Translate this page into:
Evaluation of anticarcinogenic and antioxidant properties of Eruca sativa extracts versus Ehrlich ascites carcinoma in mice
⁎Corresponding author at: Department of Community Health Sciences, College of Applied Medical Sciences, King Saud University, P.O. Box 10219, Riyadh 11433, Saudi Arabia. mfbadr@ksu.edu.sa (Mohamed Farouk Elsadek),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objective
Cancer is a major public health issue and characterized by wild cell growth and proliferative disorder. The antitumor and antioxidant role of Eruca sativa (ES) was investigated in a Ehrlich ascites carcinoma (EAC) tumor model in mice.
Methods
Twenty-four hours after EAC intraperitoneal inoculation in mice, ES extracts were administered at doses of 250 mg/kg/body weight (BW) for 9 days. Half of the mice were sacrificed on the 10th day for estimation of hematological profiles, red blood cell count, hemoglobin content, packed cell volume, mean corpuscular volume and mean corpuscular hemoglobin. In addition, antioxidant parameters (superoxide dismutase, catalase, and glutathione peroxidase) were evaluated. The remaining mice were kept alive to assess the increase in life span. Aspartate aminotransferase, alanine transaminase, alkaline phosphatase, creatinine, and urea levels were assessed for ES antitumor effects. Results.
Oral supplementation of ES seed and leaf extracts significantly reduced tumor parameters such as tumor volume and tumor weight with remarkable enhance in tumor inhibition ratio, vice versa, increased both survival and average BW of tumor-bearing mice. ES extracts significantly increased BW and extended the life span of tumor-bearing mice. Hematological profiles were restored to normal and modulated the abovementioned antioxidant parameters in ES-treated mice compared with EAC control.
Conclusion
The current study noticeably demonstrates the antitumor activity of ES seed and leaf extracts significantly prolonged life span and normalized the hematological profiles and biochemical parameters compared with EAC mice.
Keywords
Ehrlich ascites carcinoma
Eruca Sativa
Antitumor
Antioxidant parameters
Mice
- ES
-
Eruca Sativa
- EAC
-
Ehrlich Ascites Carcinoma
- BW
-
Body Weight
- Hb
-
Hemoglobin
- TP
-
Total Protein
- CAT
-
Catalase
- AST
-
Aspartate aminotransferase
- ALT
-
Alanine aminotransferase
- ESTGs
-
Eruca Sativa-Treated Groups
- MCV
-
Mean Corpuscular Volume
- TLC
-
Total Leukocytic Count
- MID
-
Monocytes and some Eosinophils
- MST
-
Median Survival Time
- ILS
-
Increased in Life Span
- TIR
-
Tumor Inhibition Ratio
- RBC
-
Red blood cell
- ALP
-
Alkaline Phosphatase
- MDA
-
Malondialdehyde
- SOD
-
Superoxide Dismutase
- GPx
-
Glutathione Peroxidase
- MCH
-
Mean Corpuscular Hemoglobin
- PCV
-
Packed Cell Volume
- LYM
-
Lymphocytes
- GRA
-
Neutrophils, Eosinophils, and Basophils
Abbreviations
1 Introduction
Cancer is a major public health issue and characterized by wild cell growth and proliferative disorder (Hanahan and Weinberg 2011). This disease is commonly diagnosed by molecular signal failure during apoptosis in cancer cells. Hence, unrestrained proliferation and deferral cell death by apoptosis finally cause tumor growth (Silva et al., 2014). Recently, cancer remains the leading cause of death in developed and developing countries. Globally, 7.6 million individuals died of cancer in 2008, and approximately 64% were from developing countries (Jemal et al., 2011). Several studies have confirmed numerous deaths due to cancer occur annually (Weinberg, 2011). Oxygen-centered free radicals (ROS) or oxidative stress is closely associated with several diseases, including cancer (Ďuračková, 2010). Excessive production of ROS during cancer plays a vital function in tissue damage and cell death (Islam et al., 2013). Several studies have documented that ROS is extremely harmful in cells, which leads to damage in protein DNA and lipids in many disease conditions, such as cancer, diabetes, hypertension, renal toxicity, and hepatotoxicity (Noda and Wakasugi 2001). Oxidative damage during tumor conditions is closely associated with abnormal oxidation and reduction states and creation of active oxygen (Noda and Wakasugi 2001). Therefore, many researchers have focused to develop a natural treatment for the tumor without side effects.
Plants and its ingredients are important natural sources used to manage or treat several diseases, including cancer, without any harmful effects. Therefore, recent new findings are focusing on these natural sources to manage several diseases, such as cytotoxicity, cardiovascular diseases, and cancer (Gonzales and Valerio 2006). The plant Eruca sativa (ES) (syn. E. vesicaria subsp. sativa (Miller) Thell.), commonly named rocket salad, is a feeble, edible annual plant that is primarily cultivated in Mediterranean countries and Western Asia (Pasini et al., 2011) and has been used as herbs, nutritional source, and medical plant. An ES green leaf has a spicy hot taste and is used as a salad vegetable worldwide (Kim et al., 2004). ES salad has several health-endorsing agents, including fibers, proteins, calcium, iron, magnesium, vitamins A and C, carotenoids, and flavonoids. Some of these ingredients are known to be powerful antioxidants (Michael et al., 2011). Moreover, ES has high levels of glucosinolates, flavonoids, and phenolics. Flavonoids and phenolic compounds have antioxidant-associated anticancer properties and also prevent the risk of cardiovascular and cognitive diseases (Lamy et al., 2008). It has several medicinal and therapeutic properties, including tumorigenesis inhibitor and hepatoprotective and anti-ulcer impact (Alqasoumi et al., 2009).
ES exerts an advantageous antidiabetic effect in rats by plummeting the oxidative stress process. Alcoholic seed extract possesses forceful antioxidant and renal protective and diuretic activities (Alam et al., 2007; Ansari, 2014).
Hence, No detailed investigation has addressed the potential effects of ES on EAC in vivo. This study was planned to evaluate the possible antitumor activity of ES seeds and leaves extracts against Ehrlich Ascites Carcinoma “EAC” in addition antioxidant status in experimental mice.
2 Materials and methods
2.1 Materials
2.1.1 Chemicals
Unless stated otherwise, all chemicals and biochemical kits used for determination were of analytical grade and procured from Sigma Chemicals Co., USA.
2.1.2 Plant materials and extracts
ES seeds and leaves were collected locally. The plant was identified and confirmed by Food Technology Research Institute, Giza, Egypt. Seeds and leaves were dried at shade after wash and minced before extraction. Moreover, 500 g of seed and leaf powder was extracted with 450 mL ethanol. Then, the extract was concentrated under vacuum and dried in desiccators. The dried extract was used for further assessment.
2.1.3 Animals and tumor cells
The transplantable murine tumor cells, namely EAC cells, were obtained from the National Cancer Institute of Cairo University. The EAC cells were maintained in the ascitic form in vivo by sequential passages in Swiss mice by means of intraperitoneal transplantation of 2 × 106 cells/mouse/0.2 mL once at the beginning of the trial. Ascitic fluid was drawn out from EAC-bearing mice 8 days after transplantation. The freshly drawn fluid was diluted with ice cold sterile normal saline, and the tumor cell count was adjusted to 2 × 106 cells/mL by sterile normal saline.
2.2 Methods
2.2.1 In-vitro antioxidant assay
2.2.1.1 Estimation of total phenolic content
Total phenolic content of the extracts were determined using the Folin–Ciocalteu micro-method (Slinkard and Singleton 1977). Briefly, 20 µl of ethanol extract were mixed with 1.16 mL distilled water and 100 µl of Folin–Ciocalteu reagent, followed by addition of 300 µl of Na2CO3 solution (20%) after 1 min and before 8 min. afterwards, the mixture was located in a shaking incubator at 40 C° for 30 min and its absorbance was measured at 760 nm in a Cintra 20 (GMBH, Germany) double beam spectrophotometer. The phenolic content was defined as gallic acid equivalents using the following linear equation based on the calibration curve: A = 0.98C + 9.925 × 10-3; R2 = 0.9996, where A is the absorbance and C is concentration as gallic acid equivalents (µg/g).
2.2.1.2 Determination of total antioxidant activity
The total antioxidant activity of ES extracts were detected using the phosphomolybdenum complex method (Prieto et al., 1999); 0.4 mL of sample extract (100 μl/mL methanol) was mixed with 4 mL of phosphomolybdenum complex containing 0.6 M sulphuric acid, 2 mM sodium phosphate, and 4 mM ammonium molybdate. Test tubes were capped and positioned in hot water for 90 min at 95 °C. Samples were cooled to room temperature and the absorbance was measured at 695 nm on a spectrophotometer (TU-1800; Human Corporation). Antioxidant activity was expressed as the mg ascorbic acid equivalent per mL (mg AE/mL).
2.2.2 Experimental design
Eighty Swiss male albino mice were obtained from the College of Pharmacy, King Saud University, and kept in an animal house in the Department of the Community Health Sciences, College of Applied Medical Sciences, King Saud University, Riyadh, KSA. Subsequently, 25–30-g male Swiss albino mice were separated and classified into four groups (n = 20) fed with basal diet. Except group 1, all animals were infused once with 2 × 106 EAC cells intraperitoneally in the abdominal area to determine the cytotoxic carcinoma. Group 1 was administered only normal saline. Group 2 did not receive any treatment (disease control). Group 3 and 4 were orally administered once daily ES leaf and seed extract, respectively. The optimal dose of the extracts was selected as 250 mg/kg per mice after literature review (Alqasoumi, 2010; Lamy et al., 2008), administered for 9 successive days. On the 10th day, the animals were sacrificed and blood was collected to study the cancer pathological biochemical markers and hematological indicators. The median survival time (MST) and changes in BW were determined in all groups. The MST of the treated groups was matched with that of the control group by measuring the increased in life span (ILS). The study protocol was approved by the Ethical Committee of the College of Applied Medical Sciences, King Saud University, Saudi Arabia (ethics number: CAMS 22-37/38). Research principles and ethical guidelines of the KSU-CAMS Research Ethics Committee were strictly observed for all animal experiments. All animals were terminated initially using isoflurane, followed by cardiac puncture and cervical dislocation.
2.2.3 Antitumor determinations
Tumor volume was estimated by collecting the ascitic fluid from the peritoneal cavity, and volume was calculated by collecting it in a graduated centrifuge tube (Kundusen et al., 2011).
Tumor Weight was measured by calculating the weight of the mice pre and post ascitic fluid collection from the peritoneal cavity after dissecting the mice (Kundusen et al., 2011). Also, tumor inhibition ratio (TIR) was calculated according to by the following equation (Kapoor et al., 2014)
2.2.4 Determination of hematological parameters
Collected blood was used for the estimation of hemoglobin (Hb) content and red blood cell (RBC) count (D’Armour et al., 1965).
2.2.5 Estimation of biochemical parameters and antioxidant assays
The blood collected was allowed to clot and serum was collected at 2500 rpm for 15 min for analyses of AST, ALT, ALP, Creatinine and urea by using Sigma Aldrich kits, USA. Contrastingly, the liver was excised and cleaned by normal ice cold saline and then rinsed with 10% KCl solution. Afterwards, tissues were sliced up and homogenised in an optimal buffer (pH 7.0) in cooling mode to get 20% homogeneity (w/v) then centrifuged at 1000 rpm in a cold centrifuge for 10 min at 0° C, the supernatants were excluded for multiple biochemical determinations. The antioxidant assay of liver tissue was measured such as MDA (Ohkawa et al., 1979) Lipid peroxidation (LPO) was determined by quantifying malondialdehyde (MDA) that formed in terms of thiobarbituric acid reactive substances (TBARS), superoxide dismutase (SOD) activity (Kakkar et al., 1984) calorimetrically was determined using the inhibition method of nitroblue tetrazolium (NBT)-bathocuproine sulfonate (BCS) reduction, catalase (CAT) (Aebi, 1983) was assessed on the basis of reduction of dichromate in acetic acid to chromic acetate. One unit of CAT activity is defined as the amount of enzyme that degrades one mmol H2O2/min. Glutathione peroxidase (GPx) (Rotruck et al., 1973) Glutathione peroxidase (GPx) was determined with slight adjustments as the GPx activity unit is taken as the amount of enzyme which consumes 1/ µmol NADPH/min. and total protein (TP) (Lowry et al., 1951).
2.2.6 Histological assays
Carcinomas and normal liver and kidney tissues were examined according to Yılmaz et al. (2019) with some modifications, liver and kidney tissues were fixed with 10% phosphate buffered neutral formalin, dehydrated in alcohol (50–100%) grade, and stored in paraffin. Thin sections with thickness of 5 μm were cut and stained with regular hematoxylin and eosin stain for photo microscopic evaluation. The initial examination was qualitative, in order to determining histopathological lesions.
2.2.7 Statistical analysis
Received data are presented as mean ± standard error of the mean. One-way analysis of variance and Duncan’s multiple range test have been used in the analysis. P-values < 0.05, 0.01, and 0.001 indicated statistical significance.
3 Results
3.1 Determination of total antioxidant activity and phenols of ES extract
The main bioactive characteristics of the ES extracts were revealed in Table 1 as total phenols and total antioxidant activity, The mean total phenol levels in leaf and seed extracts were expressed as gallic acid equivalent per 100 mL as 1.634 and 2.132 mg·mL−1 respectively, while total antioxidant activity were expressed as ascorbic acid equivalent as 1.431 and 1.722 mg·mL−1, respectively. *Expressed as gallic acid equivalent per 100 mL red grape seed extract. **Expressed as ascorbic acid equivalent.
Properties Plant parts
Total phenols (mg/mL−1) *
Total antioxidant activity (mg/mL−1) **
Leaves
1.634 ± 0.014
1.431 ± 0.17
Seeds
2.132 ± 0.012
1.722 ± 0.13
3.2 Effect of ES on body weight gain, food consumption and feed efficiency ratio
As a significant reduction of BW was obtained in tumor mice compared to the normal group. Table 2 shows that ES extract of seeds or leaves was administered to tumor mice, and the results showed significantly increased BW with maximum gain of 37.46% and 31.39%, respectively, compared to the EAC group. *ESTGs, Eruca sativa-treated groups. Significant with control (-ve) group * P < 0.05 ** P < 0.01 ***P < 0.001 Values with the same letters in column indicate nonsignificant difference (P > 0.05) and vice versa
Properties Plant parts
Body weight gain %
Food consumption (g / day)
Feed efficiency ratio (FER)
Normal control
46.11 ± 4.12a
24.71
0.098 ± 0.002a
Induced control
11.11 ± 0.78b**
19.77
0.029 ± 0.004d***
*ESTGs
Leaves
31.39 ± 3.21a
20.55
0.072 ± 0.006c**
Seeds
37.46 ± 2.17a
21.34
0.086 ± 0.006b*
3.3 Effect of ES on tumor volume, tumor weight, and tumor inhibition ratio of Ehrlich ascites carcinoma mice
Intraperitoneal administration of ES leaves and seeds extracts significantly decreased the mean of the tumor volume with 24.5% and 40.8% respectively, tumor weight with 22.6% and 35.4% correspondingly as compared to the EAC control mice as shown in Table 3. *ESTGs means Eruca Sativa treated groups. **Significant with control (+ve) group * P < 0.05 ** P < 0.01 *** P < 0.001. ***Values with the same letters in column indicate non-significant difference (P > 05) and vice versa.
Parameters Groups
Tumor volume (mL)
Tumor weight
Tumor Inhibition Ratio
Normal Control
–
–
–
Induced Control
3.6 ± 0.18a
5.22 ± 0.25a
–
ESTGs
Leaves
2.72 ± 0.17b*
4.04 ± 0.22b
0.22a*
Seeds
2.13 ± 0.15b*
3.37 ± 0.22b*
0.35a*
3.4 Effect of ES on life span analysis of Ehrlich ascites carcinoma mice
Experimental and normal mice life span analysis of normal, tumor, and treated group is presented in Table 4. The survival time, life span ratio, and mean survival time of tumor mice significantly increased compared with those in normal mice. ES extract of seeds or leaves administered to tumor mice significantly restored the survival time, life span ratio, and mean survival time near those of normal mice. *ESTGs, Eruca Sativa-treated groups. Significant with control (-ve) group *P < 0.05 **P < 0.01 ***P < 0.001. Values with the same letters in column indicate nonsignificant difference (P > 05) and vice versa. T/C = treated mice vs induced control.
Properties Plant parts
Survival time (days)
Mean survival time (days)
Increasing life ratio %
T/C %
Normal control
45
45
0
–
Induced control
15–21
18
0
–
ESTGs
Leaves
24–36
30
66.66
166.66
Seeds
27–41
34
88.88
188.88
3.5 Effect of ES on blood erythrogram of Ehrlich ascites carcinoma mice
Blood erythrogram status of normal, tumor, and treated groups are shown in Table 5. The blood erythrogram status, such as RBCs, Hb, and PCV, in tumor mice statistically significantly increased in tumor mice compared to that of normal mice. ES extract-administered group had reduced blood erythrogram status compared to tumor control mice. The MCV and MCH levels significantly decreased in tumor mice and significantly increased in tumor mice administered with ES extract seeds or leaves. *ESTGs, Eruca sativa-treated groups. RBC, red blood corpuscles; MCV, mean corpuscular volume; Hb, Hemoglobin; MCH, mean corpuscular hemoglobin; PCV, packed cell Volume; Significant with control (-ve) group * P < 0.05 ** P < 0.01 *** P < 0.001. Values with the same letters in column indicate nonsignificant difference (P > 0.05) and vice versa.
Parameters
Groups
RBCs (×106 µl)
Hb (g %)
PCV (%)
MCV (fl)
MCH (pg)
Normal control
10.35 ± 0.26a
13.84 ± 0.17a
48.48 ± 0.81a
45.91 ± 0.36c
14.11 ± 0.27c
Induced control
7.86 ± 0.19c***
10.90 ± 0.20c***
37.56 ± 0.76c***
50.58 ± 0.39a***
15.82 ± 0.22a***
ESTGs
Leaves
8.78 ± 0.40b*
11.93 ± 0.19b*
39.72 ± 0.44b*
47.06 ± 1.48b*
15.22 ± 0.33ab**
Seeds
9.01 ± 0.20ba**
12.37 ± 0.13ba**
41.26 ± 0.69ba**
47.73 ± 1.23b*
14.90 ± 1.44bc**
3.6 Effect of ES on leukogram profile of Ehrlich ascites carcinoma mice
Leukogram parameters, such as TLC, lymphocyte, MID, and GRA of normal, tumor, and treated groups are shown in Table 6. TLC, lymphocyte, MID, and GRA levels were high in tumor mice, and treatment with ES extract of seeds or leaves significantly reversed these parameters compared with the tumor control group. *ESTGs, Eruca sativa-treated groups. TLC, total leukocytic count; LYM, lymphocytes; MID, monocytes and some eosinophils; GRA, neutrophils, eosinophils, and basophils. Significant with control (-ve) group *P < 0.05 **P < 0.01 ***P < 0.001. Values with the same letters in column indicate non– significant difference (P > 0.05) and vice versa.
Parameters
Groups
TLC (×103 /µL)
Lymphocyte (×103 /µL)
MID (×103 /µL)
GRA (×103 /µL)
Normal control
8.82 ± 0.29c
6.99 ± 0.42c
0.56 ± 0.03c
0.34 ± 0.02c
Induced control
12.66 ± 0.21a***
9.73 ± 0.07 a***
1.39 ± 0.01 a***
0.67 ± 0.04 a***
ESTGs
Leaves
9.37 ± 0.21b*
7.61 ± 0.32b*
0.82 ± 0.04b*
0.50 ± 0.02b*
Seeds
10.31 ± 0.33b*
8.12 ± 0.21b*
0.94 ± 0.04b*
0.43 ± 0.03 bc**
3.7 Effect of ES levels on serum kidney functions and liver biomarkers of Ehrlich ascites carcinoma mice
AST, ALT, and ALP liver enzymes activities of normal, tumor, and treated groups are shown in Table 7. The liver marker enzymes were shown to be increased in tumor mice, and these enzyme activities were reversed toward normal mice after treatment with ES extract of seeds or leaves. Urea and creatinine levels of normal, tumor, and treated groups are presented in Table 7. Urea and creatinine levels were enhanced in tumor mice. These abovementioned parameters significantly decreased after treatment with extract compared to those in tumor control mice. *ESTGs, Eruca sativa-treated groups. Significant with control (-ve) group *P < 0.05 **P < 0.01 ***P < 0.001. Values with the same letters in column indicate nonsignificant difference (P > 0.05) and vice versa.
Groups
AST (U/L)
ALT (U/L)
ALP (IU/L)
Creatinine (mg/dL)
Urea (mg/dL)
Normal control
55.41 ± 2.1c
45.11 ± 5.31c
40.21 ± 4.15c
0.66 ± 0.04c
38.39 ± 0.50c
Induced control
165.46 ± 1.3a***
146.21 ± 1.13a***
110.27 ± 3.71a***
1.06 ± 0.02a***
64.62 ± 0.83a***
ESTGs
Leaves
70.62 ± 1.9b*
59.61 ± 6.20b*
61.91 ± 6.21b*
0.84 ± 0.03b*
50.55 ± 2.22b*
Seeds
63.27 ± 1.6bc**
54.35 ± 5.66bc**
55.11 ± 5.31bc**
0.71 ± 0.04bc**
46.63 ± 3.34bc**
3.8 Effect of ES levels on liver tissues lipid peroxide MDA, GPx, SOD, and CAT of Ehrlich ascites carcinoma mice
The impacts of Eruca Sativa leaves and seeds administration on liver tissues lipid peroxidation, glutathione peroxidase and enzymatic antioxidants, SOD and CAT, levels of ehrlich ascites carcinoma rats are presented in Table 8 *ESTGs, Eruca sativa-treated groups. Significant with control (-ve) group * P < 0.05 ** P < 0.01 *** P < 0.001. Values with the same letters in column indicate nonsignificant difference (P > 0.05) and vice versa.
Groups
CAT(µ/mg)
SOD(µ/mg)
GPX(µ/mg)
MDA(mmol/L)
Normal control
1.35 ± 0.03a
53.17 ± 5.10a
48.46 ± 4.40a
8.64 ± 1.15b
Induced control
0.63 ± 0.02d***
24.19 ± 2.11c***
20.01 ± 2.60c***
16.52 ± 2.11a***
ESTGs
Leaves
0.86 ± 0.04bc**
43.64 ± 3.14b*
34.27 ± 3.61b*
9.10 ± 1.03b*
Seeds
1.03 ± 0.05b*
45.23 ± 3.12b*
36.48 ± 4.52b*
9.58 ± 1.10b*
SOD, CAT, and GPx levels significantly (p < 0.05) decreased in tumor mice, whereas MDA level significantly increased due to effect of ehrlich ascites carcinoma. These abnormal lipid peroxidation and antioxidant statuses were significantly (p < 0.05) reversed to near normal after treatment with Eruca Sativa seeds and leaves extracts respectively.
3.9 Effect of ES on liver and kidney histopathological examination of Ehrlich ascites carcinoma mice
Microscopically, as shown in Fig. 1, liver of mice from normal group revealed the normal histological formation of hepatic lobule. Whereas, intraperitoneal injection of EAC led to development of severe infiltration invasion by parenchymal cells with infiltration of the portal tract by chronic inflammatory cells and acute congestion of blood vessel, these were manifest as cell proliferation and proximal regions of necrosis. Administration ES leave extract to EAC showed moderate infiltration of tubule interstitial accompanied by congestion of vessel and mild compression of capillary luminal, resulted in moderate regression of tumor development. Examined sections from mice with EAC administrated ES seed extract revealed a high regression of tumor development, observed in slight vacuolization of hepatocytes with minimal infiltration of interstitial by inflammatory cells, in addition, Decline in cell proliferation and proximal regions of necrosis.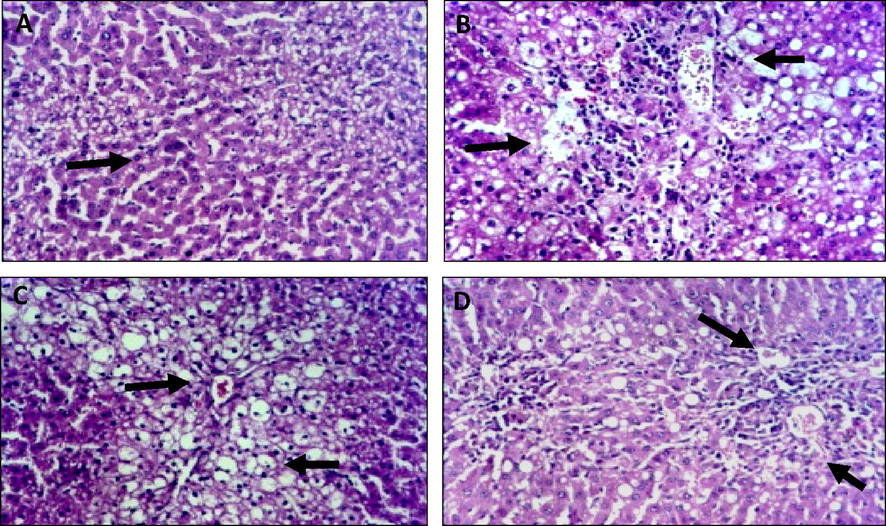
Histological findings of liver of normal, Ehrlich Ascites Carcinoma, and Eruca Sativa treated groups’ tissues. Normal control, showing no histopathological changes. A. Mice of EAC induced control without treatments showing severe infiltration invasion by parenchyma cells with infiltration of portal tract by chronic inflammatory cells and acute congestion of blood vessel. B. ESTG with leaves showing mild infiltration with congestion. C. ESTG with seeds showing mild degenerative with individual coagulative necrosis.
Microscopically, as shown in Fig. 2, kidneys of mice from normal group revealed the normal histological formation or renal parenchyma. Vice versa, intraperitoneal injection of EAC led to progress of severe infiltration and necrosis with intense infiltration on the tubule interstitial by chronic inflammatory cells, accompanied by vacuolar degeneration of epithelial lining renal tubules as well as vacuolar degeneration of endothelial lining glomerular tufts.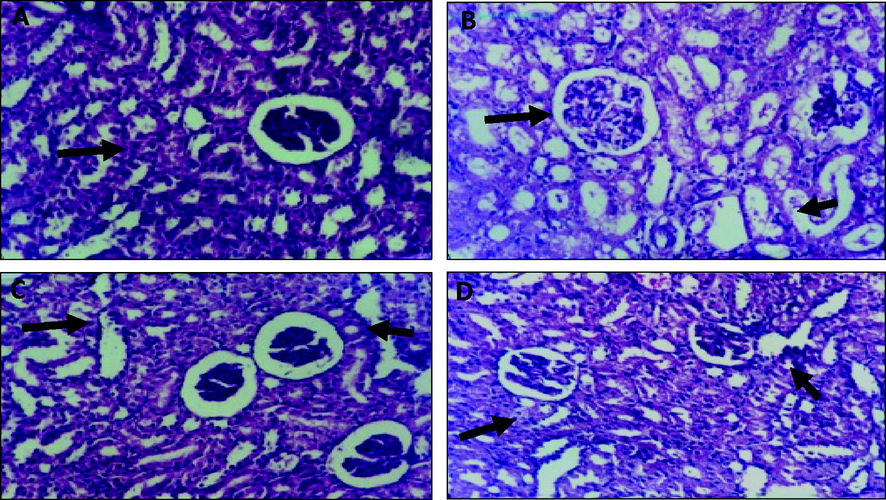
Histological findings of Kidney of normal, Ehrlich Ascites Carcinoma, and Eruca Sativa treated groups’ tissues. A. Normal control, showing no histopathological changes. B. Mice of EAC induced control without treatments showing severe necrosis with intense infiltration on the tubule interstitial by chronic inflammatory cells. C. ESTG with leaves showing moderate infiltration of tubule interstitial accompanied by congestion of vessel and mild compression of capillary luminal. D. ESTG with seeds showing minimal infiltration of interstitial by inflammatory cells.
Administration ES leave extract to EAC expressed moderate infiltration of tubule interstitial accompanied by congestion of vessel and mild compression of capillary luminal. Examined sections from mice with EAC administrated ES seed extract revealed minimal infiltration of interstitial by inflammatory cells. Moreover, slight hypertrophy of glomerular tuft as well as presence of eosinophilic protein cast in the lumen of some renal tubules.
4 Discussion
Recently, researchers have focused on some herbs for alternative cancer therapies due to less toxicity and cost benefits. Consequently, we also made an attempt to assess the effect of ES leaf or seed extract on tumor-induced mice. The Ehrlich tumor is a hastily growing carcinoma, and this ideal model has been used mostly by researchers to evaluate the pathophysiology of tumor and discover an anti-tumorigenic drug (Segura et al., 2000). Therefore, we have used this cancer-induced model to assess the effect of ES leaf or seed extract.
The percentage of BW in the Ehrlich group decreased and increased with ES extract treatment compared to the tumor group. Significant differences were not found between normal and ES extract-treated groups (p > 0.05).
One of the main criteria for assessing successful anticancer and antitumor compound is it should be willing to extend lifetime and reduce the leukocyte count (Kundusen et al., 2011). It was observed in our study that there was statistically noticeable difference in tumor volume and weights, for EAC induced control and ES treated groups. The anticancer stimulatory effects were observed by increasing the percentage in overall life span and the tumor suppression ratio (Kapoor et al., 2014).
Animal life span prolongation is noted as a reliable marker for the representation of the effectiveness of an anticancer agent (Haldar et al., 2010). The causative factor of life span abnormalities of tumor mice might be increased volume of nutritional fluid and cessation of the tumor growth. With the administration of the extract to the tumor mice, the ILS in mice might be due to a decrease in nutritional fluid volume, parallel to the results of a previous finding (Gupta et al., 2004). Anemia is an important symptom for a widespread hematological cancer, and this occurrence has been increased by administration of chemotherapy/radiotherapy. Hemoglobin and red blood cells have decreased in tumor cells due to the destruction and/or lack of ability of the bone marrow (Gaspar et al., 2015). The lifespan was increased, and the Hb content and RBC, PCV, MCV, and MCH levels returned to normal levels after treatment with the extract. Hence, our results provide a supportive hematopoietic protective effect of ES (Pandya et al., 2013). Experimental and human studies have documented that the tumors affect the functions of vital organs, especially the liver (Rubbi and Milner 2003). Decrease oxidative stress plays an important role in reduction of hepatic cell injury or damage. Diminished antioxidant status and frequent generation of free radicals are well known evidence in carcinogenesis (Szatrowski and Nathan 1991). It proved that oxidative stress is involved in the pathogenesis of tumor-induced liver oxidative damage; then the treated antioxidant potential sources protect against that tumor-induced liver oxidative damage. Therefore, decreased MDA level was shown after treatment with ES extract, which prevented cellular injury. Enzymatic antioxidants play a vital function in cells for free radical elimination. It has been stated that SOD activity decreases in tumor mice might be due to the diminished activity in the mitochondria, and loss of Mn2þ containing SOD activity in tumor cells finally results in diminished activity of total hepatic SOD (Rushmore and Picket 1993). SOD activity also declined in carcinoma mice decline which might be due to a loss of mitochondria as cited by other study (Sahu et al., 1977). In this study showed that extract enhanced the antioxidant status as compared with tumor mice, which is confirmed the antioxidant property of ES. The higher levels of hepatic ALT, AST, and ALP activities were found in tumor mice in our study due to toxic activity of tumor hepatic cells, which leads to increase in cell permeability or destruction in liver. Significant diminished activities of liver ALT, AST, ALP, creatinine, and urea levels were found in tumor mice after treatment with extract. Additionally, this study has concluded that tumor mice exhibited increased levels of liver enzymes, which are noted in cell damages, whereas these above mentioned markers were reversed to near normal after treated with extract indicated in hepatoprotective effect. Furthermore, histogram studies for liver and kidney tissues revealed that infusing EAC cells to the healthy mice causes acute cytotoxic cells offensive for the connective tissue capsule (Aldubayan et al., 2019), which resulted in aggressive congested blood vessel and chronic inflammatory collection in liver and severe necrosis with acute intense infiltration on the tubule accompanied by chronic inflammation of kidney tissues, contrarily, administration of ES leaf and seed extracts modulated the intensive clustering in the tumor control group. This improvement in the morphological form of the affected cells resulted in mild inflammation and necrosis after treatment with the extracts.
5 Conclusion
Our current study noticeably demonstrates the antitumor activity of ES extract of seeds and leaves, significantly increasing life span and normalizing the hematological profiles and biochemical parameters compared with EAC mice. Our studies concluded that the rocket salad with higher phenolic content more effectively inhibits the growth of EAC. Hence, antitumor and antioxidant effects of ES against EAC could be attributed to its glucosinolate, flavonoid, and phenolic contents.
6 Compliance with ethical standards
The Author(s) declare(s) that the work is in compliance with ethical standards.
7 Availability of data and materials
All the data is contained in the manuscript.
Author contribution
MF and BA designed the study, conceived the study and analyzed the results. MF and ME conceived an initial part of the study, performed the experiment, histology and helped in compiling the results. BA and ME performed tissue histology and helped in writing the results. MF, BA wrote the paper with input from all other authors. MF, BA and ME made substantial contribution in interpretation of data and revising the manuscript for intellectual content. All authors read and approved the final manuscript. Authors also declare that all data were generated in-house and that no paper mill was used.
Acknowledgement
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saudi University for supporting of this research through the research group project no (RG-1439-81).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Aebi HE. “Catalase,” in Methods in Enzymatic Analysis, H. O. Bergmeyer, Ed., vol. 3, pp. 273–386, Academic Press, New York, NY, USA; 1983.
- Eruca sativa seeds possess antioxidant activity and exert a protective effect on mercuric chloride induced renal toxicity. Food Chem. Toxicol.. 2007;45(6):910-920.
- [Google Scholar]
- Antineoplastic activity and curative role of avenanthramides against the growth of ehrlich solid tumors in mice. Oxid. Med. Cell. Longevity. 2019;2019:1-12.
- [Google Scholar]
- Rocket, “Eruca sativa”: a salad herb with potential gastric anti-ulcer activity. World J. Gastroenterol.. 2009;15(16):1958.
- [CrossRef] [Google Scholar]
- Carbon tetrachloride-induced hepatotoxicity: protective effect of “Rocket” Eruca sativa L. in rats. Am. J. Chin. Med.. 2010;38(01):75-88.
- [Google Scholar]
- Ameliorative effect of Eruca sativa extracts on glucose and urinary volume in streptozotocin-induced diabetic rats. Int J Biol Pharm Allied Sci.. 2014;3:1092-1100.
- [Google Scholar]
- The Manual for Laboratory Works in Mammalian Physiology (third edition). Illinois: Chicago, University of Chicago Press; 1965.
- Gaspar, B.L., Sharma, P., Das, R., Anemia in malignancies: pathogenetic anddiagnostic considerations. Hematology. 201; 1: 18-25.
- Medicinal plants from Peru: a review of plants as potential agents against cancer. Anti-Cancer Agents Med. Chem.. 2006;5:429-444.
- [Google Scholar]
- Antitumor activity and anti–oxidant status of Caesalpinia bonducella against Ehrlich ascites carcinoma in Swiss albino mice. J. Pharmacol. Sci.. 2004;94(2):177-184.
- [Google Scholar]
- Chemopreventive role of Indigofera aspalathoides in 20-methylcholanthrene-induced carcinogenesis inmouse. Toxicol. Environ. Chem.. 2010;92:1749-1763.
- [Google Scholar]
- Evaluation of antioxidant and anticancer properties of the seed extracts of Syzygium fruticosum Roxb. growing in Rajshahi, Bangladesh. BMC Complement Altern Med.. 2013;13(1)
- [CrossRef] [Google Scholar]
- A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Bio. 1984;21:130-132.
- [Google Scholar]
- Anticancer effect of dl-glyceraldehyde and 2-deoxyglucose in ehrlich ascites carcinoma bearing mice and their effect on liver, kidney and haematological parameters. Indian J. Clin. Biochem.. 2014;29(2):213-220.
- [Google Scholar]
- Isolation and structural elucidation of 4- (beta-D-glucopyranosyldisulfanyl) butyl glucosinolate from leaves of rocket salad (Eruca sativa L.) and its antioxidative activity. Biosci. Biotechnol. Biochem.. 2004;68:2444-2450.
- [Google Scholar]
- Antitumor activity of Citrus maxima (Burm.) Merr. leaves in Ehrlich's Ascites Carcinoma cell-treated mice. ISRN Pharmacol.. 2011;2011:1-4.
- [Google Scholar]
- Antigenotoxic properties of Eruca sativa (rocket plant), erucin and erysolin in human hepatoma (HepG2) cells towards benzo(a)pyrene and their mode of action. Food Chem. Toxicol.. 2008;46(7):2415-2421.
- [Google Scholar]
- Studies on the chemical constituents of fresh leaf of Eruca sativa extract and its biological activity as anticancer agent in vitro. J. Med. Plants Res.. 2011;5:1184-1191.
- [Google Scholar]
- Assay for lipid peroxidation in animal tissue by thiobarbituric acid reaction. Anal. Biochem.. 1979;95:351-358.
- [Google Scholar]
- Antitumor and antioxidant status of Terminalia catappa against Ehrlich ascites carcinoma in Swiss albino mice. Indian J. Pharmacol.. 2013;45(5):464.
- [CrossRef] [Google Scholar]
- Rocket salad (Diplotaxis and Eruca spp.) sensory analysis and relation with glucosinolate and phenolic content. J. Sci. Food Agric.. 2011;91(15):2858-2864.
- [Google Scholar]
- Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal. Biochem.. 1999;269(2):337-341.
- [Google Scholar]
- Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179(4073):588-590.
- [Google Scholar]
- Glutathione-S-transferase, structure, regulation and therapeutic implication. J. Biol. Chem.. 1993;268:11475-11478.
- [Google Scholar]
- Superoxide dismutase activity of Ehrlich ascities tumor cells. J. Natl Cancer Inst.. 1977;58:1125e8.
- [Google Scholar]
- Ehrlich ascites tumor unbalances splenic cell populations and reduced responsiveness of m T cells to Staphylococcus aureus enterotoxin B stimulation. Immunomol Lett.. 2000;74:111-115.
- [Google Scholar]
- Ethanolic extract of Mimosa caesalpiniifolia leaves: Chemical characterization and cytotoxic effect on human breast cancer MCF-7 cell line. South African J Bot.. 2014;93:64-69.
- [Google Scholar]
- Total phenol analysis; automation and comparison with manual methods. Am. J. Enol. Viticulture. 1977;28:49-55.
- [Google Scholar]
- Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res.. 1991;51:794-798.
- [Google Scholar]
- Investigating the anti-tumoral effect of curcumin on the mice in which Ehrlich ascites and solid tumor is created. Iran J. Basic Med. Sci.. 2019;22:418.
- [Google Scholar]







