the soil's physical structure and fertility, increasing plant tolerance to nematode infection and fostering plant development. Earlier research also supports our findings (Bello et al., 2006). They observed that Tamarindus indica, Cassia sieberiana, Cassia siamea, and Dolnix regia were effective in suppressing the nematode population and preventing M. incognita eggs from hatching. These plants are nematicidal due to isothiocyanates, tannins, phenolics, alkaloids, terpenes, thiophenes, and glucosides (Khan et al., 2018). The earlier study investigated that Brassica macrocarpa leaves are nematotoxic to root-knot nematode (Argento et al., 2019). According to this study, of all botanicals that were chosen, the treatment of S. dulcis + Li had the most significant effect on decreasing the pathological parameters in terms of egg masses per root, number of root galls, and nematode populations. Earlier studies have confirmed the findings of our investigation (Khan et al., 2021) and (Hussain et al., 2018). Plant nematicidal properties vary based on plant species, the plant tissue utilised, the stage of plant development, the application technique, and the kind of worms being examined (Chitwood 2002). There are metabolites present in decayed leaves with ovicidal or larvicidal activity that may be responsible for suppressing nematode growth. Insufficient juvenile penetration, feeding, and reproduction delays may reduce root-knot proliferation. The plant may have developed well due to the presence of fewer nematodes. The plant grows well and healthy when there are few interruptions to its development (Naz et al., 2013). Many naturally occurring substances suppressing Meloidogyne spp. have been identified, one of which is a glycoside called asparagusic acid derived from the plant Asparagus officinalis (Chitwood 2002). Nonacosane-10-ol and 23a-homostigmast-5-en-3b-ol are two examples of newly discovered nematicidal compounds isolated from the Fumaria parviflora Lam's roots (Naz et al., 2013). Our results indicate that applying botanicals into the soil significantly improved the plant length, weight, and biochemical parameters compared to the control. Different studies confirm our results (Hasan et al., 2021, Khan et al., 2021). There is a possibility that enhanced soil nitrogen availability as a result of the breakdown of botanicals is responsible for the improved carrot growth in treated soil. The addition of botanicals to soil promotes the growth of roots. The soil's texture changed, and its nutrient content rise when we applied herbal treatments. In our experiment, increasing soil nutrients may also strengthen nematode defences and reduce nematode infection. Previous research also observed a similar result (Khan et al., 2021).
Nitrate reductase (NR) is the primary enzymatic generator of nitric oxide in plant cells. It regulates plants' growth and resistance to environmental and biotic stress. This was reported that nematode infestation causes a decrease in biochemical parameters such as in chlorophyll, carotenoids, and NRA due to a lower rate of photosynthesis. The previous report obtained a similar result (Berger et al., 2007). It reported that the rate of photosynthesis is reduced if plants come into contact with pathogens. The utilization of leaves as soil amendment increases these parameters (Hasan et al., 2021). The study shows that an increased phenolic content in the untreated inoculated control (UIC) may be due to the release of conjugated phenols from glycosidic phytochemicals by hydrolytic enzymes during nematode root colonization. The earlier experiment confirmed the increase in phenol content (Sithole et al., 2022). It has been believed that phenolic compounds that may be detected in the feeding site of root-knot nematodes are connected with the hypersensitive response (Oliveira et al., 2019). Nematode-infected plants have higher total phenolic contents, which triggers different chemical synthesis pathways for defense, which show a resistance response mechanism. In this experiment, the treatment of botanicals decreased the phenolic content compared to the respective control (UIC), which agrees with the earlier study (Khanna et al., 2019). Our results agree with the previous report (Rahman et al., 2020), in which a similar effect was reported when they used amendment of Sargassum ilicifolium with or without biocontrol agent Pseudomonas aeruginosa for assessing induced systemic resistance in soybean against the root-knot nematode.
We have also performed the GC–MS analysis of the two most effective plant species to determine the phytochemicals responsible for controlling root-knot nematode. When we compared the GC–MS profile of L. indica and S. dulcis, the former showed the maximum effectiveness in declining the population of M. incognita. Phytol might be the major phytochemical responsible for killing the population of M. incognita. The ethylene signaling pathway has been identified as the mechanism by which phytol causes root-knot nematode resistance in Arabidopsis (Fujimoto et al., 2021). It was noted that soil treatment with botanicals leads to a significant decrease in the nematode population, comparable to earlier findings (Oluwatayo et al., 2019).
The present study hence demonstrates that botanicals enhance plant growth and biochemical parameters to inhibit the establishment of root-knot nematodes and nematode populations. Therefore, it is possible to explore these plant species as an organic amendment for sustainable agriculture which will help in reducing the application of toxic chemical nematicides.
5 Conclusion
According to the data, the use of certain botanicals as organic amendments into the soil, rather than the more conventional chemical nematicides, seems to be an effective method for eradicating root-knot nematode, M. incognita. These botanicals promote organic farming and sustainable nematode management. We report 59 compounds from L. indica and S. dulcis . In both these species L. indica has a greater percentage (95.57%) of metabolomic compounds responsible for suppressing M. incognita. The combined treatment of L. indica and S. dulcis was most effective in managing the nematodes among all treatments. Phytol was the main metabolite responsible for controlling M. incognita, whereas other metabolites were in smaller percentages. As a result, compared to S. dulcis , L. indica significantly limits the population of this root-knot nematode.
Acknowledgement
This project was supported by Researchers Supporting Project number (RSP2023R230) King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Root-knot nematode parasitism and host response: molecular basis of a sophisticated interaction. Mol. Plant Pathol. 2003;4(4):217-224.
- [Google Scholar]
- Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat. Biotechnol.. 2008;26(8):909-915.
- [Google Scholar]
- Enhancing greenhouse tomato-crop productivity by using Brassica macrocarpa guss. Leaves for controlling root-knot nematodes. Agronomy. 2019;9(12):820.
- [Google Scholar]
- Strategies for transgenic nematode control in developed and developing world crops. Curr. Opin. Biotechnol.. 2012;23(2):251-256.
- [Google Scholar]
- Effects of some plant extracts on larval hatch of the root-knot nematode, Meloidogyne incognita. Arch. Phytopathol. Plant Protect.. 2006;39(4):253-257.
- [Google Scholar]
- Plant physiology meets phytopathology: plant primary metabolism and plant–pathogen interactions. J. Exp. Bot.. 2007;58(15–16):4019-4026.
- [Google Scholar]
- Plant nematodes of agricultural importance: a color handbook. Elsevier; 2007.
- Phytochemical based strategies for nematode control. Annu. Rev. Phytopathol.. 2002;40(1):221-249.
- [Google Scholar]
- Cobb, N. A., 1918. Estimating the Nema Population of Soil, with Special Reference to the Sugar-beet and Root-gall Nemas, Heterodera Schachtii Schmidt and Heterodera Radicicola (Greef) Müller: And with a Description of Tylencholaimus Aequalis N. Sp, U.S. Government Printing Office.
- Systematic sequencing of renal carcinoma reveals inactivation of histone modifying genes. Nature. 2010;463(7279):360-363.
- [Google Scholar]
- Nematicides: history, mode, and mechanism action. Plant Science Today.. 2019;6(2):91-97.
- [Google Scholar]
- Morphological comparison of Meloidogyne female head structures, perineal patterns, and stylets. J. Nematol.. 1980;12(4):300.
- [Google Scholar]
- Phytol, a constituent of chlorophyll, induces root-knot nematode resistance in Arabidopsis via the ethylene signaling pathway. Mol. Plant Microbe Interact.. 2021;34(3):279-285.
- [Google Scholar]
- Nematode control by the use of antagonistic plants. Agriculturae Conspectus Scientificus. 2018;83(4):269-275.
- [Google Scholar]
- Use of weed plants against Meloidogyne incognita in spinach involves reduction of gall disease from roots. Acta Agriculturae Scandinavica, Section B—Soil & Plant. Science. 2021;71(6):498-506.
- [Google Scholar]
- Efficacy evaluation of Azadirachta indica, Calotropis procera, Datura stramonium and Tagetes erecta against root-knot nematodes Meloidogyne incognita. Pak. J. Bot.. 2011;43(1):197-204.
- [Google Scholar]
- Microenvironmental alteration by the use of some plants for the effective control of root-knot nematode (Meloidogyne incognita) on brinjal. Plant Prot.. 2018;2(3)
- [Google Scholar]
- Nemato-toxic analysis of several chopped plant leaves against Meloidogyne incognita affecting tomato In vitro and In pots. Bioinformation. 2022;18(4):354-363.
- [Google Scholar]
- Management of southern blight of bell pepper by soil amendment with dry biomass of Datura metel. J. Plant Pathol.. 2021;103(3):901-913.
- [Google Scholar]
- Nitrate reductase assay in intact plant tissues. Biochem. Biophys. Res. Commun.. 1971;43(6):1274-1279.
- [Google Scholar]
- Evaluation of nematicidal effects of Cannabis sativa L. and Zanthoxylum alatum Roxb. against root-knot nematodes, Meloidogyne incognita. Crop Prot.. 2012;39:52-56.
- [Google Scholar]
- Screening of carrot cultivars against root-knot nematode Meloidogyne incognita. Indian Phytopathology.. 2018;71(3):415-421.
- [Google Scholar]
- Assessment of nematicidal efficacy of chitosan in combination with botanicals against Meloidogyne incognita on carrot. Acta Agriculturae Scandinavica, Section B—Soil & Plant. Science. 2021;71(4):225-236.
- [Google Scholar]
- Impact of plant growth promoting rhizobacteria in the orchestration of Lycopersicon esculentum Mill. resistance to plant parasitic nematodes: a metabolomic approach to evaluate defense responses under field conditions. Biomolecules. 2019;9(11):676.
- [Google Scholar]
- Phytochemical and antimicrobial study of an antidiabetic plant: Scoparia dulcis L. J. Med. Food. 2006;9(3):391-394.
- [Google Scholar]
- Gas chromatography mass spectrometry–based metabolite profiling in plants. Nat. Protoc.. 2006;1(1):387-396.
- [Google Scholar]
- In vitro and in planta nematicidal activity of Fumaria parviflora (Fumariaceae) against the southern root-knot nematode Meloidogyne incognita. Plant Pathol.. 2013;62(4):943-952.
- [Google Scholar]
- A review of isothiocyanates biofumigation activity on plant parasitic nematodes. Phytochemistry Reviews. 2017;16(5):827-834.
- [Google Scholar]
- Mechanisms of nematode suppression by organic soil amendments—a review. Appl. Soil Ecol.. 2010;44(2):101-115.
- [Google Scholar]
- Impact of phenolic compounds on Meloidogyne incognita in vitro and in tomato plants. Exp. Parasitol.. 2019;199:17-23.
- [Google Scholar]
- Nematicidal effect of some botanical extracts for the management of Meloidogyne incognita and on growth of tomato. Asian J Agricul Horticul Res.. 2019;4(2):1-8.
- [Google Scholar]
- Evaluation of systemic defense responses in soybean induced by sargassum ilicifolium and endophytic pseudomonas aeruginosa against root knot nematode. Int J Biol Res.. 2020;8(1):11-20.
- [Google Scholar]
- Food phenolics. Technomic Pub. Co.; 1995.
- Gas chromatography–mass spectrometry (GC–MS) profiling reveals substantial metabolome diversity in seabuckthorn (Hippophae rhamnoides L.) berries originating from different geographical regions in the Indian Himalayas. Phytochem. Anal. 2022;33(2):214-225.
- [Google Scholar]
- Nematodes: a threat to sustainability of agriculture. Procedia Environ. Sci.. 2015;29:215-216.
- [Google Scholar]
- Effects of Botanicals on Growth and Phytochemistry of the Nematode-Infected Pelargonium sidoides and GC–MS Profiling of Cucurbita maxima Seeds. J. Plant Growth Regul. 2022:1-17.
- [Google Scholar]
- Comparative preliminary phytochemical analysis various different parts (Stem, Leaf and Fruit) of Cayratia trifolia (L.). Indo American Journal of. Pharm. Res.. 2015;5(1):218-223.
- [Google Scholar]
- Lindenbergia Indica: Present Status And Future Perspectives. Biotech Today: An International Journal of Biological Sciences.. 2014;4(2):31-33.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102911.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







