Translate this page into:
Evaluation and chemical profiling of different Centaurea iberica extracts and investigation of different in vitro biological activities
⁎Corresponding authors. javed89qau@gmail.con (Javed Iqbal), sobia.kanwal@aiou.edu.pk (Sobia Kanwal),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In almost all cultures medicinal plants are used as a rich source of phytochemicals and nutraceuticals components and have played promising role in maintaining and improving human health. The current study was aimed to unveil biological evaluation of Centaurea iberica. Plant extracts were prepared in five different solvents viz. methanol, ethanol, n-hexane, ethyl acetate and chloroform. The antioxidant potentials (2,2-diphenyl, 1-picrylhydrazine scavenging activity, total reducing power) of the plant revealed that the methanolic extract showed significant DPPH (2,2-diphenyl, 1-picrylhydrazine) scavenging activity (IC50 value 77.20 ± 8.81), reducing power (20.45 ± 0.54 AAE mg/g). In case of antibacterial assay, highest zone of inhibition (20 ± 1.5 mm) was recorded in methanolic extract against Klebsiella pneumonia. Likewise, for antifungal activity the highest zone of inhibition was recorded for methanolic extract (18 ± 2.4 mm) against Aspergillus niger. Moreover, 50 % inhibition was observed for methanolic extract against targated PC3 (prostate cancer cell line) cell lines and 3T3 cell lines. In the light of present study, it is recommended that further in vitro and in vivo studies should be performed using different animal models and characterization should be done to isolate different compounds that could be used to treat different types of ailments.
Keywords
Antibacterial
Antifungal
Anticancer
Antioxidant
Centaurea iberica
1 Introduction
Medicinal plants have strong therapeutic purposes and have been used since centuries for the preparation of functional drugs. Plants are in use as medicine for human beings at least 60,000 years back to the middle Paleolithic period (Zahra et al., 2022). The 21st century is considered as the century of biology, drove and powered by technological advances and scientific knowledge (Lenzner et al., 2019). Medicinal plant cultivation has started about 5000 years ago in Egypt, India and China and approximately 2500 years ago in Greece and Central Asia (Jamshidi et al., 2018). Humans in the initial era slowly realized the importance of plants for different purposes including, food, shelter, clothing, fertilizers, flavors, fragrances (Anand et al., 2019). Medicinal plants are being used for the service of humanity and are very important, irrespective of the region and era throughout the globe. Plants were, are, and will remain beneficial ever for various purposes including, nutritional, cultural, social, religious, environmental, and human well-being especially for the people living in low-income countries like Pakistan, where people are facing poverty, poor health, malnutrition and unemployment (Ullah, 2010). Following the ancient time when plant-based medicines were used for the treatment, demand for herbal medicine and herbal products has increased gradually. For primary healthcare, approximately 80 % of the Asian and African population is dependent upon traditional medicine as herbal medicines are economical and are without side effects (Sahoo et al., 2010). Chinese, Egyptians and Greeks are considered the earliest people who used plants as medicine since more than centuries. The knowledge regarding medicinal plants has been transferred gradually from one generation to other and has been updated gradually with modern technologies and facilities (Schippmann et al., 2006). Because of quality, safety and effectiveness, herbal medicines became an important part of therapeutics in developing countries (Jamshidi et al., 2018). Medicinal plants have very important role in treating various fatal diseases. All parts of the plants contain various active compounds which can be used as a remedy for different diseases (Shinwari et al., 2017). Phytochemicals also known as bioactive nutrients or compounds which naturally found in plants which are released or produced by the plants for health benefits (Zahra et al., 2021, Zahra et al., 2022).
To check the medicinal importance of the plants, present research study was conducted to analyze the phytochemical composition and biological potential of C. iberica (Asteraceae). This plant is native to District Chakwal, Pakistan and is frequently found in the northern areas of Pakistan (Nasir and Sikander, 2006). Different chemical substances such as steroid, volatile constituents, flavones, terpenoids, fatty acids, lactones have been reported. Centaurea species had widely been part of traditional therapeutics for the treatment of various health issues including, digestive, gynecological and dermatological problems (Khammar and Djeddi, 2012). It is an important part of Turkish traditional medicine and have shown significant biological activities such as wound healing, ease the inflammation and pain in patients having rheumatoid arthritis, headache, and high fever and for quick wound healing (Koca et al., 2009, Khan et al., 2011). Centaurea iberica is a rich source of different phytochemicals (lignans anthocyanins, flavonoids, phenolic acids, and stilbenes), xanthophylls and carotenes and exhibited several medicinal properties including anti-cancer, cytotoxicity, anti-inflammatory, and antioxidant (Alper et al., 2021). The Centaurea iberica has also shown antifungal and anti-microbial potential due to the presence of high amount of sesquiterpene lactones (Aktumsek et al., 2013). The current study was conducted to explore the phytochemical composition and biological potentials of asynthesized C. iberica mediated extracts.
2 Materials and methods
2.1 Plant collection and extract preparation
The medicinal plant Centaurea iberica Trevir. ex Spreng. was collected in its flowering season from the mountainous region of Murree (33.9070° N, 73.3943° E) Pakistan with dominant surrounding flora Acacia modesta, Diosypros lotus etc) and was taxonomically identified consulting literature review and flora of Pakistan. The study was conducted at Plant Biochemistry and Molecular Biology lab, QAU Islamabad. After collection and identification, whole plant was thoroughly washed by running tap water and was shade dried. Next, the plant extract was grounded into fine powder. Further, five different solvents including two polar (ethanol and methanol) and three non-polar (ethyl acetate, n-hexane and chloroform) were used to prepare extracts. The extracts were prepared using 250 mL of each solvent. Further, extracts were filtered and the filtrate was placed in fume hood for drying. After drying, the extracts were scratched and were shifted into eppendorf’s tube and finally placed at 4 °C. The mixtures were used for further quantitative and qualitative analysis.
2.2 DPPH free radical scavenging assay
A method which was outlined by Clarke et al. (2013) was used for DPPH determination with minor changes. Plant extract of 10 µL and 190 µL of DPPH was poured in microtiter plate to make a volume of 200 µL. Ascorbic acid was used as positive and DMSO as negative control. Afterwards, the incubation of reaction mixture was done at room temperature in darkness for half an hour. Changes in the color of mixture indicates oxidation potential of plants. Finally, absorbance of the experimental sample was recorded at 517 nm using formula below.
2.3 Reducing power assay
Protocol by Ravisankar et al. (2014) was used to analyze the reducing power of different plant extracts. To achieve this purpose, 100 µL of stock solution of each plant sample was taken and was added with 1 % solution of potassium ferricyanide (250 µL) solution and 0.2 M phosphate buffer (200 µL) solution. Incubation of these mixtures were done for 20 min at 50 °C. For the acidification of solution, 200 µL trichloroacetic acid was added. Centrifugation of the resultant mixtures was performed for 10 min at 300 rpm. Supernatant (150 µL) was taken and 50 µL (0.1 %) ferric chloride was added in it. Later on, the reaction mixtures of 200 µL was shifted into 96 wells plate and absorbance was recorded at 630 nm. To determine the reducing power of the experimental sample Gallic acid (GA) was taken as positive control and reducing potential was recorded as GA equivalents mg/g of extract.
2.4 Antibacterial activity
Disc diffusion method performed by Razmavar et al. (2014) was used to evaluate the antibacterial potential of the plant samples. For antibacterial assay, 100 µL of each bacterial inoculum was smoothly spread on media using sterile cotton swabs. Different concentrations were pre-pared as stock dilutions. Paper discs of 6 mm diameters were soaked with 25 µL of the stock solution from each extract and were placed on petri plates containing bacterial inoculum. For positive control Oxytetracycline was used followed by incubation at 24 hr at 37 °C. Antibacterial activity of selected plant extracts was checked by calculating their respective MIC values.
2.5 Antifungal activity
For the assessment of antifungal potential of each plant sample disc diffusion method was done as described by Sharma et al. (2012). Sabouraud dextrose agar media (65 g) per 1000 mL of distilled H2O was used for the growth of fungal cultures and its pH was maintained to 6.5. Filter paper Whatman # 1 was used for the preparation of round discs of about 6 mm in size. The experiment was performed in sterilized conditions. Media was first autoclave and it was poured and solidified. Each sample solution of about 5 µL along with 2 µL of standard drug was loaded on filter discs and it was kept on the plated media (SDA). Parafilm was used to cover the plates and were incubated for 24 hr at 28 °C. The next day, clear zones of inhibition were recorded and measured by mean of Vernier caliper. Minimum inhibitory concentration (MIC) was observed.
2.6 Anticancer activity on PC3 and 3T3 cell lines
Prostate cancer (PC3) activity of the plant was detected by protocol outlined by Asadi-Samani et al. (2018) and for 3T3 cell lines method outlined by Mossam et al. (1983) was used. For evaluating cells viability, the anticancer assay was performed in 96 wells micro plates in which MTT was used as a standard. The PC3 (Prostrate cancer) and 3T3 (Mouse fibroblast) cells were cultured using DMEM media, 5 % FBS, 100 IU/mL penicillin, 100 µg/mL of streptomycin was added in flask, and incubated in 5 % CO2 incubator at 37 °C. After 24 hr of incubation, old media was removed and cells were replaced with fresh media (200 µL) containing different concentrations of plant extracts (ranges from 1 to 30 µM). After 48 hr, 200 µL of medium contains MTT (0.5 mg/ml) was loaded into each well and were incubated for 4 hr. Absorbance of the plates were recorded at 570 nm using 96 well plate to determine the (IC50) value of the experimental values against for PC3 cell lines and 3T3 cell lines.
2.7 Statistics
Each experiment was carried out in triplicate. The descriptive statistics were applied using MS excel (2016). Results of biological evaluation and antioxidant were subjected to Analysis of variance (ANOVA) and least significant difference (LSD), by using Statistics version 8.1. Graph pad prism version 5.01 was used for calculating IC50 values.
3 Results and discussion
3.1 Antioxidant assays.
It is one of the important biological activity which shows capacity of the extract to scavenge the free radical species which may cause harmful effects on body, if they are present in high concentrations inside the body. Oxidative stress caused by ROS is one of the main cause of various diseases. Plants contains natural antioxidant potential which may help in scavenging the ROS and thus can be used in therapeutic preparations. For the detection of antioxidant potentials, various assays were performed which includes DPPH, and total reducing power (TRP).
3.2 DPPH free radical scavenging assay
Five different concentrations of plant extracts were used in free radical scavenging assay. It was noticed that CIE exhibited the lowest IC50 value (82.01 ± 8.9 µg/mL). Ascorbic acid was taken as standard with IC50 value (9.187 ± 0.473 µg/mL). Whereas, CIM showed value of 77.20 ± 8.81 µg /mL as shown in Table 1. The maximum IC50 values (127.6 ± 11.6 µg /mL, 179.4 ± 2.27 µg /mL, 294.8 ± 2.97 µg/mL) were noted for CIA, CIC and CIH, respectively. The CIE exhibited dose dependent activity at 1000 µg/mL (75.10 %) and (49.01 %) at 62.5 µg/mL (Fig. 1). For CIA the maximum scavenging activity were recorded at 1000 µg/mL (72.30 %) minimal activity (43.55 %) at 62.5 µg/mL. The CIC scavenged 65.52 % DPPH free radical at 1000 µg/mL and 32.87 % at 62.5 µg/mL. CIH showed maximum scavenging effect (66.20 %) at 1000 µg/mL and minimum scavenging effect at 62.5 µg/mL (33.81 %). CIM (74.21 %) showed maximum % inhibition at 1000 µg/mL respectively and minimum % inhibition was recorded at 62.5 µg/mL (48.53 %).
Sr. #
Plant Extract
IC50 (µg/mL)
1
CIE
82.01 ± 8.9
2
CIA
127.6 ± 11.6
3
CIH
294.8 ± 2.97
4
CIC
179.4 ± 2.27
5
CIM
77.20 ± 8.81
6
Ascorbic Acid
9.187 ± 0.473
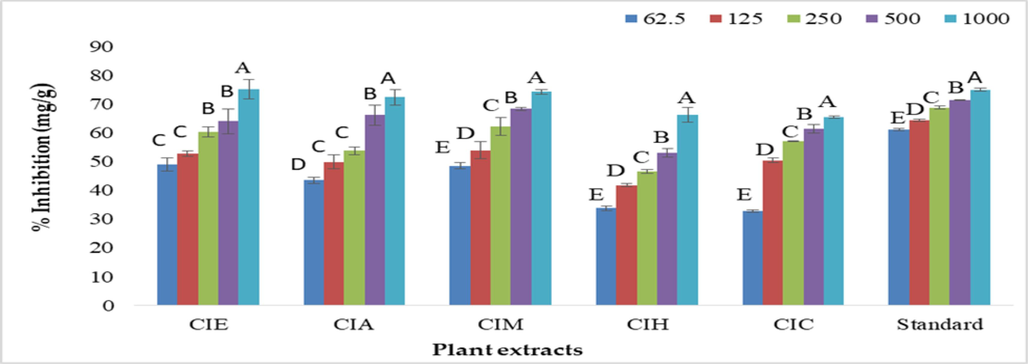
DPPH scavenging potential of C. iberica mediated extracts at various concentrations. Key: CIE: C. iberica ethanolic extract; CIA: C. iberica ethyl acetate extract; CIH: C. iberica n-hexane extract; CIC: C. iberica chloroform extract; CIM: C. iberica methanolic extract.
3.3 Total reducing power (TRP)
The TRP is also known as phospho-molybdenum assay. TRP was evaluated using equation obtained from Gallic acid calibration curve (y = 0.0004x + 0.2492), and results were expressed as Gallic acid equivalent (GAE mg/g). The highest value was shown by CIM (20.45 ± 1.6 mg/g) followed by CIA (17.13 ± 0.8 mg/g), CIE (16.40 ± 3.0 mg/g), CIC (12.6 ± 2.4 mg/g) and the minimum value was recorded by CIH (8.9 ± 2.4 mg/g) as shown in the Fig. 2.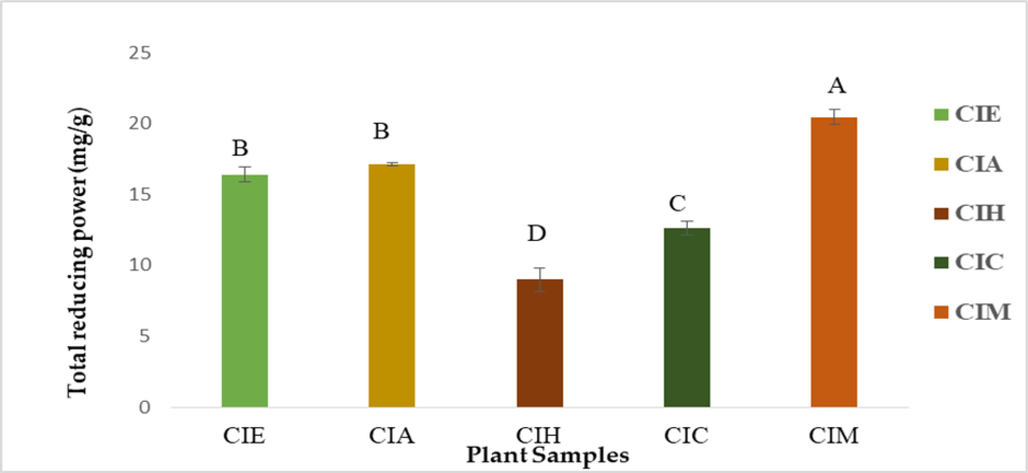
Total reducing power of various extracts of C. iberica. Key: CIE: C. iberica ethanolic extract; CIA: C. iberica ethyl acetate extract; CIH: C. iberica n-hexane extract; CIC: C. iberica chloroform extract; CIM: C. iberica methanolic extract. Error bars = Standard error.
3.4 Antibacterial activity
Antibacterial activity was performed on two extracts of C. iberica which includes one polar CIM (C. iberica methanolic extract) and one non polar CIH (C. iberica n-hexane extract) by using disc diffusion assay. For this, S. aureus was used as a gram-positive bacteria and E. coli and K. pneumonia were used as gram negative bacteria. Oxytetracycline was taken as a standard and for negative control DMSO was used. All plant extracts were examined at four different concentrations (250, 100, 33.33, 11.11 µg/mL) and then MIC was recorded. Results revealed that the selected plant extracts showed resistance against three bacterial strains (Table 2). CIM showed maximum zone of inhibition against K. pneumonia (ATCC 1705) (20 ± 1.5 mm) followed by S. aureus (ATCC 6538) (19 ± 1.24 mm) and E. coli (ATCC 33456) (14 ± 1.16 mm). In case of CIH, maximum antibacterial activity was observed against K. pneumonia (12 ± 1.94 mm) followed by E. coli (6 ± 1.04 mm). However, CIH did not showed any zone of inhibition against S. aureus. Hence, CIM plant extract revealed significant antibacterial activity as compared to n-hexane extracts when tested against three bacterial strains. Results are expressed as mean ± SD (n = 3). Key: CIM: C. iberica methanolic extract; CIH: C. iberica n-hexane extract;
Microorganisms
E. coli
ATCC33456
K. pneumonia
ATCC 1705
S. aureus
ATCC6538
Plant Extracts
ZOI (mm)
MIC (µg/ml)
ZOI (mm)
MIC (µg/ml)
ZOI (mm)
MIC (µg/ml)
CIM
14 ± 1.16
100
20 ± 1.5
33.33
19 ± 1.24
33.33
CIH
6 ± 1.04
12 ± 1.94
100
NI
DMS (Negative Control)
NI
NI
NI
Oxytetracycline (Standard)
30 ± 1.5
28 ± 3.5
32 ± 2.8
3.5 Antifungal activity
Antifungal assay of methanol and n-hexane extracts of C. iberica was done using agar disc diffusion method. Selected plant extracts were examined using four different concentrations (250, 100, 33.33, 11.11 µg/mL). Total three fungal strains (C. albicans, M. racemosus, and A. niger) were used to examined antifungal activity of these extracts (Table 3). Chloramphenicol was taken as a standard and for negative control DMSO was used. Maxi-mum zone of inhibition was produced by CIM extract of C. iberica against A. nigar (FCBP 0918) (18 ± 2.4 mm) followed by M. racemosus (FCBP 0300) (16 ± 2.5 mm) and C. albicans (FCBP 478) (15 ± 2.6 mm). However, minimum zone of inhibition was determined by CIH against C. albicans (14 ± 2.6 mm) and A. niger (12 ± 1.5 mm). No zone of inhibition was formed by CIH against M. racemosus. Key: CIM: Centaurea iberica methanolic extract; CIH: Centaurea iberica n-hexane extract;
Fungal strains
M. racemosus
FCBP 0300
C. albicans
FCBP 478
A. niger
FCBP 0918
Plant Extracts
ZOI (mm)
MIC (µg/ml)
ZOI (mm)
MIC (µg/ml)
ZOI (mm)
MIC (µg/ml)
CIM
16 ± 2.5
33.33
15 ± 2.6
100
18 ± 2.4
100
CIH
NI
–
14 ± 2.6
100
12 ± 1.5
33.33
DMSO (Negative Control)
NI
–
NI
–
NI
–
Chloramphenicol (Standard)
28 ± 2.5
–
32 ± 3.5
–
31 ± 1.8
–
3.6 Anticancer assay
Anticancer effect of the methanolic extract of C. iberica was tested against 3T3 cell line and PC3 cell line using MTT assay. For this, MTT was taken as a standard against 3T3 cell lines and PC3 cell lines. The results revealed significant anticancer activity of the extract towards PC3 cell lines (5.0 % inhibition) compared to the 3T3 cell lines (30.4 % inhibition) respectively. IC50 values also confirmed highest activity against PC3 cell lines (89.9 %) while it was found to be lowest in case of 3T3 cell lines i.e < than 50 % (Table 4). Key CIM: C. iberica methanolic extract; standard: MTT against 3T3 cell line and PC3 cell line.
Anticancer assay
3T3 cell line
PC3 cell line
% inhibition
IC50
% inhibition
IC50
Plant extract
30.4 %
< 50
5.0 %
89.9 %
Standard
89.9 %
0.8 ± 0.14
Inactive
1.9 ± 0.4
4 Discussion
Medicinal plants play a very promising role in the treatment of a variety of diseases (Naseer et al., 2022, Ijaz et al., 2023). The nature has blessed medicinal plants with a wide range of bioactive phytochemicals (Abbasi et al., 2022, Zahra et al., 2023). The present study was designed to explore the biological potential of C. iberica. The current research, study was designed to explore the phytochemical composition and biological potentials of C. iberica. Besides beneficial microorganisms, various pathogenic organisms are also widespread in nature that lead to serious health issues. Hence, antimicrobial activity of two extracts of C. iberica (CIM and CIH) were tested against one-gram positive bacteria (S. aureus), two gram negative bacteria (E. coli and K. pneumonia) and three fungal strains (C. albicans, M. racemosus and A. niger) using disc diffusion assay. Selected plant extracts were examined at four different concentrations (250, 100, 33.33, 11.11 µg/mL) and oxytetracycline and chloramphenicol were used as postive control (standard). Antibacterial results revealed that the methanolic plant extract showed maximum zone of inhibition against K. pneumonia (ATCC 1705) 20 ± 1.5 mm, S. aureus (ATCC 6538) 19 ± 1.24 mm and E. coli (ATCC 33456) 14 ± 1.16 mm. However, n-hexane extracts formed less than 14 mm zone of inhibition against all bacterial strains. Similarly, in case of antifungal activity, maximum zones were revealed by CIM against all fungal strains as compared to n-hexane extracts which revealed less than 14 mm zones of inhibition. Altogether, the methanol extracts of C. iberica were found to be very effective against wide range bacterial and fungal strains than n-hexane extracts. Previously, Albayrak et al. (2017) reported significant antibacterial activity (7.00 ± 0.00 mm) of C. aksoyi against E. coli and 7.00 to 11.00 mm inhibition zones were detected by C. amaena against various gram positive and gram-negative bacterial strains. Similarly, according to Barbour et al. (2004) C. erengoides and C. aintensis exhibits 66.66 % and 88.8 % antibacterial activity respectively. From the obtained results, it can be suggested that antimicrobial activity in the examined species might be due to the presence of bioactive compounds present in Centaurea species and hence can be utilized in various industries (Barbour et al., 2004). To the best of our knowledge and literature surveys, this is the first study reporting the antimicrobial activity of C. iberica extracts.
Medicinal plants are considered as the rich source of antioxidants and reduces the oxidative stress to great extent due to the presence of different phenolic compounds. These species can be used to cure various human ailments such as diabetes, cancer and heart ailments (Ali et al., 2022, Gul et al., 2022). In present studies, antioxidant activity of C. iberica extracts was examined via two assays viz. DPPH and TRP assays. Results revealed that CIM extracts exhibited maximum % inhibition (97.14 %) at 1000 µg/mL and IC50 value of 1.54 µg/mL followed by CIH extract which displayed 94.65 % inhibition and IC50 value of 36.61 µg/mL at 1000 µg/mL. Highest TRP (20.45 ± 1.6 GAE mg/g) was also noted in CIM extracts as compared to other extracts of C. iberica. Current research confirmed that the and TRP values are greatly influenced by the use of different solvents (Kumar and Jain, 2015; Mehwish et al., 2019). Previously, Zengin et al. (2011) reported 44.0 % DPPH inhibition (137.06 IC50) and 39.70 mg AAE/g TAC value in the methanol extract of C. urvillei. Similarly, Escher et al. (2018) observed that C. cyanus possess 51–62 % DPPH (2,2-diphenyl, 1-picrylhydrazine) inhibition, 487 to 846 mg GAE/100 g reducing capacity and 1134 to 1359 mg AAE/100 g TAC activity.
Cancer is one of the major causes of death in the worldwide and has been reported for centuries that plants have anticancer properties and they are important source of anticancer agents (Cragg and Newman, 2013). The Centaurea genus contains anticancer properties due to the availability of phytochemicals (Alper and Gunes, 2019). Hence, present study was carried out to observe the anticancer activity of CIM extracts against 3T3 and PC3 cell lines. Results revealed that CIM extract exhibited 30.4 % inhibition against 3T3 cell lines and 5.0 % inhibition against PC3 cell lines. Previously similar experiment was conducted on various species of Centaurea including C. solstitialis, C. kilaea, C. cuneifolia, C. salicifolia and C. stenolepis (Sekerler et al., 2018). According to their results Centaurea genus have good potential as an anti-cancer agent. The plant demand is increasing day by day due to their less side effects on normal. Numerous species were studied from Asia and Africa, the herbal therapies against cancer are common in undeveloped countries (Azmi et al., 2006). Chemotherapy is not as safe as plant based medicines, therefore the mandate for substitute care with naturally derived anticancer active agents with plants being the favorite cause (Uddin et al., 2021). According to the results CIM is not a potential candidate for insecticidal. However, on the basis of overall results it can be suggested that C. iberica is highly enriched with secondary metabolites and thus is effective against ailments including cancer and inflammation (Koca et al., 2009 and Ochwang et al. (2009).
5 Conclusion
In current study, antioxidant, antifungal, anti-bacterial, anticancer and antioxidant activities were carried out using different extracts of C. iberica. Among all the plant extracts, antioxidant activity was found to be maximum in the methanolic extract. Further, methanolic extract has shown promising antimicrobial activities as compared to other extracts. Therefore, it can be assumed from the biological activities of selected plant exhibits significant phyto-compounds that can be utilized effectively against various pathogens for defensive purposes. In future, different other in vitro and in vivo studies should be carried out to further confirm the bio-efficacy of selected plant extract and compound characterization will help in isolating various novel compounds that could be used to treat different types of ailments.
Ethics approval
Not Applicable.
Consent to participate
All authors consent to participate in this manuscript.
Consent for publication
All authors consent to publish this manuscript in Saudi Journal of Biological Science.
Data availability statement
Data will be available on request to corresponding or first author.
Code availability
Not Applicable.
Author contributions
H.B., J.I and T.M. generated the idea, performed research work and assisted with writing-original draft and editing. Supervision and experimental facilities were provided by T.M. B.A.A., S.K., M.T. helped with software, formal analysis, manuscript editing and revision. M.Z.A. helped with funding acquisition, review and editing with All authors have read and agreed to the published version of the manuscript.
Acknowledgments
This work was funded by Researchers Supporting Project number (RSPD2023R728), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Rhamnella gilgitica functionalized green synthesis of ZnONPs and their multiple therapeutic properties. Microsc. Res. Tech.. 2022;85(6):2338-2350.
- [Google Scholar]
- Assessment of the antioxidant potential and fatty acid composition of four Centaurea L. taxa from Turkey. Food Chem.. 2013;141(1):91-97.
- [Google Scholar]
- Comparison of phenolic components and biological activities of two Centaurea species obtained by three extraction techniques. Asian Pac. J. Trop. Med.. 2017;10(6):599-606.
- [Google Scholar]
- Chemical characterization and evaluation of the nephroprotective potential of Parrotiopsis jacquemontiana (Decne) Rehder and Periploca hydaspidis Falc crude extract in CCl4-induced Male Sprague-Dawley Rats. Saudi Journal of Biological Sciences. 2022;29(2):702-712.
- [Google Scholar]
- Assessment of Antioxidant and Cytotoxic Activities and Identification of Phenolic Compounds of Centaurea solstitialis and Urospermumpicroides from Turkey. Braz. Arch. Biol. Technol.. 2021;64
- [Google Scholar]
- A comprehensive review on medicinal plants as antimicrobial therapeutics: Potential avenues of biocompatible drug discovery. Metabolites. 2019;9(11):258.
- [Google Scholar]
- A screening of growth inhibitory activity of Iranian medicinal plants on prostate cancer cell lines. Biomedicine. 2018;8(2)
- [Google Scholar]
- Azmi, A.S., Bhat, S.H., Hanif, S., & Hadi, S.M. 2006. Plant polyphenols mobilize endogenous copper in human.
- Screening of selected indigenous plants of Lebanon for antimicrobial activity. J. Ethnopharmacol.. 2004;93(1):1-7.
- [Google Scholar]
- High correlation of 2, 2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging, ferric reducing activity potential and total phenolics content indicates redundancy in use of all three assays to screen for antioxidant activity of extracts of plants from the Malaysian rainforest. Antioxidants. 2013;2(1):1-10.
- [Google Scholar]
- Chemical study, antioxidant, anti-hypertensive, and cytotoxic/cytoprotective activities of Centaurea cyanus L. petals aqueous extract. Food Chem. Toxicol.. 2018;118:439-453.
- [Google Scholar]
- Phytochemistry, biological activities and in silico molecular docking studies of Oxalis pes-caprae L. compounds against SARS-CoV-2. Journal of King Saud University-Science. 2022;34(6):102136
- [Google Scholar]
- Rosmarinic acid and its derivatives: Current insights on anticancer potential and other biomedical applications. Biomed. Pharmacother.. 2023;162:114687
- [Google Scholar]
- Medicinal plants: Past history and future perspective. Journal of Herbmed Pharmacology. 2018;7(1)
- [Google Scholar]
- Pharmacological and biological properties of some Centaurea species. Eur. J. Sci. Res.. 2012;84(3):398-416.
- [Google Scholar]
- Phytochemical analysis of selected medicinal plants of Margalla Hills and surroundings. Journal of Medicinal Plants Research. 2011;5(25):6055-6060.
- [Google Scholar]
- In vivo anti-inflammatory and wound healing activities of Centaurea iberica Trev. ex Spreng. J. Ethnopharmacol.. 2009;126(3):551-556.
- [Google Scholar]
- Appraisal of total phenol, flavonoid contents, and antioxidant potential of folkloric Lanneacoromandelica using in vitro and in vivo assays. Scientifica. 2015;2015(1):1-13.
- [Google Scholar]
- A framework for global twenty-first century scenarios and models of biological invasions. Bioscience. 2019;69(9):697-710.
- [Google Scholar]
- In vitro antileishmanial and antioxidant potential, cytotoxicity evaluation and phytochemical analysis of extracts from selected medicinally important plants. Biocatal. Agric. Biotechnol.. 2019;19:101117
- [CrossRef] [Google Scholar]
- Rapid colorimetric assay for cellular growth and survival. J Immunol Methods. 1983;55:65.
- [Google Scholar]
- Deciphering chemical profiling, pharmacological responses and potential bioactive constituents of Saussurea lappa Decne. Extracts through in vitro approaches. Saudi Journal of Biological Sciences. 2022;29(3):1355-1366.
- [Google Scholar]
- Noxious weeds of winter crops in District Chakwal, Pakistan. Int. J. Agric. Res.. 2006;1(5):480-487.
- [Google Scholar]
- Antioxidant activites and phytochemical analysis of methanol extract of leaves of Hypericum hookerianum. Int. J. Pharm. Sci. Res.. 2014;6(4):456-460.
- [Google Scholar]
- Razmavar, S., Abdulla, M. A., Ismail, S. B., & Hassandarvish, P. 2014. Antibacterial activity of leaf extracts of Baeckea frutescens against methicillin-resistant Staphylococcus aureus. BioMed Research International, 2014.
- A comparison of cultivation and wild collection of medicinal and aromatic plants under sustainability aspects. Frontis 2006:75-95.
- [Google Scholar]
- Anticancer, Antioxidant and Anti-Inflammatory Activities of Chloroform Extracts from Some Centaurea Species. In Multidisciplinary Digital Publishing Institute Proceedings. 2018;25(2):1542.
- [Google Scholar]
- Phytochemical analysis and antifungal potential of Durantaerecta against some phytopatogenic fungi. Int. J. Pharm. Sci. Res.. 2012;3(8):2686.
- [Google Scholar]
- Quantitative analyses of medicinal plants consumption among the inhabitants of Shangla-Kohistan areas in Northern-Pakistan. Pak. J. Bot.. 2017;49(2):725-734.
- [Google Scholar]
- Green synthesis of nickel oxide nanoparticles using leaf extract of Berberis balochistanica: Characterization, and diverse biological applications. Microsc. Res. Tech.. 2021;84(9):2004-2016.
- [Google Scholar]
- Antimicrobial, cytotoxic, antioxidants, enzyme inhibition activities, and scanning electron microscopy of Lactuca orientalis (Boiss.) Boiss. Seeds. Microscopy Research and Technique. 2021;84(6):1284-1295.
- [Google Scholar]
- Scanning electron microscopy of Sophora alopecuroides L. seeds and their cytotoxic, antimicrobial, antioxidant, and enzyme inhibition potentials. Microsc. Res. Tech.. 2021;84(8):1809-1820.
- [Google Scholar]
- A comprehensive review on traditional uses, phytochemistry and pharmacological properties of Paeonia emodi Wall. ex Royle: current landscape and future perspectives. Chin. Med.. 2023;18(1):23.
- [Google Scholar]
- Antioxidant Properties of Methanolic Extract and Fatty Acid Composition of Centaurea urvillei DC. subsp. hayekianaWagenitz. Records of Natural Products. 2011;5(2)
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102992.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







