Translate this page into:
Estimating abundance of some wild faunal elements of Jasrota Wildlife Sanctuary, India
⁎Corresponding author. atharscorp@gmail.com (Athar Noor)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Perpetually increasing human population has kept natural resources and biodiversity under a continuum of anthropogenic pressures compelling wildlife managers to keep a count of what and how many are there to be conserved and protected. We present here baseline information about the abundance of some wild faunal species counted on predetermined belt transects of varied lengths on three consecutive days during summer 2012 in the Jasrota Wildlife Sanctuary, Jammu & Kashmir. Rhesus macaque (Macaca mulatta) was the most abundant species with the highest mean ecological density (individuals/km2) of 146.9 mean ± 1.47 90% CI followed by the red jungle fowl (Gallus gallus) (46.46 ± 0.84 90% CI). Amongst the ungulate species observed, the muntjac (Muntiacus muntjak) had the highest mean density (9.49 ± 1.52 90% CI). Bounded Count method used to estimate population size produced the highest estimate for macaques as 135 individuals (90% CI: lower bound = 117 and upper bound = 279) whereas the smallest population estimate obtained was 5 individuals (90% CI: lower bound = 3 and upper bound = 21) for the wild pig. The highest grouping tendency was found for the rhesus macaque (10.88 mean ± 5.74 SD) followed by the red jungle fowl (2.65 ± 2.07) and the Indian peafowl (2.26 ± 1.72). The Indian muntjac was observed either solitary or in very small groups (1.26 ± 0.45). The abundance estimates obtained in the study area can be considered conservative and may be helpful in developing future management strategies. We discuss about the limitations and precision of the abundance estimates obtained.
Keywords
Abundance
Estimators
Fauna
Group size
Population
Jasrota Wildlife Sanctuary
1 Introduction
The Himalaya is the highest and the youngest mountain system in the world (Devan, 1988). The formation (∼70 million years ago) of the Himalaya resulted in new barriers and corridors leading to the creation of ideal habitats for a variety of floral and faunal species. The richness in biological diversity in this region owing to its variable climatic conditions and habitats (Rau, 1975), ultimately led it to become one of the global biodiversity hotspots (Myers et al., 2000). The western Himalaya contains several species which are endemic to this region only. Of the 137 species of endangered Himalayan plants listed so far in the Red Data book, 56 species are from the Western Himalaya (IUCN, 2015). 11 species of endemic birds including the Cheer pheasant (Catreus wallichi) and the Western Tragopan (Tragopan melanocephalus) (Statersfield et al., 1998) are found in this region. Endemic mammals like Kashmir markhor (Capra falconeri), Asiatic ibex (Capra sibirica), Kashmir red deer (Cervus elaphus hanglu), Tibetan antelope (Pantholops hodgsonii) and Eurasian lynx (Lynx lynx) are found exclusively in the Western Himalaya (Macdonald, 2001; Rodgers and Panwar, 1988).
In the face of continuous anthropogenic pressures due to the increasing human population, the fragile Himalayan ecosystem is undergoing rapid degradation which has serious long term repercussions. The factors attributable to this environmental degradation include unsustainable harvesting of biological resources like firewood, non timber forest products (NTFP), timber, large-scale developmental projects, extensive livestock grazing, illegal extraction of rare and threatened plants and poaching of endangered animals. Moreover, all these pressures have resulted in fragmentation and degradation and even loss of wildlife habitats making some of the forests empty of their wildlife in south-east Asia (Datta et al., 2008; Steinmetz et al., 2013) as well as the Himalaya (Shehzad et al., 2014).
Biological diversity is viewed as the potential resource capital of a state or region that possesses it. Loss of the biodiversity worldwide has become a major political and social concern (Lele et al., 2010) with in situ conservation prevailing as the model adopted to reduce biodiversity loss (Eken et al., 2004). Effective conservation and management of biodiversity is of paramount importance and requires prior knowledge of species diversity, distribution and abundance so as to detect significant changes for appropriate management interventions. Consequently, efficient and reliable methods for rapid assessment of species richness and abundance are required in determining conservation priorities (Silviera et al., 2003).
The basic information pertaining to distribution, abundance and ecology for many species in the Himalayan ecosystem is limited due to rugged terrain, low accessibility, extreme weather conditions, etc. (Schaller, 1977) leaving a void in the sound understanding of wildlife ecology. We do not even know the status of some existing species and lag behind in exploring and reporting unrecorded species in the state. Keeping these points in mind the present study was undertaken to quantify the faunal diversity of a north western Himalayan protected area. We document the estimates of abundance or abundance indices of some faunal elements that were observed during the survey in the Jasrota Wildlife Sanctuary, Jammu and Kashmir (J&K), India. This study may serve as baseline information for future management interventions as we provide abundance estimates of the species observed during the survey.
2 Material and methods
2.1 Study area
Jasrota Wildlife Sanctuary (hereafter JWS; 10.04 km2; 32° 27′–32° 31′ N, 75° 22′–75° 26′ E; elevation 356–643 m above sea level) is situated on the right bank of the Ujh river (District Kathua, J&K) (Fig. 1). The climate is generally dry sub-humid (average annual precipitation around 1000 mm). Summer runs from April to mid-July, with maximum summer temperatures ranging between 36 °C and 42 °C. Winter runs from November to February and spring between mid-February and mid-April. The vegetation is comprised of broad-leaved associates, namely Lannea coromandelica, Dendrocalamus strictus, Acacia catechu, A. arabica, Dalbergia sissoo, Bombax ceiba, Ficus religiosa, Zizyphus jujuba, etc. along with shrubs like Adhatoda vasica, Lantana camara, Parthenium hysterophorus, Calotropis procera, etc.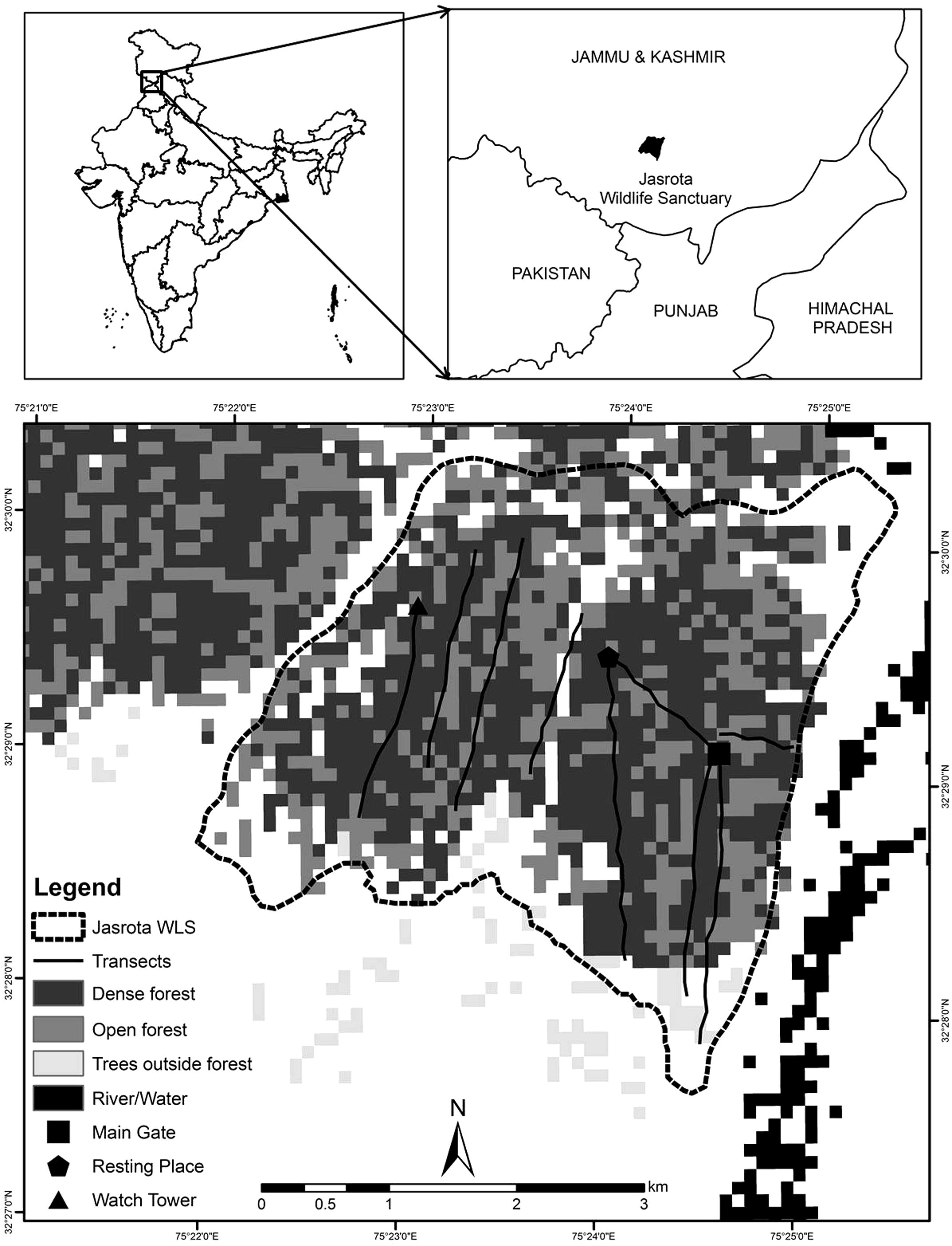
Map showing the sampled transects in the Jasrota Wildlife Sanctuary, Jammu and Kashmir.
The area comprises a small population of ungulates such as Indian muntjac (Muntiacus muntjak), spotted deer or chital (Cervus axis), sambar (Rusa unicolor) and wild pig (Sus scrofa). The JWS is believed to be the northern most limit of some species (e.g. chital and sambar) distribution range in the wild in the J&K state. The sanctuary is a home to more than 50 species of birds including genetically threatened red jungle fowl (Gallus gallus).
2.2 Sampling
We conducted a three-day (June 29–July 1, 2012) sampling using belt transects (Sutherland, 1996) in the study area. A total of nine transects were pre-marked by the Forest Department prior to the survey covering almost all major habitat types/vegetation of the study area. Since, the study area mostly consisted of forest and detections beyond 20 m were not possible, we fixed the width of each transect to total 0.04 km but the length was variable (1.5–2.8 km). A 2–3 member team (pre-trained and acclimatized with sampling methodology) walked transects in the morning hours (0600–0800 h) to observe/record the number of animals of different species. Each transect line was walked once a day for three consecutive days in order to maximize data collection.
2.3 Data analysis
Data obtained through this sampling were used to estimate encounter rates (defined as the total number of individuals of a species observed during a sampling day divided by the total distance (km) walked during that period) as an index of abundance. The abundance analysis was undertaken adopting three approaches.
2.3.1 Density estimation
We calculated animal densities per unit area on a given day as the total number of groups of a species seen on a particular day divided by the total area of all transects following Hilaluddin and Naqash (2013). We first estimated the mean group densities ( , number of groups per km2) and its standard error ( ) for each species. From this, the mean ecological density ( , number of individuals per km2) and its standard error ( ) were derived using the following equations (see Drummer, 1987; Karanth and Sunquist, 1992): where = mean group size of a species, = standard error of the mean group size and = number of groups of a species detected.
2.3.2 Bounded Count method
This method (Regier and Robson, 1967) assumes that all animals are counted without duplication during a survey of the population and that the process is repeated independently. Population is assumed to be closed during the course of the surveys i.e. the population is constant in size throughout the study (Overton, 1969).
Bounded Count estimator (NBC) was calculated using the formula: where Xm = largest of the m counts recorded, Xm−1 = second largest count recorded. Confidence interval for NBC was calculated following Robson and Whitlock (1964): where , (level of significance used) was 0.1 (90%).
3 Results
3.1 Sampling
A total distance of 54.3 km was walked and 46.8 man-hours were spent on predetermined transects during the whole survey exercise. Of all the 12 species recorded during the survey, rhesus macaque was the most sighted species with the highest number of individuals observed (N = 294) followed by the red jungle fowl (N = 93) (Table 1). The least recorded species included the Indian porcupine (N = 4), jungle cat (N = 3) and the Indian pangolin, palm civet and mongoose with only one individual sighted of each (Table 1). Therefore, these least observed species were excluded from further analysis.
Species
N
Mean ± SE
Range
Rhesus macaque
Macaca mulatta
294
5.41 ± 0.622
4.30–6.46
Red jungle fowl
Gallus gallus
93
1.71 ± 0.139
1.54–1.98
Indian peafowl
Pavo cristatus
24
0.44 ± 0.177
0.11–0.71
Indian muntjac
Muntiacus muntjak
19
0.29 ± 0.092
0.27–0.38
Chital
Cervus axis
7
0.13 ± 0.048
0.05–0.22
Indian hare
Lepus nigricollis
6
0.11 ± 0.084
0.00–0.27
Wild pig
Sus scrofa
5
0.09 ± 0.036
0.05–0.16
Indian porcupine#
Hystrix indica
4
0.08 ± 0.066
0.00–0.22
Jungle cat#
Felis chaus
3
0.03 ± 0.018
0.00–0.05
Indian pangolin#
Manis crassicaudata
1
–
–
Palm civet#
Paradoxurus hermaphroditus
1
–
–
Mongoose#
Herpestes sp.
1
–
–
3.2 Index of species abundance
In terms of encounter rates, the rhesus macaque had the highest mean (±SE) encounter rate (5.41 ± 0.62 individuals/km) during the survey period followed by red jungle fowl (1.71 ± 0.13 birds/km) and Indian peafowl (0.44 ± 0.17 birds/km). The least encountered species was wild pig (0.09 ± 0.03 animals/km) (Table 1). The mean (±SD) group size was recorded the highest for rhesus macaque (10.88 ± 5.74) followed by red jungle fowl (2.65 ± 2.07) and the Indian peafowl (2.26 ± 1.72). The Indian muntjac (1.26 ± 0.45) and the Indian hare (1.20 ± 0.44) were found to have the smallest grouping tendency (Fig. 2).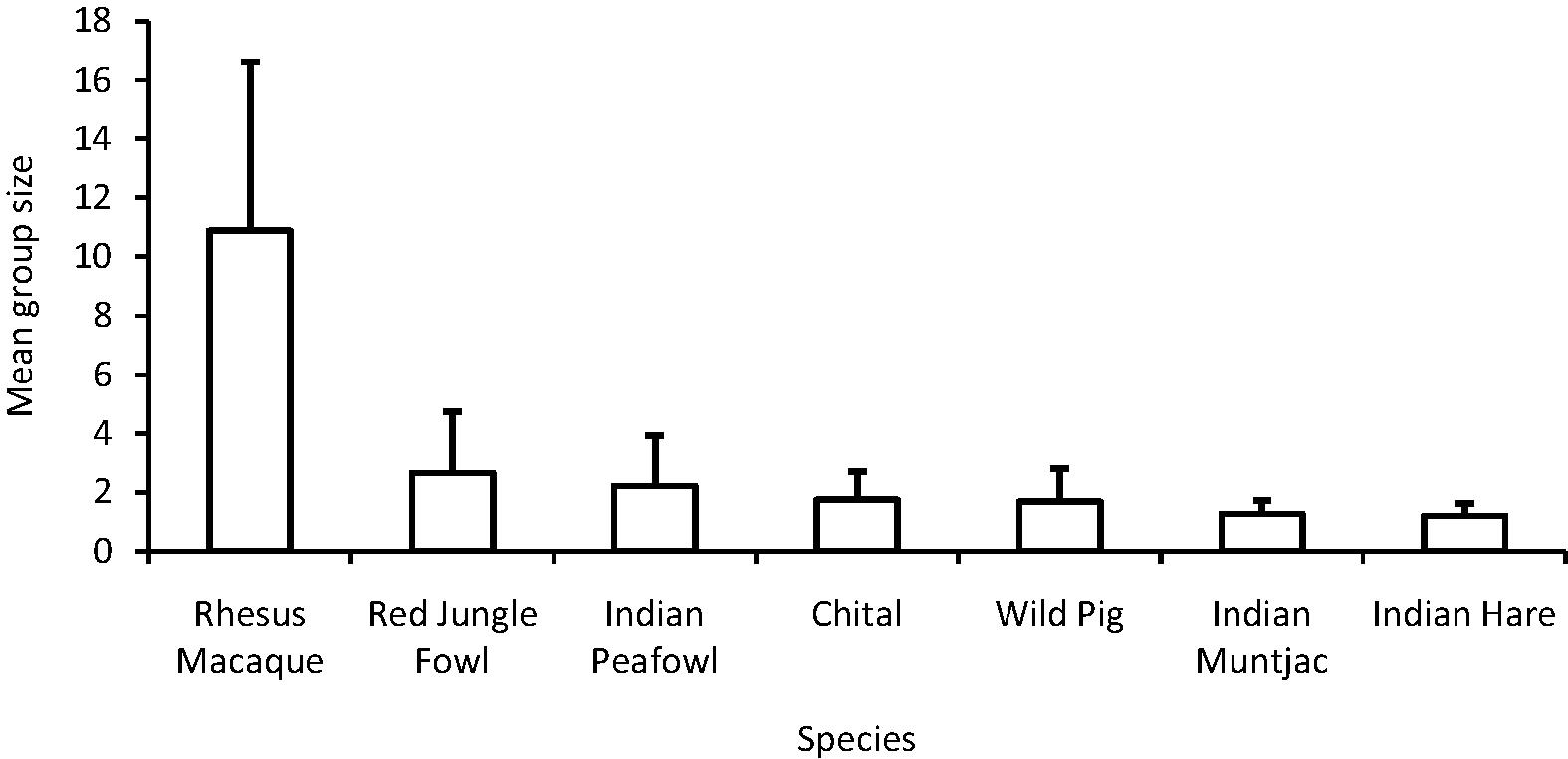
Mean group size (±SD) of species observed during the survey in Jasrota Wildlife Sanctuary.
3.3 Density estimates
In the JWS, rhesus macaque was found to be the most abundant species and had the highest mean (±SE) animal density (146.9 ± 1.12 individuals/km2) followed by red jungle fowl (46 ± 1.68 individuals/km2) (Table 2). The highest mean group density gets exchanged between these two species with the highest being for the red jungle fowl (17.49 ± 0.50 groups/km2) followed by the rhesus macaque (13.49 ± 0.87 groups/km2). Amongst ungulates, muntjac had the highest density with 9.49 ± 2.11 animals/km2 followed by chital (3.50 ± 1.00 individuals/km2) and wild pig (2.50 ± 0.58 individuals/km2). Between the two avian species recorded in the survey, the ecological density of the Indian peafowl (9.98 ± 2.11 individuals/km2) was lower than the red jungle fowl (Table 2).
Species
n
Dg
SeDg
D
90% CiD
% CV
Indian muntjac
15
07.49
0.87
09.49
8–11
15.8
Chital
4
02.00
0.50
03.50
1–6
24.7
Rhesus macaque
27
13.49
0.87
146.90
131–163
1.0
Indian peafowl
9
04.50
0.87
09.98
6–14
15.0
Red jungle fowl
35
17.49
0.50
46.46
44–49
1.9
Wild pig
3
01.50
0.00
02.50
3–3
0
3.4 Population estimates
The maximum population estimated by the Bounded Count estimator (NBC) was that of rhesus macaque (NBC = 135; lower bound = 117 and upper bound = 279), followed by red jungle fowl (NBC = 43; lower bound = 36 and upper bound = 99). The smallest population size estimated, in the sanctuary, was that of wild pig (NBC = 5; lower bound = 3 and upper bound = 21) (Fig. 3).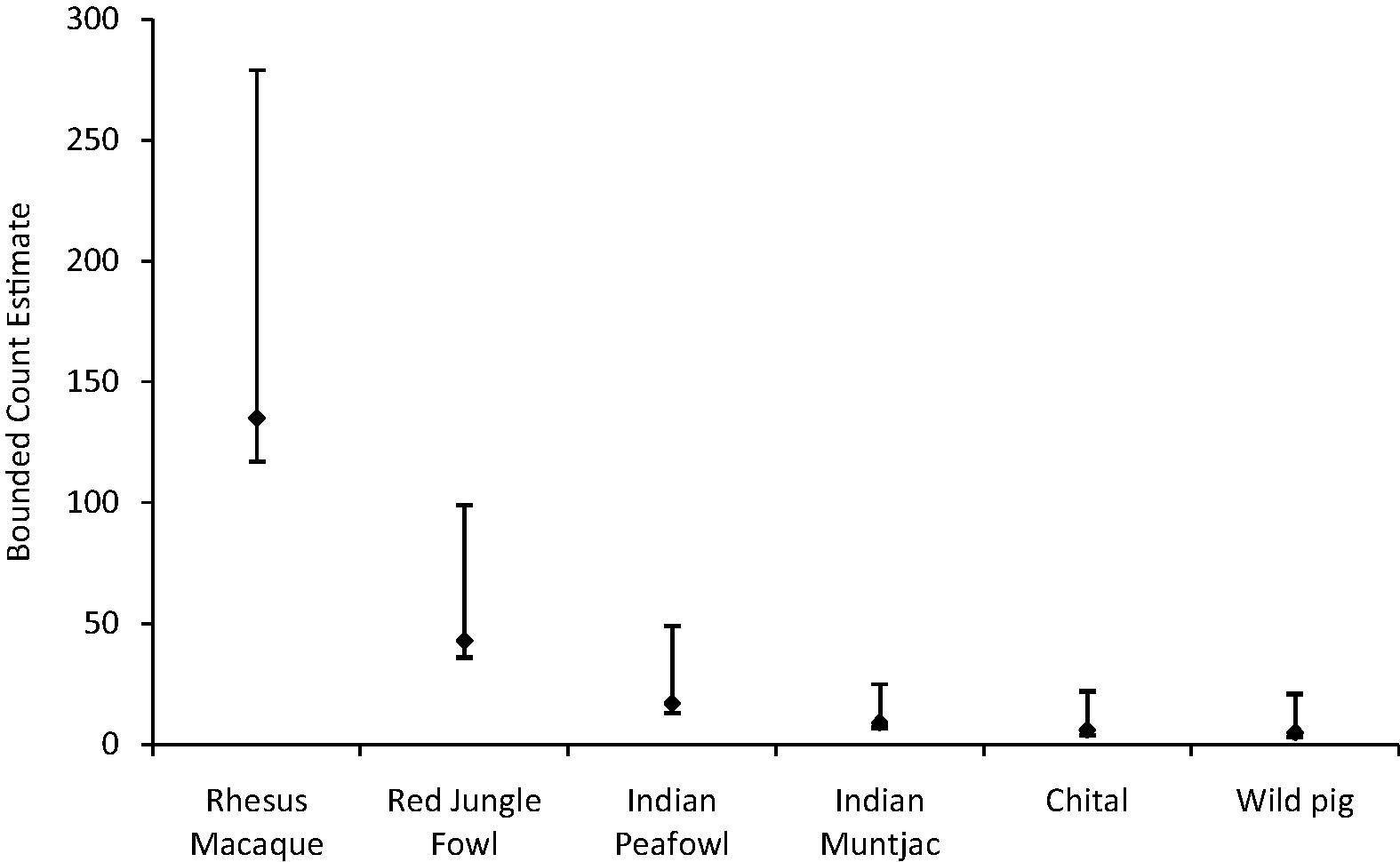
Bounded Count estimates of population of species observed during the survey in Jasrota Wildlife Sanctuary.
4 Discussion
The JWS is a small protected area (PA) which supports several wildlife species, though in less abundance except a few, most of which have been recorded here in this study. Out of these species, rhesus macaque was the most encountered species that had the highest grouping tendency i.e. forms large groups. Our density estimates of macaques are very high in comparison to those reported from other PAs within the same state (17.18 individuals/km2 in Nandini Wildlife Sanctuary (WS); Hilaluddin and Shawl, 2006: 7.1 individuals/km2 in Kishtwar National Park (NP); Hilaluddin and Naqash, 2013) or elsewhere (1.92 individuals/km2 in Kugti WS; Hilaluddin, 2007: 4.4 individuals/km2 in Pakke Tiger Reserve (TR); Selvan, 2013). Similar is the case with the red jungle fowl which had a higher density in comparison to other studies (12.9 individuals/km2 in Pakke TR; Selvan, 2013). In case of Indian peafowl, the density was still higher than that in a few studies (for example, 6.29 individuals/km2 in Satpura TR; Gurjar et al., 2013) but quite a low from others (for example, 60.16 individuals/km2 in Sariska TR; Mondal, 2011). As far as ungulate densities in the JWS are concerned, the muntjac had the highest density in comparison to chital and wild pig. The density estimates of the muntjac were even higher than those reported in several studies conducted in similar Himalayan ecosystems and ranged between 1.24 and 6.6 individuals/km2 (Dinerstein, 1979; Tamang, 1982; Thapa, 2011 and references therein). The density estimate of chital in the JWS is quite low since the species is not much abundant in the sanctuary. In similar ecosystems, estimates of density as high as 267 individuals/km2 (Wegge and Storas, 2009) and as low as 18.6 individuals/km2 (Singh et al., 2005), have been reported. We did not estimate densities of species which had very low sample sizes or because of their ecological behaviour for example, the Indian hare is mostly a nocturnal species due to which its detection during survey period was very low leading to a large variance and very low precision in density estimate.
The crude density estimates in our study area are higher than most of the studies elsewhere in similar Himalayan ecosystem settings. We attribute this huge difference in macaque densities between ours and Hilaluddin and Shawl (2006) to two main factors; first being larger widths of transects (100 m) in comparison to ours (20 m) which increased the size of the sampled area thereby reducing the density estimates. Secondly, there is a good population of the macaques inside the sanctuary, since there is a temple inside the sanctuary which remains opened year round for the devotees who consider the rhesus macaques sacred as per the Hindu mythology (Pragatheesh, 2011). There is a great reverence for macaques all across the country and the Hindu devotees occasionally feed the macaques also. This occasional food provisioning might be a reason that attracts the macaques to the sanctuary, thus inflating their numbers. This artificial feeding has been considered to change the overall behaviour of the macaques including the relationship with the humans i.e. making them more tolerant to human presence (Southwick et al., 1965, 1976). In such a scenario where human presence is high in such a small area, it is expected that the animals living inside the protected area get adapted to human activities and rarely show serious concerns.
We caution against direct comparisons with other studies as the methods used in density estimation are largely based on distance sampling i.e. accounting for distances between objects seen and the transect line, which is more robust technique which we could not use because of several limitations including undulating terrain, time and large number of observations (∼40) required for distance sampling (Burnham et al., 1980). Furthermore, there is an inverse relation between the density and the area used in density estimates, which is based on distances of objects seen from the transect line (effective strip width) in distance sampling (Burnham et al., 1980). Though, we fixed a reasonable 20 m as effective width on both sides of transect lines our impression is that, it reduced the area of the sampling units (transects) in which we counted all species groups and thus increased the density estimates. Still, we could achieve reasonable levels of precision (CV < 10–20%) in most cases only because of data collected across temporal replications. Our own subjective impression is that these densities may have been overestimated as a result of sampling error, and the true densities may be closer to the lower confidence limits of the species.
Our impression and prior information subjectively obtained from locals and the sanctuary officers in the study area as well as long experience of the frontline staff suggests that the area does not support large populations of ungulates therefore, we believe that more than half of the population of the species was observed during a survey day. Although, the estimates obtained through the Bounded Count method appear to be less precise as larger and asymmetrical confidence intervals were obtained we believe it could be an artefact of small number of replicates. Large confidence intervals can be associated with large variances which can be influenced by two factors; one of which is clustering of animal groups and secondly, proportion of area surveyed. Thus, for species with highly clustered distributions (except for the muntjac and red jungle fowl all species showed highly contagious distribution), increasing the proportion of the study area surveyed could help in achieving acceptable variance estimate (Taylor and Pollard, 2008). However, from the management point of view the point estimates obtained seemed reasonable but more precise estimates are warranted for future ecological research purposes through more robust sampling protocols.
We do not recommend uncritically applying the methods of this study to other areas as conditions will vary. We also value the uncorrected raw counts as rough indices to population trend, at least when they can be repeated through time under similar conditions. Since, estimating detection probability is not possible everywhere, our study should remind wildlife managers to interpret raw counts of the species with the likelihood of negative biases and low precision firmly in mind.
To conclude, given the logistical as well as methodological limitations, our abundance estimates are conservative as far as the minimum number of individuals of a species present is concerned. We acknowledge that the precision of the estimates can be enhanced by employing better and more robust methods and protocols than the ones used in this study, but that will come at an additional cost; therefore, there needs to have a trade-off between cost and reliability of estimates. One plausible method could be increasing the number of the replicates spatially (more number of shorter, say, 1 km long transects) as well as temporally i.e. more number of repetitions of transects. Despite being smaller in size the study area appears to support a good and unique assemblage of mammalian as well as avian fauna. The mammals of the JWS though small in population, represent 4.3% of the total mammals (372 species) recorded by Hosetti (2002) from India. The observed faunal diversity in the relatively small study area underlies the importance of this area for biodiversity conservation. Furthermore, under the current situation and limitations it is pertinent to have long term monitoring programmes with robust methodologies in order to have precise abundance estimates of the wildlife so as to develop strong conservation and management strategies in the sanctuary, otherwise this forest would also be another empty forest (Datta et al., 2008; Steinmetz et al., 2013; Shehzad et al., 2014).
Acknowledgements
We are thankful to the forest guards and the ground staff of the Jasrota Wildlife Sanctuary and others from the same region of the State Department of Wildlife Protection who contributed in data collection. We thank the Jammu & Kashmir Government and the Department of Wildlife Protection for granting the necessary permission and help in conducting the counting exercise. We also thank two anonymous reviewers for their helpful comments in improving the manuscript. We also acknowledge the support extended by the Wildlife and Environmental Conservation Society, India (WECS).
References
- Estimation of density from line transect sampling of biological populations. Wildl. Monogr.. 1980;72:202.
- [Google Scholar]
- Empty forests: large carnivores and prey abundance in Namdapha National Park, north-east India. Biol. Conserv.. 2008;141:1429-1435.
- [Google Scholar]
- State of Himalayas – ecology, environment, geography, resource and publications: a call for action. In: Himalayas: Ecology and Environment. Delhi, India: Mittal Publications; 1988. p. :183.
- [Google Scholar]
- An ecological survey of Royal Karnali-Bardia wildlife reserve, Nepal. Biol. Conserv.. 1979;18:5-38.
- [Google Scholar]
- Program Documentation and User’s Guide for SIZETRAN. Houghton.: Michigan Technological University; 1987. (p. 26)
- Key biodiversity areas as site conservation targets. Bioscience. 2004;54:1110-1118.
- [Google Scholar]
- Density of the Indian Peafowl Pavo cristatus in Satpura Tiger Reserve, India. Podoces. 2013;8:12-18.
- [Google Scholar]
- Hilaluddin, 2007. Impact of large mammal and pheasants hunting on their populations in the western Indian Himalaya. Oriental Bird Club, U.K. and Wildlife Conservation Society, Bangalore.
- Densities and population sizes of large mammals in Kishtwar high altitude national park, Jammu and Kashmir, India. Indian For.. 2013;139:872-878.
- [Google Scholar]
- Hilaluddin, Shawl, T., 2006. Survey and census report of Nandani Wildlife Sanctuary. Jammu East Wildlife Division, Department of Wildlife Protection, Government of Jammu and Kashmir.
- Glimpses of Biodiversity. Delhi: Daya Publishing House; 2002.
- IUCN, 2015. International Union for the Conservation of Nature. Red List of Threatened Species.
- Population structure, density and biomass of large herbivores in the tropical forests of Nagarahole, India. J. Trop. Ecol.. 1992;8:21-35.
- [Google Scholar]
- Beyond exclusion: alternative approaches to biodiversity conservation in the developing tropics. Curr. Opin. Environ. Sustainability. 2010;2:1-7.
- [Google Scholar]
- The New Encyclopedia of Mammals. Oxford: Oxford University Press; 2001.
- Mondal, K., 2011. Ecology of leopard (Panthera pardus fusca) in Sariska Tiger Reserve, Rajasthan, India (Ph.D. thesis). Saurashtra University, Rajkot, India, p. 235.
- Estimating the numbers of animals in wildlife populations. In: Wildlife Management Techniques (3rd ed.). Washington, D.C., USA: The Wildlife Society; 1969. p. :403-455.
- [Google Scholar]
- Effect of human feeding on the road mortality of Rhesus Macaques on National Highway-7 routed along Pench Tiger Reserve, Madhya Pradesh, India. J. Threat. Taxa. 2011;3(4):1656-1662.
- [Google Scholar]
- High Altitude Flowering Plants of West Himalaya. Howrah, India: BSI; 1975. (p. 241)
- Estimating population number and mortality rates. In: The Biological Basis of Freshwater Fish Production. Oxford, United Kingdom: Blackwell Scientific Publications; 1967. p. :31-66.
- [Google Scholar]
- Planning a Protected Area Network in India. Vol vol. 1. Dehradun, India: Wildlife Institute of India; 1988. (p. 341)
- Mountain Monarchs: Wild sheep and Goats of the Himalaya. Chicago: University of Chicago Press; 1977. (p. 425)
- Selvan, K.M., 2013. Ecology of sympatric large carnivores in Pakke Tiger Reserve, Arunachal Pradesh (Ph.D. thesis). Saurashtra University, Rajkot, Gujarat, p. 216.
- Forests without prey: livestock sustain a leopard Panthera pardus population in Pakistan. Oryx. 2014;1–6
- [CrossRef] [Google Scholar]
- Camera trap, line transect census and track surveys: a comparative evaluation. Biol. Conserv.. 2003;114:351-355.
- [Google Scholar]
- Distribution and abundance of ungulates. In: The Relationship among the Large Herbivores, Habitats and Peoples in Rajaji-Corbet National Parks, Uttaranchal, Northern India. Dehra Dun: Wildlife Institute Publication; 2005. p. :150-203.
- [Google Scholar]
- Southwick, C.H., Beg, M.A., Siddiqi, M.R. 1965. Rhesus Monkeys in north India. In: Primate Behavior: Field Studies of Monkeys and Apes. New York, pp. 111–159
- Effects of artificial feeding on aggressive behaviour of rhesus monkeys in India. Anim. Behav.. 1976;24(1):11-15.
- [Google Scholar]
- Endemic Bird Areas of the World. Priorities for Biodiversity Conservation. Cambridge, U.K.: Birdlife International; 1998.
- Tigers, leopards, and dholes in a half-empty forest: assessing species interactions in a guild of threatened carnivores. Biol. Conserv.. 2013;163:68-78.
- [Google Scholar]
- Why census? In: Ecological Census Techniques: A Handbook. Cambridge, U.K: Cambridge University Press; 1996. p. :1-9.
- [Google Scholar]
- Tamang, K.M., 1982. The status of the tiger (Panthera tigris tigris) and its impact on principal prey populations in the Royal Chitwan National Park, Nepal (Ph.D. thesis). Michigan State University, USA.
- Evaluation of two methods to estimate and monitor bird populations. PLoS One. 2008;3:e3047.
- [CrossRef] [Google Scholar]
- Thapa, T.B., 2011. Habitat suitability evaluation for leopard (Panthera pardus) using remote sensing and GIS in and around Chitwan National Park, Nepal (Ph.D. thesis). Saurashtra University, Rajkot, Gujarat, p. 228.
- Sampling tiger ungulate prey by the distance method: lessons learned in Bardia National Park, Nepal. Anim. Conserv.. 2009;12:78-84.
- [Google Scholar]







