Translate this page into:
Evaluating antibody response pattern in asymptomatic virus infected pregnant females: Human well-being study
⁎Corresponding authors. mushahid@ksu.edu.sa (Shahid Mahboob), d.men@wh.iov.cn (Men Dong), qiuxianghuang2001@163.com (Qiuxiang Huang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
The ongoing SARS-CoV-2 pandemic infecting millions of people globally has given rise to serious public health threats. The need for early detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in asymptomatic pregnant women is compelling to detect vertical transmission timely. Here, 11 SARS-CoV-2 asymptomatic pregnant cases from Wuhan China were investigated. All the patients were initially tested negative for SARS-CoV-2 on RT-PCR, so a chest CT scan was performed. Also, serum antibody (IgM and IgG) titers were estimated. CT scan of patients revealed typical abnormalities related to SARS-CoV-2, indicating ground-glass opacity and infection lesions suggesting viral pneumonia. Elevated IgM and IgG antibodies levels (p < 0.001) were also noticed in infected patients. Hence, CT imaging and serum antibody response are valuable in the early detection of SARS-CoV-2 in asymptomatic pregnant patients. These might serve as prognostic markers for healthcare professionals, in RT-PCR negative patients, to assess the effect of given treatment by chest CT.
Keywords
Asymptomatic
SARS-CoV-2
CT scan
Pregnant women
Serum antibodies
1 Introduction
The current pandemic caused by severe acute respiratory coronavirus syndrome (SARS-CoV-2), has already become a global threat to the human population (Hamza et al., 2020; Khan et al., 2020). As of August 2020, more than nineteen million people tested positive for COVID-19 caused by SARS-CoV-2, 80 percent of whom had mild or no symptoms of the disease. There are currently 6 million people in this category. Currently, the number of asymptomatic pregnant women is increasing compared to non-pregnant infected women hospitalized patients. Several studies from Europe, China, and New York reported that asymptomatic pregnant women tested positive for SARS-CoV-2 infection in higher percentages than non-pregnant patients (Breslin et al., 2020; Schwartz, 2020; Yu et al., 2020). Recent studies have focused on symptomatic and confirmed positive COVID-19 cases in hospitals that generate a knowledge gap in the diagnosis of infection in asymptomatic or mildly symptomatic patients (Della Gatta Anna Nunzia et al., 2020). Corona virus is a highly contagious disease that can spread through contact, mouth, and droplets with severe consequences, causing respiratory diseases in infected personnel (Xu et al., 2020). Pregnancy triggers various physiological and anatomical changes in women that have made them prone to respiratory diseases (Jamieson et al., 2009). It is known that maternal physiological adjustments to pregnancy increase the risk of developing severe conditions in response to viral infections, such as influenza; preliminary data indicate that the prognosis of illness with SARS-CoV-2 in pregnant women may also be more severe (Savasi et al., 2020). Vertical transmission of the other two animal coronaviruses known to infect humans, SARS-CoV and Middle East Respiratory Syndrome (MERS), has never been documented. However, the number of cases reported for infected pregnant women was deficient and insufficient to draw firm conclusions (12 cases reported for SARS-CoV and 11 cases reported for MERS) (Wong et al., 2004a; Alfaraj et al., 2019).
Conversely, multiple reports focus on SARS-CoV-2-positive pregnant women as the number of SARS-CoV-2-positive patients increases worldwide. Therefore, pregnant women with SARS-CoV-2 are difficult to manage than non-pregnant women (Rasmussen et al., 2020). Also, Facchetti et al. (2020) revealed the potential for vertical transmission by spotting SARS-CoV-2 RNA and the expression of S and N proteins in the placenta of pregnant COVID-19 patients (Facchetti et al., 2020).
Real-time reverse transcription-polymerase chain reaction (RT-PCR) is the best method for diagnosing corona virus infection. However, the quality of sampling is the limitation of this procedure. In addition, detection by RT-PCR requires a relatively large amount of time. Consistency comparisons between RT-PCR and CT have already been studied (Ai et al., 2020; Fang et al., 2020; Kim et al., 2020). It has been suggested that a positive determination of serum antibodies (IgM and IgG) may also be deterministic evidence for SARS-CoV-2 infection (Zhang et al., 2020). This research study aimed to assess the biological aspects, radiological characteristics, and serum antibody levels of asymptomatic pregnant women with corona virus pneumonia and their correlation with diagnosis and disease progression.
2 Materials and methods
A total of 11 asymptomatic pregnant patients with corona virus obstetric pneumonia at Wuhan maternal and child health hospital were reported retrospectively from the electronic medical records database between January 2020 and March 2020. Patients ranged from 22 to 36 years of age (median, 29 years of age). The research involved patients who received low-dose CT in the chest and had available clinical records and laboratory results. Diagnosis of COVID-19 pneumonia was based on the recommendations for the diagnosis and treatment of new Coronavirus Infection (2019-nCoV) issued by the National Health Commission of China (Trial Version 5). All patients in the sample were asymptomatic of COVID-19 based on these clinical diagnostic criteria. Standard clinical classifications (mild, moderate, extreme, and critical) have been used as defined by Somatom Description AS128 (Siemens, Erlangen, Germany) or Philips Ingenuity Core128 (Philips Medical Systems, Best, Netherlands). CT scanners adopt the chest procedure, where the patient took a supine posture with raised arms. Each patient was advised to hold their breath during the image acquisition, which included the total volume of the lung. On the basis of previously published papers, four stages of pulmonary involvement were described on the basis of CT imaging in the pulmonary window14: (1) early-stage, (2) progressive stage, (3) peak stage, and (4) absorption stage. The results of the picture were decided by consensus.
Data on demographic, epidemiological, and clinical features were collected from the Medical Records System. In addition, Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) real-time reverse transcriptase-polymerase chain reaction tests (Novel Coronavirus PCR Fluorescence Diagnostic Kit [BGI Genomics, Hong Kong], Throat swab kit purchased by BGI, IgM / IgG antibody kit purchased by Zhuhai livzon diagnostics inc) were performed using nasopharyngeal and anal swab samples. Data were collected between January 2020 and March 2020. Statistical research was carried out using SPSS version 19 (IBM, Armonk, NY, USA). Categorical variables have been expressed as percentages. Continuous variables have been expressed as mean ± standard deviation. P < 0.001 was found to be statistically important.
3 Results
3.1 Clinical characteristics
Data from eleven asymptomatic pregnant patients who became positive after admission to SARS-CoV-2 were recovered from Wuhan Children Hospital, China. Initially, four (36.3%) patients were diagnosed with RT-PCR infection, while seven (63.6%) patients were tested negative but were suspected of being COVID-19 positive. Two patients were in close contact with SARS-CoV-2 positive patients due to family history. The clinical stage of the patients at the time of admission was mild or normal, as per the classification criteria. Major clinical symptoms, including cough (n = 2, 18.1%) and fever (n = 1, 9.0%) were observed in patients. Nine patients received antibiotics for the treatment of fever.
All patients were late in pregnancy (>28 weeks). The caesarean section was administered to eight patients, while the three had a vaginal delivery. Out of eleven neonates, eight (72.7%) neonates showed no symptoms, two (18.1%) neonates had nausea, cough, and fever, and one neonate (9.0%) had neonatal jaundice. The Apgar score for each neonate was 9–10 five minutes after delivery. All neonates tested negative for SARS-CoV-2 on RT-PCR. Three neonates received antibiotic therapy that was administered through the caesarean section. Eight neonates were administered to interferon suspected of having a viral infection.
All patients had been discharged from the hospital for 7–25 days. The clinical characteristics of pregnant women and neonates were shown in Tables 1 and 2, respectively.
Patient
Age, y
Gestational age, wk
Primary Symptom
Source of Infection
RT-PCR*
Treatment
Hospitalization, d
1
24
38 + 4
None
Unclear
+
Antibiotic
23
2
36
37 + 5
Fever, diarrhea, mild dyspnea
Family
–
Antibiotic
12
3
29
40
Fever
Unclear
–
Antibiotic
12
4
29
39 + 3
None
Family
+
Antibiotic
17
5
30
39 + 2
None
Unclear
+
Antibiotic
19
6
28
36 + 3
None
Unclear
–
Due to the merger of ICP, Atomo Moran and Yin Zhihuang treatment
9
7
33
37 + 5
None
Family
–
Antibiotic
17
8
22
37 + 5
None
Unclear
–
None
15
9
29
40 + 1
None
–
Antibiotic
Family
10
31
36 + 1
None
Unclear
–
None
19
11
30
40 + 4
None
Unclear
–
Cephalosporins
Patient No.
Birth weight, g*
5-minute Apgar Scores
Symptom and duration
RT-PCR**
Treatment
Hospitalization, d
1
3260
9-10′
Stuffy nose, sneezing
–
Antibiotic, nutritional myocardium, interferon
18
2
3330
9-10′
Normal
–
Nutritional myocardium
9
3
3200
9-10′
Normal
–
Nutritional myocardium
9
4
3000
9
Normal
–
Nutritional myocardium
14
5
3410
9
Vomit little coffee grounds
–
Nutritional myocardium,
17
interferon
6
2940
9-9′
Jaundice
–
Nutritional myocardium, interferon
6
7
3320
9-10′
Normal
–
Interferon
15
8
2270
9-10′
–
Cephalosporin antibiotics and nutrition therapy
13
Low weight
9
2580
9-10′
Normal
–
Interferon
15
10
3080
9-10′
Normal
–
Forcen, interferon, antibiotic (amoxicillin)
16
11
3130
9-10′
Normal
–
Interferon
17
3.2 Radiologic findings
Based on the staging of the definition of CT images. Typical abnormalities related to COVID-19 pneumoniae have been observed in negative RT-PCR patients (Fig. 1). Six (27.2%) patients showed small patchy ground glass opacity in the left lung (Fig. 1A&E). The texture of the lungs has been enhanced and blurred. Infectious lesions, considering viral pneumonia, were the flaky ground glass shadow in the lungs. Fourteen (63.6%) patients had bilateral wedge-shaped, multi-lobular sub-segmented ground glass opacity in the broncho vascular area indicating disease severity (Fig. 1B, C, D). Two (9.0%) patients had patchy consolidation and small lung lesions.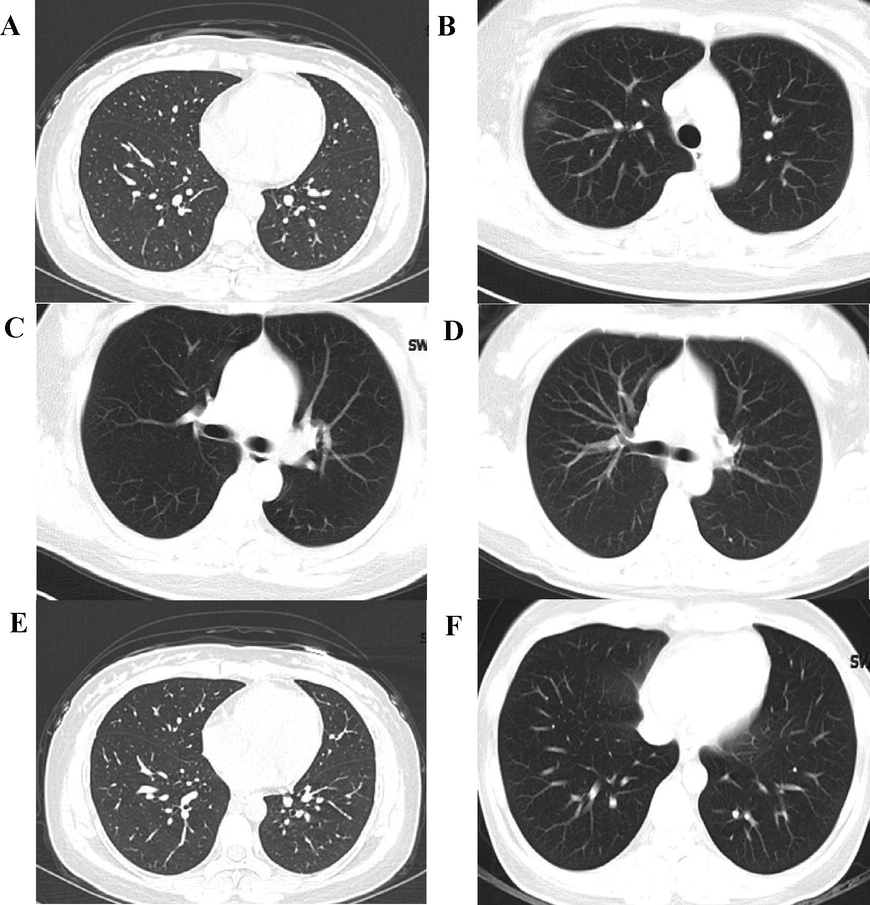
CT scan of SARS-CoV-2 infected pregnant patients. (A) 24-y-old, 38 wk; The texture of both lungs was enhanced and blurred, and patchy fuzzy “shadows were seen in the lower lobe of left lung”. The imaging diagnosis was infectious lesions in both lungs. (B) 30-y-old, 39 wk; The texture of both lungs was enhanced, and small pieces of ground glass shadow were seen in the lower lobe of the left lung. The imaging diagnosis was infection of the lower lobe of the left lung. (C) 33-y-old, 37 wk; The lung texture is clear, with patchy “ground glass shadows in the upper right lung and nodular ground glass shadows in the upper left lung.” The imaging diagnosis was infectious lesions in the upper lobes of both lungs. (D) 29-y-old, 39 wk; The texture of both lungs was enhanced without substantial infiltration. (E) 31-y-old, 36 wk; The texture of both lungs is enhanced, and the “lower lobe of the right lung is patchy ground glass shadow. The imaging diagnosis” is right lung pneumonia, suspected viral pneumonia. (F) 30-y-old, 40 wk; The texture of the lungs is clear, and the “lower lobe of the right lung has a flaky ground glass shadow”. The imaging diagnosis is ground-glass shadow of the right lower lobe, considering viral pneumonia.
3.3 Clinical characteristics & CT manifestations correlation
Eleven asymptomatic patients were at an early stage of the disease when given to a hospital with ground glass opacity and lung lesions. Later, the majority of patients became positive for SARS-CoV-2 on RT-PCR. The clinical characteristics were significantly higher (P < 0.001) in patients with leukocyte counts. Lymphocyte counts for pregnant women, and neonates were within range. In particular, more elevated IgM and IgG levels were observed in patients suspected of having COVID-19. The clinical characteristics associated with SARS-CoV-2 pneumoniae are shown in Table 3.
Laboratory Values
Pregnant women
Neonates
p-value
Leukocyte
9.871 ± 1.5 (4–10/L)
14.75 ± 5.9 (5–30*109/L)
<0.001
Lymphocyte
1.78 ± 0.6 (0.8–4/L)
3.40 ± 0.9 (2–17*109/L)
0.05
IgM
16.19 ± 22.3 (0–10 RU/mL)
3.79 ± 8.1 (0–10 RU/mL)
0.04
IgG
48.8 ± 40.2 (0–10 RU/mL)
47.2 ± 34.6 (0–10 RU/mL)
<0.001
4 Discussion
The present work highlighted the crucial role of chest CT scan and serum antibodies for early diagnosis of SARS-CoV-2 asymptomatic pregnant patients. The majority of the patients initially tested negative on RT-PCR for SARS-CoV-2 but displayed typical CT manifestations at initial examinations. The clinical characteristics of the asymptomatic patients with SARS-CoV-2 showed significant similarity with the SARS-CoV-2 positive, non-pregnant patients, which was consistent with previous investigations (Huang et al., 2020; Jin et al., 2020; Wang et al., 2020).
Albeit the specificity of RT-PCR for the detection of COVID-19 is remarkable, but its accuracy depends on sampling quality. Additionally, the longest detection time makes it difficult for rapid medical decision-making and clinical workflow during an outbreak. Our study shows that patients with positive CT and negative RT-PCR should also be kept isolated to reduce the risk of viral spread and infection.
Ample literature stipulated that neonates of pregnant patients infected with SARS or MERS did not have COVID-19 at birth, with no cases proposing vertical transmission of infection from mother to child (J. Liu et al., 2020). Nevertheless, it has been reported perinatal COVID-19 may confer detrimental effects on neonates causing respiratory and neonatal distress, premature labour, thrombocytopenia along with liver dysfunction, and even mortality (Zhu et al., 2020). In the current work, eleven newborns had negative results for SARS-CoV-2 infection, and no vertical transmission was noticed. These findings are inconsistent with Wu et al. (Wu et al., 2020). However, vertical transmission of COVID-19 in neonates from an infected mother has also been reported, which highlighted the significance of early detection and considering the pregnant mother as a high-risk group (Alzamora et al., 2020).
The features of CT imaging in SARS-CoV-2 pneumonia are not much more specific compared to both SARS and H1N1 (Wong et al., 2004b; Yuan et al., 2012). Bilateral, multifocal ground-glass opacities and peripheral subpleural distribution were observed in most of our patients in agreement with previous studies (Chung et al., 2020; Kim, 2020; K. Li et al., 2020; Song et al., 2020; Zhou et al., 2020). The CT images of some patients revealed that they were in initial or progressive stages of the disease, depicting its diversity. The presence of intralobular interstitial thickening with fibrous stripes and consolidation was seen in some patients, while others have symmetrical spheres of ground-glass opacity accompanying hydropericardium, which is a similar feature found in non-pregnant SARS-CoV-2 pneumonia patients (Chen et al., 2020; Pan et al., 2020). Beyond the diagnosis, the progressive lesions shown on CT imaging of asymptomatic SARS-CoV-2 patients help establish the prognosis. CT is valuable for surveillance of disease progression and evaluation of the efficacy of treatment.
In addition, the difference in IgM and IgG values of asymptomatic SARS-CoV-2 patients could be used as an indicator of the infection. The elevated levels of IgG antibodies were noticed in pregnant women and neonates both, in this study, which depicted vigorous immune response indicating the severity of the disease. The presence of IgM antibodies shows preliminary defense against viral infection whereas, the presence of high-affinity IgG antibody generates long-term immunity and immunological response (G. Li, et al., 2003; X. Liu et al., 2020).
5 Conclusion
Briefly, asymptomatic pregnant patients with negative RT-PCR could be at a mild or moderate stage of SARS-CoV-2 based on CT imaging and clinical characteristics. Screening of the patient’s antibodies response is an efficient approach to the timely diagnosis of SARS-CoV-2 pneumonia in asymptomatic patients. The small sample size along with the lack of a patient’s history of pregnancy stages during SARS-CoV-2 onset, are the limitations of this study. These issues will surely garner the attention of researchers in future studies.
Acknowledgments
The authors extend their appreciation to the Deputyship for Research and Innovation, ‘‘Ministry of Education” in Saudi Arabia for funding this research work through the Project no. (IFKSURP-RGP-1435-012).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;296:E32-E40.
- [CrossRef] [Google Scholar]
- Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection during pregnancy: report of two cases & review of the literature. J. Microbiol. Immunol. Infect.. 2019;52:501-503.
- [CrossRef] [Google Scholar]
- Severe COVID-19 during pregnancy and possible vertical transmission. Am. J. Perinatol.. 2020;37(08):861-865.
- [CrossRef] [Google Scholar]
- COVID-19 infection among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am. J. Obstet. Gynecol. MFM.. 2020;2:100118.
- [CrossRef] [Google Scholar]
- Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395(10226):809-815.
- [CrossRef] [Google Scholar]
- CT imaging features of 2019 novel Coronavirus (2019-nCoV) Radiology. 2020;295(1):202-207.
- [CrossRef] [Google Scholar]
- COVID19 during pregnancy: a systematic review of reported cases. Am. J. Obs. Gynecol.. 2020;223:36-41.
- [CrossRef] [Google Scholar]
- SARS-CoV2 vertical transmission with adverse effects on the newborn revealed through integrated immunohistochemical, electron microscopy and molecular analyses of Placenta. EBioMedicine. 2020;59:102951.
- [CrossRef] [Google Scholar]
- Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology. 2020;296(2):E115-E117.
- [CrossRef] [Google Scholar]
- Hamza, M., Ali, A., Khan, S., Ahmed, S., Attique, Z., Ur Rehman, S., … Munir, A., 2020. nCOV-19 peptides mass fingerprinting identification, binding, and blocking of inhibitors flavonoids and anthraquinone of Moringa oleifera and hydroxychloroquine. J. Biomol. Struct. Dyn. 1–11. https://doi.org/10.1080/07391102.2020.1778534
- Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506.
- [CrossRef] [Google Scholar]
- H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374(9688):451-458.
- [CrossRef] [Google Scholar]
- A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Mil. Med. Res.. 2020;7:4.
- [CrossRef] [Google Scholar]
- COVID-19: Clinical aspects and therapeutics responses. Saudi Pharmaceutical Journal. 2020;28(8):1004-1008. ISSN 1319-0164
- [CrossRef] [Google Scholar]
- Outbreak of novel coronavirus (COVID-19): What is the role of radiologists? Eur. Radiol.. 2020;30(6):3266-3267.
- [CrossRef] [Google Scholar]
- Diagnostic performance of CT and reverse transcriptase polymerase chain reaction for coronavirus disease 2019: a meta-analysis. Radiology. 2020;296(3):E145-E155.
- [CrossRef] [Google Scholar]
- Profile of specific antibodies to the SARS-associated coronavirus. N. Engl. J. Med.. 2003;349(5):508-509.
- [CrossRef] [Google Scholar]
- CT image visual quantitative evaluation and clinical classification of coronavirus disease (COVID-19) Eur. Radiol.. 2020;30(8):4407-4416.
- [CrossRef] [Google Scholar]
- Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS‐CoV, MERS‐CoV, and 2019‐nCoV. J. Med. Virol.. 2020;92(5):491-494.
- [CrossRef] [Google Scholar]
- Patterns of IgG and IgM antibody response in COVID-19 patients. Emerg. Microb. Infect.. 2020;9(1):1269-1274.
- [CrossRef] [Google Scholar]
- Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. 2020;295:715-721.
- [CrossRef] [Google Scholar]
- Coronavirus Disease 2019 (COVID-19) and Pregnancy: what obstetricians need to know. Am. J. Obstet. Gynecol.. 2020;222:415-426.
- [CrossRef] [Google Scholar]
- Clinical findings and disease severity in hospitalized pregnant women with coronavirus disease 2019 (COVID-19) Obstet. Gynecol.. 2020;136:252-258.
- [CrossRef] [Google Scholar]
- An analysis of 38 pregnant women with COVID-19, their newborn infants, and maternal-fetal transmission of SARS-CoV-2: maternal coronavirus infections and pregnancy outcomes. Arch. Pathol. Lab. Med.. 2020;144:799-805.
- [CrossRef] [Google Scholar]
- Emerging 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 2020;295:210-217.
- [CrossRef] [Google Scholar]
- Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069.
- [CrossRef] [Google Scholar]
- Severe acute respiratory syndrome: thin-section computed tomography features, temporal changes, and clinicoradiologic correlation during the convalescent period. J. Comput. Assist. Tomogr.. 2004;28:790-795.
- [Google Scholar]
- Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am. J. Obstet. Gynecol.. 2004;191:292-297.
- [CrossRef] [Google Scholar]
- Radiological findings and clinical characteristics of pregnant women with COVID-19 pneumonia. Int. J. Gynecol. Obstet.. 2020;150:58-63.
- [CrossRef] [Google Scholar]
- Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci. China Life Sci.. 2020;63:457-460.
- [CrossRef] [Google Scholar]
- Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study. Lancet Infect. Dis.. 2020;20:559-564.
- [CrossRef] [Google Scholar]
- Initial HRCT findings of novel influenza A (H1N1) infection. Influenza Other Respi. Viruses.. 2012;6:e114-e119.
- [CrossRef] [Google Scholar]
- Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg. Microbes Infect.. 2020;9:386-389.
- [CrossRef] [Google Scholar]
- Coronavirus disease 2019: initial chest CT findings. Eur. Radiol.. 2020;30:1-9.
- [CrossRef] [Google Scholar]
- Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl. Pediatr.. 2020;9:51-60.
- [CrossRef] [Google Scholar]







