Translate this page into:
Evaluation of amniotic fluid treatment effect on placental stem cells immunomodulatory activity
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The advent of stem cell therapy in treating various complex diseases has become a major breakthrough in clinics. Mesenchymal stem cells are well known for their pluripotency and their immunomodulation feature, therefore research on mesenchymal stem cells is widely acknowledged. Obtaining viable stem cells with a less invasive extraction method as well as ethical issues is a major challenge in stem cell research. Recently, human placental stem cells are becoming more known as the best source of stem cells due to their ease of extraction, lesser ethical constraints, and presence of mesenchymal stem cells. Naturally derived bioactive compounds to modulate the proliferation, regeneration, cell fate and immunomodulation feature of stem cells are under investigation. In the current in-vitro study, the placenta-derived mesenchymal stem cells were treated with amniotic fluid and were observed for their effects. Our results suggest amniotic fluid treatment enhanced proliferation and induced mesenchymal to the epithelial transition of stem cells. Further, we observed changes in the secretion pattern of cytokines as well as growth factors, which are involved in T-cell suppression. Notable, the of TGFβ (17.4 ± 2.3 ng/ml) and IL-10 (21.7 ± 1.9 pg/ml) with the increase in the concentration of AF whereas the TNFα (8.6 ± 2.1 pg/ml) and IL-6 (12.3 ± 1.6 pg/ml) showed a decline in expression cytokine.
Our results suggest amniotic fluid treatment can induce host cell immune suppression; however further validation studies have to be carried out.
Keywords
Amniotic fluid
Epithelial differentiation
Human placental stem cell
Immunomodulation feature
1 Introduction
Advances in stem cell research have contributed to the expansion of regenerative medicine in clinics. Several diseases, including heart diseases, blood diseases, liver disease, Duchenne muscular dystrophy, diabetes, bone disease, and renal disease, have been shown to benefit from stem cell therapy (Larijani et al., 2012). Identification of human stem cell sources that can be isolated and purified with less technical expertise, as well as ethical issues and expanding the stem cells in a controlled manner, are the two main challenges in stem cell research. In spite of having some drawbacks, investigations related to the clinical application of adult and embryonic stem cells are being studied. As damage to human embryos is inevitable during stem cell extraction, major ethical concerns must be addressed. Whereas adult stem cells have limited clinical applicability because of their diminished potential and the retention of the epigenetic changes even after reprogramming. (Matikainen and Laine, 2005).
In studies, the placenta seems to be the stem cell source (Parolini et al., 2008). Because placenta amnion, human cord blood, and umbilical cord are typically thrown away after birth, their accessibility and lack of ethical concerns make them a worthwhile alternative stem cell source. As these cells have both the features of mesenchymal and embryonic stromal cells, many preclinical studies focusing on the therapeutic application of these cells in neonatal illness models have recently been under investigation. Placental stem cell-based therapy has been under pre-clinical investigation for different diseases. For example, mesenchymal cells derived from the stem cells of the human placenta can be employed to treat pancreatic disorders because of their ability to develop human placental stem cells (hPMSCs) into insulin-producing cells (Chang et al., 2007). In the murine osteogenesis imperfecta model, injection of hPMSCs increased bone volume, ductility, and the expression of genes included in endochondral and intramembranous ossification (Jones et al., 2014). These findings point to hPMSCs' potential in cell therapy for a variety of diseases.
Recently, research was going on to identify the potential of naturally obtained compounds to regulate the cell fate of stem cells (Bejaoui et al., 2021). For example, a study reported Genistein and isoflavones in soy can induce osteogenic differentiation via an ER-dependent mechanism (Okumura et al., 2003). Similarly, proliferative effects of naturally derived compounds from medicinal plants on mesenchymal stem cells have also been reported (de la Torre et al., 2019; Potu et al., 2009). However, currently, molecules like cytokines, proteins, and recombinant growth factors are widely employed to induce stem cell differentiation, which can result in adverse effects such as toxicity, malignancy, and an increased risk of rejection (Bejaoui et al., 2021). If protocols for modulating proliferation, differentiation, and stem cell regenerative capacity can be established using naturally obtained compounds and biofluids, it will be a cost-effective method in cell therapy. In the present study, the main focus was on the effects of in-vitro application of amniotic fluid (AF) on hPMSCs. Amniotic fluid (AF) is present in the amniotic sac. It plays a crucial role in the development of the foetus throughout pregnancy. The composition of amniotic fluid varies during the course of pregnancy, the amniotic fluid is mostly made up of water from the mother from fertilisation until eight weeks. The foetus produces urine for about 10 weeks, which enters the amniotic sac. The amniotic sac expands in the second and third trimesters, and during this time the amniotic fluid is mostly composed of foetal urine. In addition to this, the amniotic fluid contains the foetus’ lung secretions, excretions from the umbilical cord, and gastrointestinal secretions.
On reviewing the literature, the current study seems to be a first attempt at studying the effect of AF on hPMSCs. So, understanding the effects of amniotic fluid on placental stem cells would be a new strategy. As the hPMSCs and AF are derived from the same individual, we hypothesise the side effects of the treatment will also be fewer.
2 2.Materials and methodology
2.1 Collection of samples
Placentas were taken from healthy human mothers (n = 12) who were having a caesarean section with healthy characteristics of both the mother (average ± standard deviation: age: 24 ± 2 years; BMI: 20.5 ± 1.9; normal conception, singleton, with no infection, gestational diabetes, and/or preeclampsia normal during delivery) and baby (weighed at the time of 3.12 ± 0.9 kg, with no anomalies or infection). According to institutional ethics guidelines, informed permission was acquired. The term placentas were collected in sterilised containers containing phosphate-buffered saline with the antibiotic–antimycotic solution and transported immediately to the laboratory for further processing. Simultaneously, informed consent was obtained for collecting amniotic fluid (AF) during amniocentesis from the participants (n = 6) who were in the second trimester (27–34 weeks) of gestational period. The health status of the participants who provided AF was recorded as healthy (normal conception, singleton, with no infection, gestational diabetes, and/or preeclampsia, and no anomalies were detected in the AF as a part of the usual investigation). The amniotic fluid was brought to the laboratory and passed through a 0.22-µm filter (Corning, NY, USA). After that, they were stored at minus eighty degrees Celsius until they were needed for the experiment. Manufacturer details of all the products used in the present study are listed in Table 1.
Product
Manufacturer
0.25 percent Trypsin-EDTA
Invitrogen, Carlsbad, CA, USA
Fetal Bovine Serum
Gibco, Rockville, MD, USA
Cell strainer
Corning, NY, USA
Tissue culture flask
Nunc, Rochester, NY, USA
Cell culture media (DMEM + 10% FBS + antibiotic–antimycotic)
Invitrogen, Carlsbad, CA, USA
0.25 percent Trypsin-EDTA solution
Nunc, Rochester, NY, USA
ELISA kits
Krishgen Biosystems, Los Angeles, CA, USA
Multiskan FC spectrophotometer
Thermo Scientific, San Jose, CA, USA
RNA purification kit
Thermo Scientific, Vilnius, Lithuania
cDNA synthesis kit
High Capacity, Applied Biosystems, Carlsbad, CA, USA
Quantitative gene analysis
SYBR Green PCR master mix (Applied Biosystems, Austin, TX, USA)
GraphPad Prism 8 software
GraphPad Software, La Jolla, CA, USA
2.2 Isolation and expansion of placental stem cells (PMSCs)
Tissue chunks from the chorionic villus region were taken out and subsequently sliced into little pieces for the enzymatic digestion procedure. The tissue was then sectioned into tiny pieces and mixed with trypsin-EDTA. The obtained digest was neutralised by passing it through a strainer with a desirable Fetal Bovine Serum (FBS) volume. Resuspending single cells in entire culture media resulted in a single cell suspension. A cell strainer of 70 µm was used to run the combination once again. Later, it was plated in a tissue culture flask fed with a cell culture medium maintained with 5% CO2 at 37 degrees Celsius for 24 h. The growth of the cell, the health of the cell, and the morphology of the cell were analysed using a phase-contrast microscope after replacing the culture medium two times per week. After seventy to eighty percent confluence, the cells were removed and transferred to a 25-cm2 large culture flask made of polystyrene. A trypsin-EDTA solution was used in the flask. Confluent PMSCs were removed and continually passaged in for expansion with trypsin-EDTA. Passage 4 cells were used in this study.
2.3 Growth curve plotting by cell counting
To determine the proliferation rate of PMSCs, 1 × 104 PMSCs at passage 4 cells were placed on the 12-well cell culture plates and separated into four groups. PMSCs were separated into four groups and given varying doses of amniotic fluid (0 percent, 5%, 10%, and 20%). The cell count was evaluated every day up to 13 days. Cell counts were collected for 13 days in order to plot the growth curve.
2.4 Flow cytometry analysis for PMSCs cell surface marker
The PMSCs were divided into four groups and given different concentrations of amniotic fluid for seven days. Control and treated PMSCs were trypsinized and washed twice with PBS for MSC-specific cell surface marker analysis.
2.5 Analysis of cytokine and growth factor levels of IL-10, IL-6, TNF-alpha, TGF-beta, EGF, IGF, FGF, and VEGF in the conditioned media by ELISA
Human ELISA kits were used to analyse at the protein level of soluble cytokines and growth factors. After processing with different concentrations of amniotic fluid in the complete medium, conditioned media from PMSCs was obtained. The procedure was carried out as per the experimental instructions mentioned by the manufacturer. A microplate photometer spectrometer was used with an absorbance level of 450 nm and observed.
2.6 Real-time quantitative polymerase chain reaction: Gene expression using quantitative analysis
Total RNA from the cells was extracted with an RNA purification kit and quantified using NanoDrop spectrophotometers. The extracted RNA (2 μg) was reverse transcribed with random primers using a cDNA synthesis kit, following the protocol given by the manufacturer (Table 1). Then, for gene expression, 20 μl of total reaction volume was utilised to determine the expression of each gene, with the specific forward and reverse primers (Table 2) added to the 100 ng of total cDNA along with the reaction master mix. The real-time qPCR 7900 HT Fast system was used to perform the reaction, and the 2–ΔΔCt method was adopted to calculate the relative expression of each target gene that was normalised with the GAPDH housekeeping gene.
Gene
Forward primer
Reverse primer
OCT4
5′-CCT GAA GCA GAA GAG GAT CAC C-3′
5′-AAA GCG GCA GAT GGT CGT TTG G-3′
SOX2
5′-GCT ACA GCA TGA TGC AGG ACC A-3′
5′-TCT GCG AGC TGG TCA TGG AGT T-3′
NANOG
5′-CTC CAA CAT CCT GAA CCT CAG C-3′
5′-CGT CAC ACC ATT GCT ATT CTT CG-3′
SSEA4
5′-TGG ACG GGC ACA ACT TCA TC-3′
5′-GGG CAG GTT CTT GGC ACT CT-3′
GAPDH
5′-GTC TCC TCT GAC TTC AAC AGC G-3′
5′-ACC ACC CTG TTG CTG TAG CCA A-3′
2.7 Analysis of statistics
Three independent values from the experiment with a mean +/- standard deviation were used to tabulate the results. A two-tailed unpaired t-test was done to compare the between-group results. It was done on GraphPad Prism 8 software. The grading of the results was given as significant at p <.05, and not significant (ns).
3 Results of the study
3.1 Isolation of PMSCs from the chorionic villus tissue
3.1.1 Morphological and growth kinetics of PMSCs treated with different AF concentrations
The PMSCs were isolated from the tissue of chorionic villus according to the standard protocol. The isolated PMSCs were expanded for further analysis. The morphological and growth curve analyses of the PMSCs were carried out. The PMSCs showed a stable “fibroblast-like” spindle morphology. With an increase in the concentration of AF, the morphology of PMSCs changed (Fig. 1A). In 20% of AF, the change in morphology of PMSCs was more obvious, and the cells showed an epithelial-like phenotype. Further, we carried out a growth curve analysis of PMSCs treated with AF. The growth curves of PMSCs treated with different concentrations AF and compared to controls are shown in Fig. 1B. We examined an increase in growth rate in PMSCs when treated with AF. The growth rate of PMSCs treated with 20% of AF was higher than the other treatments as well as the control. Our results indicate an increase in the growth rate of PMSCs, which is attributed to the AF treatment.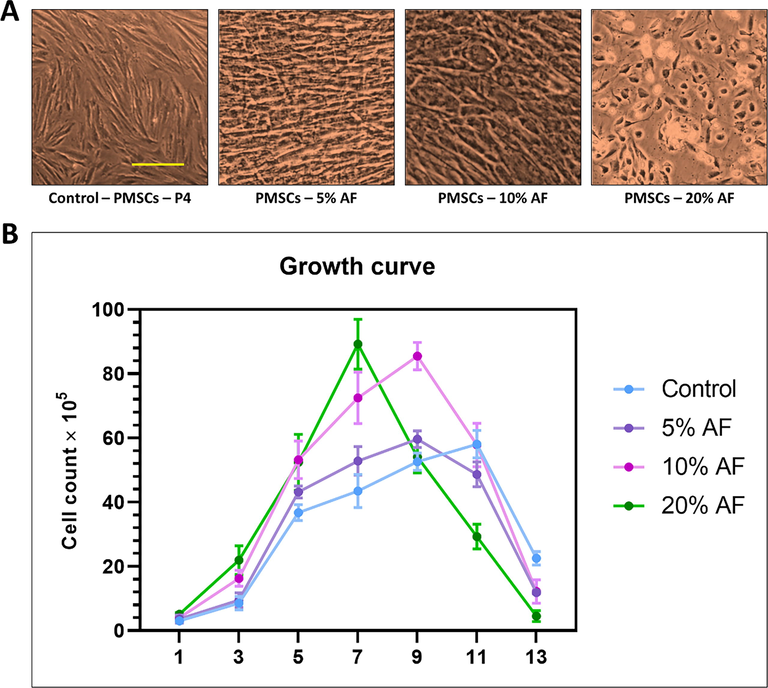
(A) Comparative morphology by photomicrographs after 7 days. (B) Population doubling time analysis by cell counting of placental stem cells after 13 days treatment with different concentrations of amniotic fluid. Scale bar: 200 μm. AF: Amniotic fluid.
3.2 Expression of epithelial and mesenchymal markers in PMSCs treated with AF
The cellular morphology characteristics of PMSCs treated with AF depicted a variable morphology from the parental cells. We observed that the treated cells showed an epithelial-like morphology, especially when treated with 20% AF. Thus, we aimed to investigate the expression of epithelial as well as mesenchymal markers in these cohorts. N-cadherin and vimentin were the mesenchymal markers used to check the gene expression. E-cadherin and cytokeratin were the epithelial markers used in the study. With the increase in the concentration of amniotic fluid, the expression of the epithelial markers increased significantly as the amniotic fluid concentration increased. Whereas there was a decline in the expression of mesenchymal markers upon AF treatment, PMSCs treated with 20% of AF showed the highest expression of ECAD and CK compared to other treated groups and controls. Similarly, the expression of mesenchymal markers was also significantly reduced in PMSCs treated with 20% AF (Fig. 2). These observations together suggest AF might have induced epithelial differentiation in PMSCs upon treatment.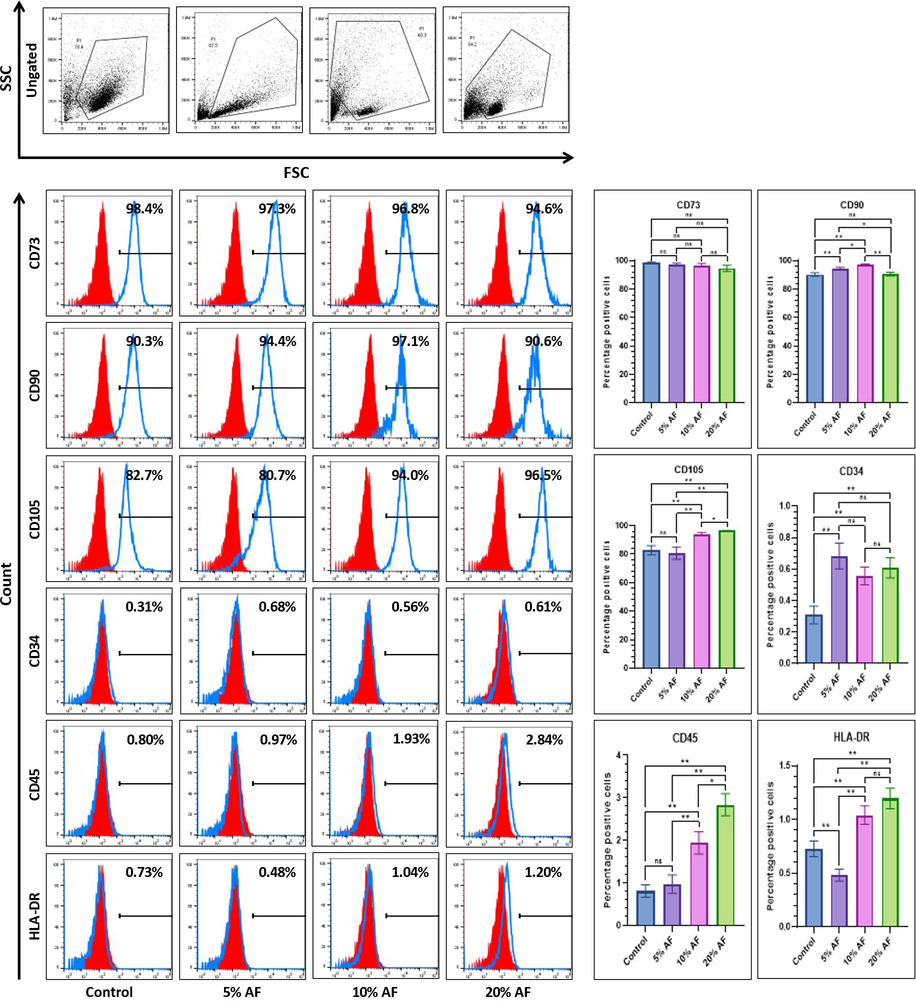
Comparative mesenchymal and epithelial marker analysis by flow cytometry of placental stem cells after 7 days treatment with different concentrations of amniotic fluid. *p<.05, **p<.01, and not significant (ns). AF: Amniotic fluid.
3.3 Expression of mesenchymal stem cell markers in PMSCs treated with AF
We further evaluated the mesenchymal stem cell surface marker expression in PMSCs treated with AF. Markers of mesenchymal stem cell for its expression was using CD90, CD105, pan-hematopoitic, and CD73, MHC Class ll molecule markers such as HLA-DR, CD45, CD34. Although the epithelial and mesenchymal markers support mesenchymal to epithelial transition there was no variation among the expression of mesenchymal stem cell marker CD34 expression among the treated cells as well as control. Similarly, we could not notice any difference in the expression of CD90 between the control and PMSCs (20% AF), while a high expression of CD105 was observed in PMSCs (20% AF). The hematopoietic stem cell markers like HLA-DR, CD45, and CD34 showed significantly higher expression in 20% of AF-treated PMSCs compared to the control (Fig. 3).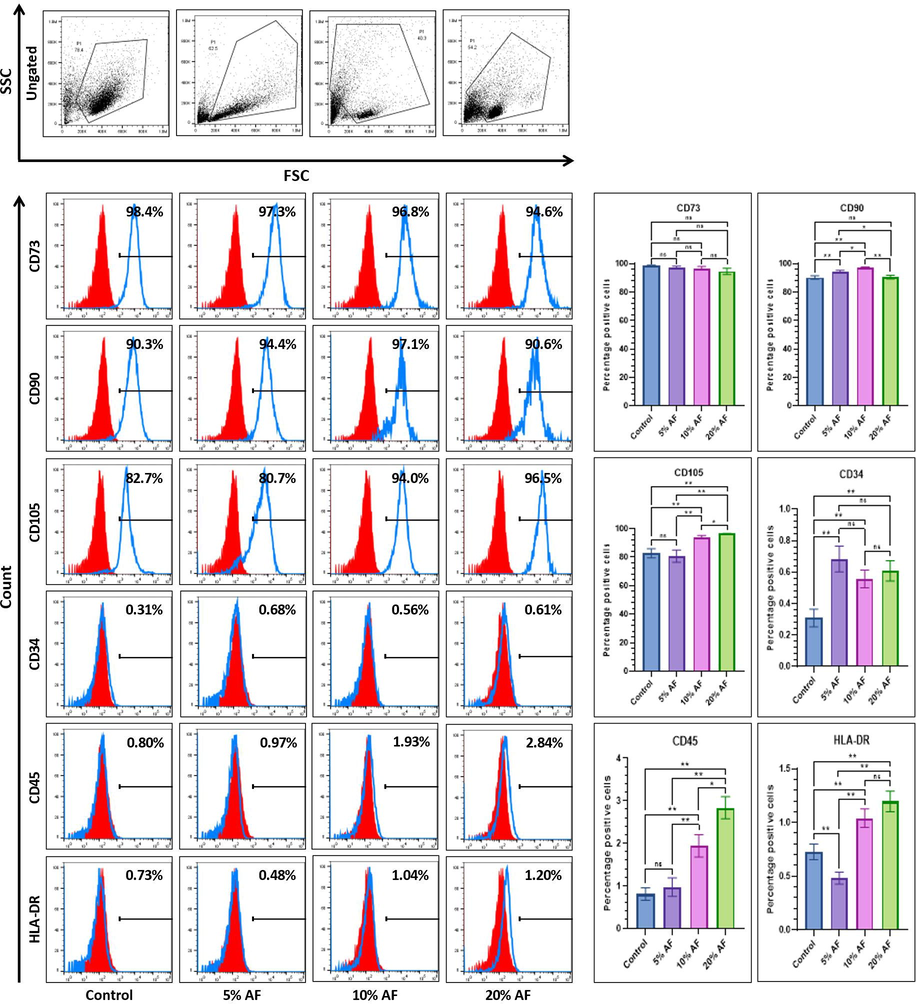
Comparative flow cytometry marker analysis for surface of MSC-specificplacental stem cells after 7 days treatment with different concentrations of amniotic fluid. *p<.05, **p<.01, not significant (ns). AF:Amniotic fluid.
3.4 Stemness and pluripotency-related transcription factor gene expression analysis of placental stem cells
RT-PCR-based comparative expression of embryonic stem cell markers (SSEA4, Oct4, SOX2, and NANOG) was also carried out in PMSCs treated with AFs. The expressions of stem cell markers like Sox2 and NANOG were down regulated upon treatment with AF, whereas no statistically significant variation was observed with Oct4 expression among the untreated and 20% treated PMSCs. PMSCs treated with 10% of AF showed high expression of Oct4. However, after AF treatment, the surface marker for pluripotency, SSEA4, increased expression. Fig. 4 shows the expression of the transcription factors during the different treatment conditions.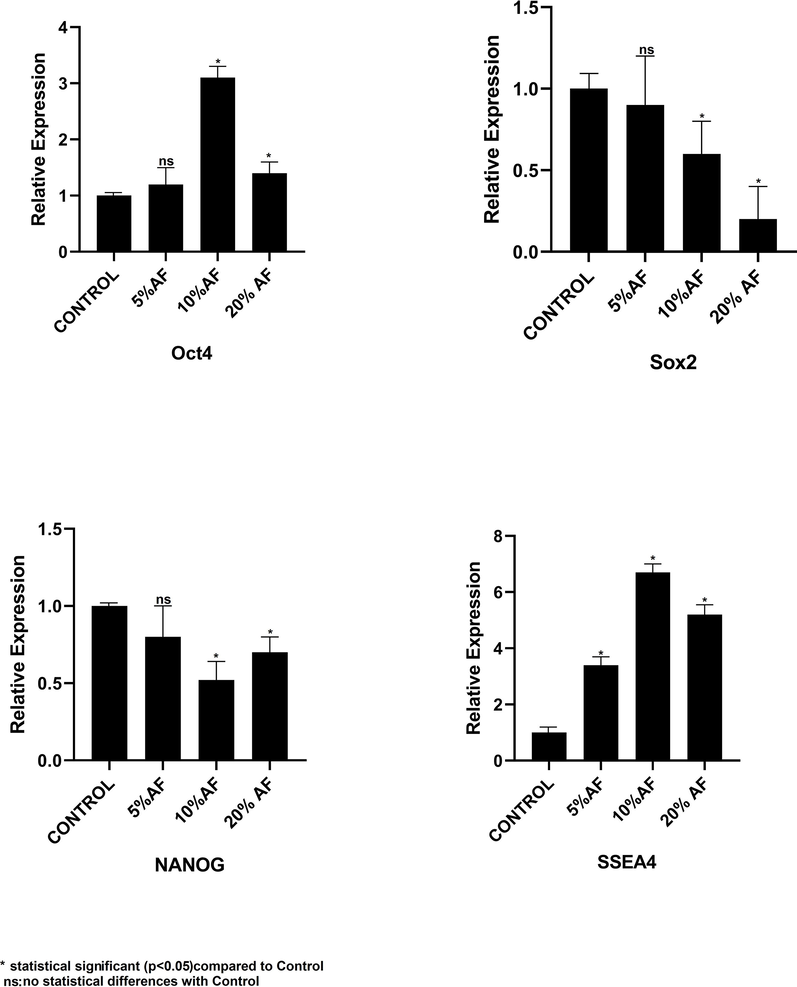
Comparative RT-qPCR-based analysis of stemness and pluripotency-related transcription factor gene expression analysis of placental stem cells after 7 days treatment with different concentrations of amniotic fluid. *p<.05, **p<.01, not significant (ns).AF: Amniotic fluid.
3.5 Comparative analysis of cytokines and growth factors secreted by the PMSCs treated with AF
Cytokines and growth factors play a crucial role in regulating and promoting the proliferation of mesenchymal stem cells. Therefore, we perform secretome analysis of PMSCs treated with different concentrations of AFs to quantify the growth factors and cytokines. ELISA-based expression analysis of TNFα, TGFβ cytokines, and interleukins IL-10, and IL-6 were included. We observed a direct correlation between TGFβ and IL-10 and the increase in the concentration of AF whereas the TNFα and IL-6 showed a decline in expression. The growth factors such as EGF and FGF were down regulated with an increase in the concentration of AF. The expression of growth factors such as vascular endothelial (VEGF) and insulin-like growth factors (IGF) in PMSCs was up-regulated by AF treatment. Fig. 5 represents the change in expression of cytokines and growth factors upon AF treatment.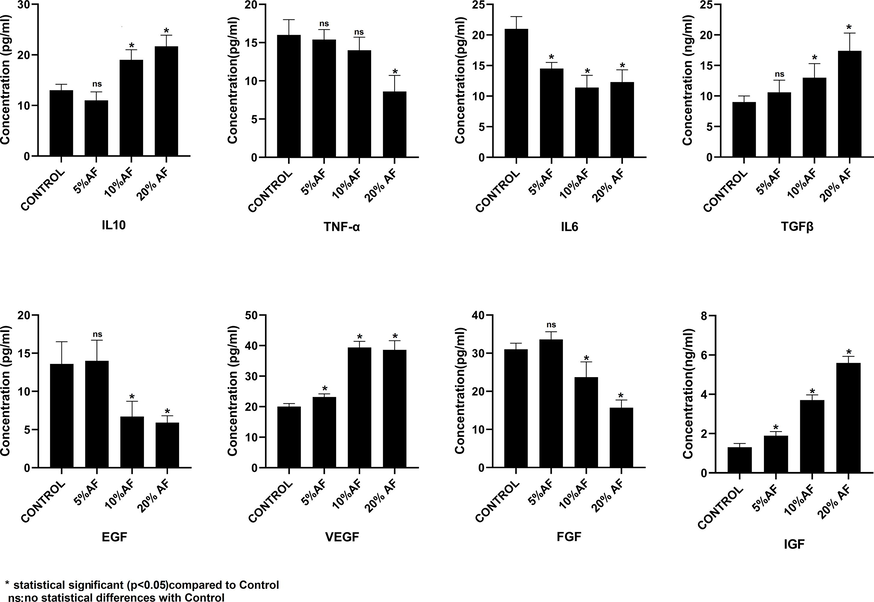
Comparative analysis of growth factors and cytokines using ELISAin conditioned media from placental stem cells after 7 days treatment with different concentrations of amniotic fluid. *p<.05,**p <.01, not significant (ns). AF: Amniotic fluid.
4 Discussion
Mesenchymal stem cells of humans belong to the category of non-hematopoietic stem cells that can be differentiated into mesodermal lineages such as ectodermal and endodermal lineages, chondrocytes, osteocytes, and adipocytes, as well. As placental stem cells have minimal ethical constraints and are less invasive to extract, they have an advantage over their counterparts, such as MSC from adipose tissue or bone marrow. PMSCs showed substantial benefits to patients with diseases like cancer, myocardial infarction, stroke and critical limb ischemia (de la Torre et al., 2019)In-vitro differentiation of PMSCs into lineages such as hepatic cells, skin cells, and islets of Langerhans-like glucagon-secreting cells in various differentiation media conditions has been reported previously (Chien et al., 2006; Mahmood et al., 2015; Suşman et al., 2010). The plant based compounds that are bioactive can intensify mesenchymal stem cell immunomodulation, rate of tissue regeneration, and differentiation (Saud et al., 2019). In this present study, we observed the in-vitro effect of amniotic fluid on the placenta-derived mesenchymal stem cells.
We have isolated the derived placental mesenchymal stem cells and treated them with different concentrations of amniotic fluid to understand its effects on PMSCs. The proliferation analysis of the PMSCs revealed that the PMSCs treated with AF proliferated faster than the control. Imposing AF can enhance cell proliferation in PMSCs. Our primary morphological observation of AF-treated PMSCs implied an acquisition of epithelial morphology. Further, we aimed to confirm this observation by checking the expression of the well-established epithelial and mesenchymal markers. AF treatment significantly increased the expression of epithelial markers like E-cadherin and cytokeratin, confirming our previous observation. Also, downregulation of N-cadherin and vimentin mesenchymal markers was observed in the study. These findings suggest that AF treatment causes a mesenchymal-epithelial transition (MET) in PMSCs.
We further investigated the change in mesenchymal stem cell surface marker expression upon AF treatment. The cluster of differentiation (CD) 105, CD73, CD90, CD29, and CD44 was expressed by mesenchymal stem cells surface markers, but not CD45, CD34, CD15, or HLA (human leukocyte antigen)-DR (Dominici et al., 2006). However, we did not observe a decline of the mesenchymal stem cell markers. We used PMSCs treated for seven days for surface marker analysis; however, due to the shorter time interval of treatment, we may not have seen a decline in surface marker expression. We have not further characterised the MET induced by AF treatment. However, interestingly, we observed an increase in hematopoietic markers such as HLA-DR, CD34, and CD45 upon AF treatment. The involvement of HLA-DR, CD34, and CD45 positive cells in immunomodulation is well known. Evidence suggests that the expression of HLA-DR on human bone marrow stem cells evades the natural killer (NK) cell-mediated killing of MSCs (Spaggiari et al., 2006). This suggests that AF treatment might be affecting the immunomodulating features of MSCs. Therefore, we further looked into the immunomodulatory changes caused by AF treatment in PMSCs.
The immunomodulatory feature of MSCs is of great interest to researchers in stem cell therapeutics. MSCs and host compatibility are the main topics of discussion in cell therapy and transplantation. It has been reported that MSCs regulate the microenvironment of host tissue by secreting cytokines, anti-inflammatory molecules and regulating the immune cells of the host (Ullah et al., 2015). We analysed the expression of cytokines like IL-10, IL-6, TNFα and TGFβ in the secretome of PMSCs cultured at different concentrations of AF. Upon AF treatment the TGFβ and IL-10 showed upregulation in expression whereas TNFα, and IL-6 got downregulated. TGFβ directly targets T cell functions (Thomas and Massagué, 2005). Similarly, antigen-presenting cells (APCs) that help in forming pro-inflammatory cytokines are suppressed by IL-10 and the functions of T cells and natural killer (NK) cells are suppressed. (Trinchieri, 2007). The cytokine TNFα, which is involved in the activation and proliferation of T- cells, is downregulated during the AF treatment (Mehta et al., 2018). Similarly, AF treatment reduced the expression of the cytokine IL-6, which promotes T cell proliferation and expansion during inflammation (Mehta et al., 2018). We further quantified the growth factors in the secretome of PMSCs treated with AF. Vascular endothelial growth factor (VEGF) and insulin growth factor (IGF) expression increased in response to treatment, whereas fibroblast growth factor (FGF) and epidermal growth factor (EGF) expression decreased. The VEGF growth factor directly suppresses the activation of T cells (Mehta et al., 2018). Collectively, our results suggest MSCs treated with AF enhanced the expression of cytokines, which can aid the MSCs to evade immune surveillance in the host microenvironment.
We carried out further analysis of pluripotent markers in PMSCs treated with AF. Oct4 didn’t show any variation in expression, however, Sox2 and Nanog showed a decline in expression, and SSEA4 showed a rise in expression. The variable expression of the pluripotency markers is currently inconclusive based on our results. Overall, our study added several advantages to establishing the use of natural AF in the differentiation of MSCs that can be useful for several applications. However, there were a few limitations, including 1) the small number of samples tested and 2) the AF and placenta were collected from different participants. Both limitations are need to considered before practical applicability.
5 Conclusion
Our results suggest that AF treatment induced an epithelial morphology and induction of epithelial markers on PMSCs. However, we could not observe a decline in the expression of mesenchymal surface markers. The AF treatment enhanced the immunomodulatory features of PMSCs by enhancing the surface marker expressions such as HLA-DR and upregulating the certain cytokines and growth factor expression. Our result suggests that PMSCs treated with AF can suppress the host T cell proliferation and activation and thus evade the immunosurveillance. This can reduce the problems such as graft rejection without using immunomodulators. However, further experiments such as co-culturing of T-cells with AF-treated cells have to be carried out to confirm this. Also, studies have to be done to understand the long-term effect of AF treatment on PMSCs as well mechanism of induction of MET, the mechanism by which AF enhance immunomodulation features have to be carried out.
Informed consent
Informed consent was obtained in accordance with institutional ethics considerations.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Regulating cell fate of human amnion epithelial cells using natural compounds: an example of enhanced neural and pigment differentiation by 3,4,5-tri-O-caffeoylquinic acid. Cell Commun. Signal.. 2021;19:26.
- [CrossRef] [Google Scholar]
- Placenta-derived multipotent stem cells induced to differentiate into insulin-positive cells. Biochem. Biophys. Res. Commun.. 2007;357:414-420.
- [CrossRef] [Google Scholar]
- In vitro differentiation of human placenta-derived multipotent cells into hepatocyte-like cells. Stem Cells. 2006;24:1759-1768.
- [CrossRef] [Google Scholar]
- de la Torre, P., Jesús Pérez-Lorenzo, M., I. Flores, A., 2019. Human Placenta-Derived Mesenchymal Stromal Cells: A Review from Basic Research to Clinical Applications, in: Stromal Cells - Structure, Function, and Therapeutic Implications. IntechOpen. https://doi.org/10.5772/intechopen.76718.
- Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317.
- [CrossRef] [Google Scholar]
- Potential of human fetal chorionic stem cells for the treatment of osteogenesis imperfecta. Stem Cells Dev.. 2014;23:262-276.
- [CrossRef] [Google Scholar]
- Larijani, B., Ensieh Nasli Esfahani, P.A., Nikbin, B., Kamran Alimoghaddam, S.A., Malekzadeh, R., Nika Mojahed Yazdi, M.G., Yahya Dowlati, Mohammad Ali Sahraian, A.G., 2012. Stem cell therapy in treatment of different diseases. Acta Med. Iran. 50
- In vitro differentiation potential of human placenta derived cells into skin cells. Stem Cells Int.. 2015;2015:1-11.
- [CrossRef] [Google Scholar]
- Placenta—an alternative source of stem cells. Toxicol. Appl. Pharmacol.. 2005;207:544-549.
- [CrossRef] [Google Scholar]
- Osteogenic effect of genistein on in vitro bone formation by rat bone marrow cell culture - for development of advanced bio-artificial bone. Key Eng. Mater.. 2003;254–256:1071-1074.
- [CrossRef] [Google Scholar]
- Concise review: isolation and characterization of cells from human term placenta: outcome of the first international workshop on placenta derived stem cells. Stem Cells. 2008;26:300-311.
- [CrossRef] [Google Scholar]
- Petroleum ether extract of Cissus quadrangularis (Linn.) enhances bone marrow mesenchymal stem cell proliferation and facilitates osteoblastogenesis. Clinics. 2009;64:993-998.
- [CrossRef] [Google Scholar]
- A review on the effect of plant extract on mesenchymal stem cell proliferation and differentiation. Stem Cells Int.. 2019;2019:1-13.
- [CrossRef] [Google Scholar]
- Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood. 2006;107:1484-1490.
- [CrossRef] [Google Scholar]
- Placental stem cell differentiation into islets of Langerhans-like glucagon-secreting cells. Rom. J. Morphol. Embryol.. 2010;51:733-738.
- [Google Scholar]
- TGF-β directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. 2005;8:369-380.
- [CrossRef] [Google Scholar]
- Interleukin-10 production by effector T cells: Th1 cells show self control. J. Exp. Med.. 2007;204:239-243.
- [CrossRef] [Google Scholar]
- Human mesenchymal stem cells - current trends and future prospective. Biosci. Rep.. 2015;35
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102648.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







