Translate this page into:
Essential oil from the rhizomes of the Saudi and Chinese Zingiber officinale cultivars: Comparison of chemical composition, antibacterial and molecular docking studies
⁎Corresponding author at: College of Pharmacy, PO Box 620, PC 130, National University of Science and Technology, Muscat, Oman. shahalam@nu.edu.om (Shah Alam Khan) sakhan@omc.edu.om (Shah Alam Khan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Background
Essential oils have been known to possess useful biological activities and are widely used in aromatherapy owing to their peculiar aroma. The present study compared the essential oil composition isolated from the Chinese and Saudi ginger cultivars and determined their antimicrobial activity.
Methods
The volatile compounds were extracted by a hydrodistillation method and the composition was analysed by gas chromatography coupled with mass spectrometry (GC–MS). The antibacterial activity of oils was tested against the pathogenic Gram positive and Gram negative bacterial strains. Potential bioactivities were predicted with the help of Prediction of activity spectra for substances (PASS) software and the molecular docking studies on the mycobacterial protein were carried out using Pyrex with Vina wizard tool.
Results
A total of 43 and 30 volatile components were identified in the Chinese and Saudi varieties respectively. α–zingiberene in the Chinese and ar-curcumene in Saudi ginger oil were found to be the major volatile constituents. Ginger oil showed better antibacterial activity against Gram positive than Gram negative bacteria. Molecular docking studies showed ar-curcumene to have high binding affinity for the inhibition of bacterial protein.
Conclusion
The presence of the biological active compounds such as α–zingiberene, ar-curcumene and β-sesquiphellandrene makes ginger essential oil a potential source of antimicrobial agents and as an additive/preservative in the food industry.
Keywords
Antibacterial activity
Essential oil
GC-MS
Ginger
Molecular docking
- Amu
Atomic mass unit
- DDS
Drug discovery studio
- DMSO
Dimethyl sulfoxide
- EM
Electron multiplier
- GC-MS
Gas chromatography coupled with mass spectrometry
- mm
millimetre
- PASS
Prediction of activity spectra for substances
- PDB
Protein data bank
- SMILES
Simplified molecular-input line-entry system
Abbreviations
1 Introduction
Since the ancient time, humans are using essences or the volatile oils isolated from the various parts of the aromatic plants in food, medicinal and cosmetic applications (Bakkali et al., 2008). Chemically, essential oils are mixture of hydrocarbons and their oxygenated derivatives (Al-Breiki et al., 2018). These oils have been shown to possess useful biological activities. Now a days, essential oils are widely used in the aromatherapy owing to their peculiar aroma (Ali et al., 2013). GC–MS is an excellent hyphenated analytical technique which combines two instruments with different techniques in one i.e., the gas chromatograph and the mass spectrometer The gas chromatograph separates complex mixture into the individual components based on their differential affinity to the stationary phase and the mass spectrometer which is used as a detector, characterize and identify the separated constituents based on their molecular weight (Niessen, 2001; Danish and Nizami, 2019). Because of the accuracy in analysis and ease of operation, GC–MS is widely used for the identification and quantification of volatile constituents of an essential oil. It enables industrial chemists to confirm botanical source, check purity and identify the contaminants or adulterants in the essential oil (Brown, 1960). A number of studies have reported the analysis and characterization of chemical constituents of essential oils using GC–MS (Mothana et al., 2013; Patra et al., 2015).
Zingiber officinale Rosc., known as ginger in English, Adrak in Hindi, Shengjiang in the Chinese and Zangabeel in the Arabic, belongs to the family of Zingiberaceae. It is an herbaceous aromatic plant that grows to about 1 m in height. The rhizome or the underground stem is the most commonly used part of the ginger in food products as a spice, cosmetics and in medicine (Chen et al., 2000; Li, 2008.). It is very rich in minerals, vitamins, lipids, proteins, carbohydrates (starch) and essential oil. Ginger has been used therapeutically and traditionally for its antimicrobial, anti-inflammatory and anti-oxidant properties since the old age (Shukla and Singh, 2007). Various traditional system of medicine also use ginger alone or in combination with the other herbs to treat hypertension, arthritis, ulcer, symptoms associated with cold/flu, vomiting, nausea, migraine headache etc (Vasanthi and Parameswari, 2010). The aroma and pungent taste of fresh ginger is due to the presence of volatile constituents including monoterpenoids, sesquiterpenes, sesquiterpenoids and some non volatile compounds like gingerols, shagaols, zingerone etc (Jolad et al., 2004). Ginger volatile oil because of its aroma is also used widely in flavour and perfumery industries. Numerous studies have reported the chemical composition and antimicrobial activity of ginger oil (Wohlmuth et al., 2006; Sultan et al., 2005; Ahmed et al., 2002). These studies have confirmed the presence of several identical constituents such as α–zingiberene, ar-curcumene and β-sesquiphellandrene in significant amounts along with variation in content and chemical composition (Liu et al., 2019).
In the Arab world, ginger tea and Arabic ginger qahwa (coffee) are among the most popular hot beverages. Its aqueous decoction is used by herbal healers to treat respiratory infections and stomach disorders. The mixture of honey and crushed grated ginger juice is also used for the treatment of sore throat and common cold (Divakar et al., 2016). The Chinese ginger is abundantly available and sold in the vegetable/supermarkets in the gulf countries and is also preferred by the locals over the ginger varieties from other countries. We, therefore, chose Chinese ginger and Saudi ginger to study the differences in the chemical composition and antimicrobial activity of their volatile oils, if any. Hence, we undertook this study and for the first time report herein the comparison of the chemical composition of the Chinese and Saudi ginger volatile oils vis a vis evaluation of their in vitro antimicrobial activity supported with the molecular docking studies of the major constituents.
2 Materials and methods
2.1 Collection of the plant material
The fresh rhizomes of the Chinese and Saudi ginger were purchased from the local farms of Al Hasa, Saudi Arabia and from the vegetable section of the Carrefour supermarket in Muscat. They were kept in a refrigerator to prevent the drying of the ginger rhizome. The samples were identified by a faculty member in the College of Pharmacy, National University and the plant specimen was deposited in the Pharmacy lab for the future reference.
2.2 Isolation of essential oil
The fresh ginger was cleaned under tap water to remove soil and other impurities. Approximately 349.5 g of Chinese ginger and 462.0 g of Saudi ginger were chopped into small pieces and made into a paste by using domestic blender. The ginger essential oil was isolated by the clevenger apparatus. The oily layer was separated following hydrodistillation for 6 h (Mohammed et al., 2019).
The oil was dried over anhydrous sodium sulphate and then the percentage (%) yield of each essential oil was calculated with respect to the dried weight taken. The oils kept in air tight plastic bottles were stored in a refrigerator until further use.
2.3 GC- MS analysis
The chemical volatile composition of essential oil was analyzed on a Perkin Elmer Clans 600 GC System, fitted with HP-5MS capillary column (30 m X 0.25 mm i.d X 0.25 μm film thickness; maximum temperature, 350 °C), coupled to a Perkin Elmer Clarus 600C MS. Standard operating conditions were used for the analysis viz., carrier gas- helium (99.9999% purity); flow rate- 1.0 mL/min; injection temperature-290 °C, transfer line temperature- 280 °C and ion source temperature- 270 °C; ionizing energy-70 eV; injected sample volume-1 uL; split ratio of 150:1. The oven temperature program was set to 60 °C at a rate of 3 °C/ min −280 °C held for 2 min. Electron multiplier (EM) voltage was obtained from auto-tune. The mass spectra were scanned in the range of 40–550 amu to obtain the data for identification. The identity of the compounds was established by comparing the obtained spectrum with the mass spectrum libraries.
2.4 Antibacterial activity
The antimicrobial activity was determined by the well diffusion method using Muller Hinton Agar nutrient media. Four previously sterilized nutrient agar plates were used to culture the Staphylococcus aureus (ATCC 29213) and Escherichia coli (ATCC 8739) bacteria. Two culture plates each were swabbed with the S. aureus and E. coli. Five holes of uniform diameter were made in each plate with the help of a cork borer under aseptic conditions. Three different concentrations of the each oil (2.5, 5.0, and 10 μL) were tested for the antibacterial activity. A volume of 10 µL of each concentrations of the Chinese and Saudi ginger essential oils were added to the holes in Gram negative and Gram positive cultured petri plates. Dimethyl sulfoxide (DMSO) was used as a diluent to make up the volume to 10 μL. A disc of Ampicillin (25 µg) was added as a reference antibiotic and DMSO (10 µL) was used as a negative control. Afterwards, the cultured plates were incubated for 24 h at 37 °C. Finally the zone of inhibition for each concentration of ginger volatile oil was measured the next day.
2.5 In silico prediction of bioactivity and molecular docking studies
The five major chemical constituents of the ginger volatile oils were identified with the help of gas chromatogram. The simplified molecular-input line-entry system (SMILES) notations of selected chemical constituents were fed in to the PASS online software to predict their biological activity spectrum. The X- ray crystal structure of mycobacterial protein enoyl reductase (PDB: 4TZK) was downloaded from RCSB protein data bank. The molecular docking studies of the major constituents on to the mycobacterial protein was performed using Pyrex with Vina Wizard tool to find out the binding energy and to know the various ligand–receptor interactions responsible for the antibacterial activity of ginger volatile oil. Drug discovery studio (DDS) was used for the visualization of docking poses.
3 Results and discussion
3.1 Percentage (%) yield of isolated ginger volatile oil
Hydrodistillation method is one of the common and easiest methods employed to extract the essential oil. The Chinese and Saudi ginger produced the 0.14% and 0.2% v/w of essential oil and clearly indicates low yield as well as variation in % yield. The fresh ginger contains 1–2% of volatile oil, the low yield suggest either the ginger rhizomes were not mature enough, either dried or extraction method was not appropriate. The Chinese oil was noticed to be of dark yellow in colour while the Saudi ginger oil was of light yellowish- green tint. The variation in the yields of oils could be due to the difference in geographical conditions, cultivation time, harvesting time, the origin of the ginger, and other environmental factors (Onyenekwe and Hashimoto, 1999).
3.2 Chemical composition of ginger volatile oils by GC–MS analysis
Chemical composition of volatile oils isolated from the Chinese and Saudi ginger cultivars were analyzed with the help of GC–MS chromatogram (Figs. 1 and 2). The volatile compounds were identified by comparing the retention time and molecular weight with the reference compounds in the NIST library.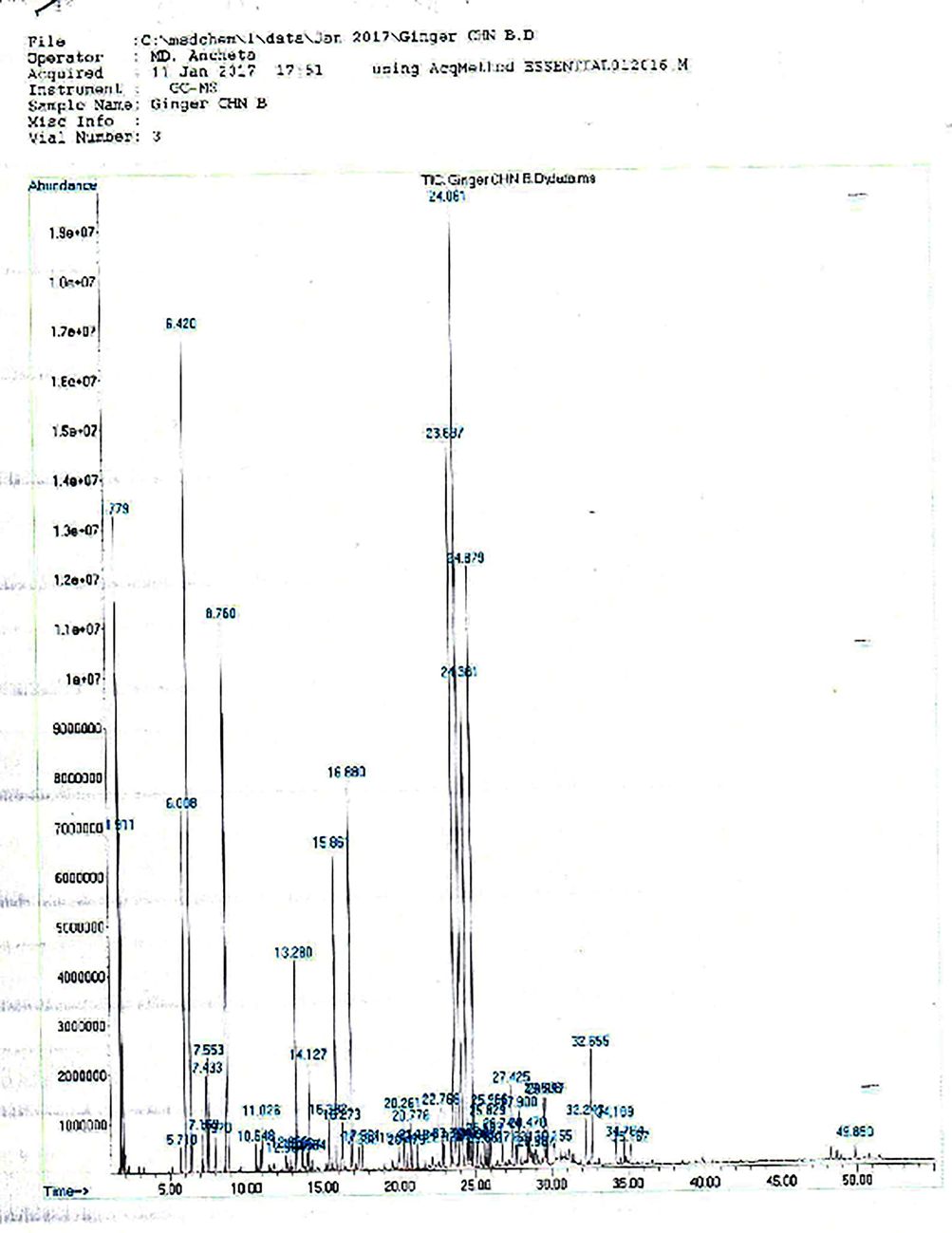
Chromatogram of Chinese ginger oil.
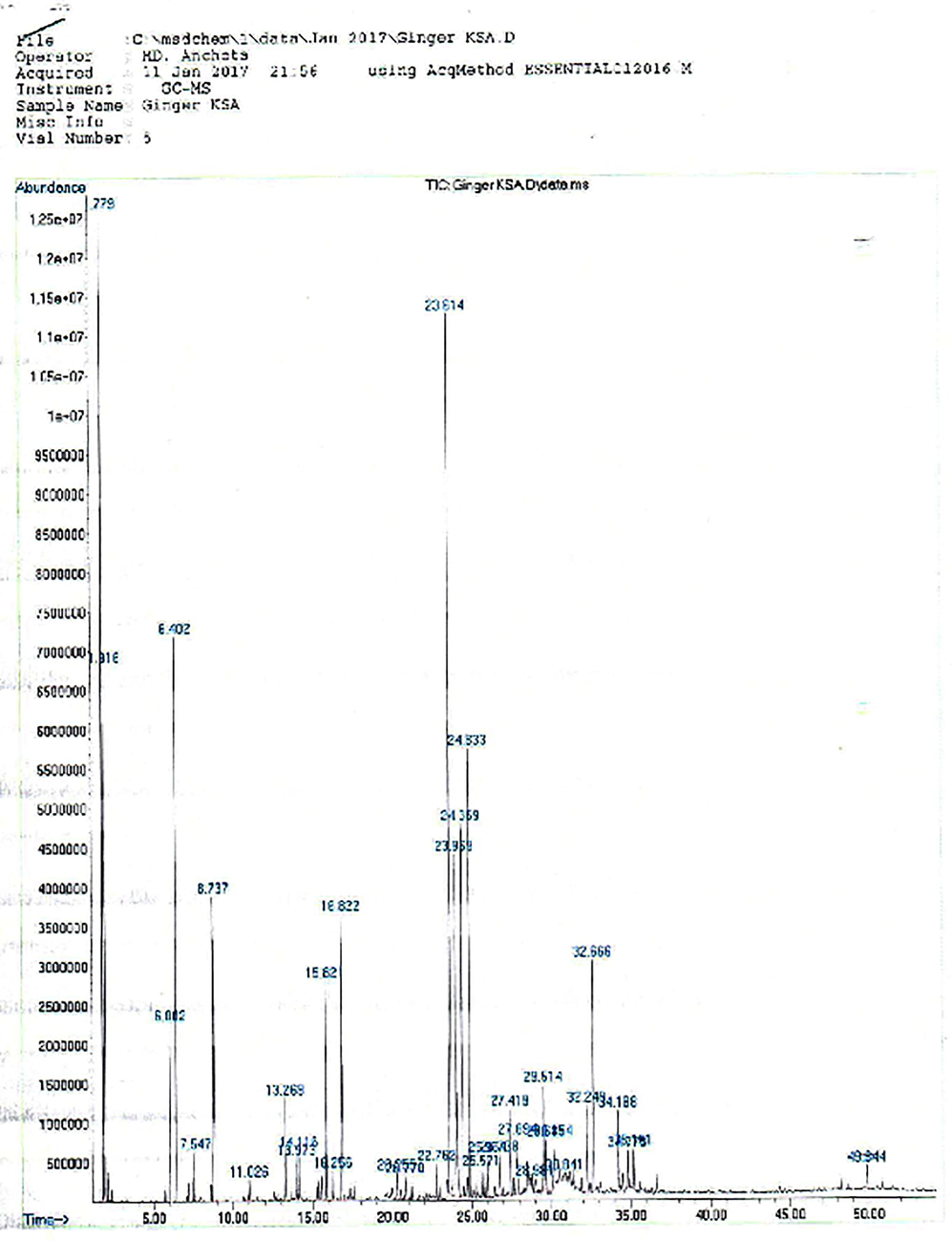
Chromatogram of Saudi ginger oil.
The Chinese and Saudi varieties were found to contain 43 and 30 volatile components and the results are presented in the Table 1. The volatile components of ginger oil belong to monoterpenoid, sesquiterpene and sesquiterpene alcohol, etc. The five major volatile constituents identified in the Chinese and Saudi oils include α-zingiberene (17.94% & 7.68%), β-phellandrene (10.81% & 7.11%), ar-curcumene (10.31 & 15.78%), β-sesquiphellandrene (7.69% & 6.99%) and β-bisabolene (7.59% & 7.33%) respectively (Figs. 3 and 4). As it can be seen from the Table 1, α–zingiberene (monocyclic sesquiterpene hydrocarbon) is the major constituent of the Chinese rhizome variety while Saudi ginger was noted to be of ar-curcumene chemotype. Our results of the Chinese chemotype are in agreement with the study of Toure and Xiaoming. They detected 48 volatile constituents in the Chinese ginger oil and identified α-zingiberene as the major constituent with a little higher content than obtained in the current study (Toure and Xiaoming, 2007). Some of the volatile constituents such as α-pinene, L-camphene, β-myrcene, β-linalool, L-borneol, citral, geraniol, β-farnesene, β-elemene, elemol and trans-nerolidol, etc., are common in both the volatile oils.
S. No
Ginger volatile oil (Chinese)
Ginger volatile oil (Saudi)
Compound name
Retention time (min)
% Composition
Compound name
Retention time (min)
% Composition
1.
L. Cyclene
5.710
0.18
α–Pinene
6.002
1.84
2.
α – Pinene
6.008
2.57
L. Camphene
6.402
6.25
3.
L. Camphene
6.420
7.26
β-Myrcene
7.547
0.55
4.
β-Pinene
7.169
0.30
β-Phellandrene
8.737
7.11
5.
β-Myrcene
7.553
0.83
β-Linalool
11.026
0.43
6.
β-Phellandrene
8.760
10.81
L. Borneol
13.269
1.61
7.
α –Terpinolene
10.648
0.23
L. Cryptone
13.973
0.68
8.
L-Linalool
11.026
0.71
α-Terpineol
14.116
0.71
9.
L. Camphor
12.554
0.16
β-Citral (z- citral)
15.821
3.16
10.
L-Borneol
13.280
2.18
Trans–Geraniol
16.256
0.54
11.
α (−)- Terpinen-4-ol
13.664
1.30
E- Citral
16.822
4.35
12.
L-Cryptone
13.984
0.18
α–Cubebene
20.255
0.42
13.
β – Citronellol
15.392
0.61
β–Elemene
20.770
0.42
14.
Trans–Citral
15.861
3.78
(Z) β- Farnesene
22.762
0.51
15.
Trans-Geraniol
16.273
0.61
ar- Curcumene
23.614
15.78
16.
β-Citral
16.880
6.62
L-Zingiberene
23.969
7.68
17.
Bornyl acetate
17.332
0.23
β-Bisabolene
24.369
7.33
18.
Methyl nonyl ketone
17.561
0.23
β-Sesquiphellandrene
24.833
6.99
19.
α-Longipinene
19.924
0.36
Elemol
25.571
0.38
20.
α–Copaene
20.261
0.57
Trans-Nerolidol
25.954
0.67
21.
Geranyl acetate
20.479
0.21
(+)-α –Bisabolol
27.419
2.11
22.
β-Elemene
20.776
0.65
Thujopsen
27.894
1.77
23.
β -Farnesene
22.767
0.58
α -Farnesene
28.981
0.39
24.
(−) Alloaromadendrene
22.928
0.53
Cis-Geranylacetonesqualene
29.685
0.77
25.
β-Panasinsene
23.374
0.38
β-Nerolidol
30.154
1.45
26.
ar-Curcumene
23.637
10.31
(−)-Caryophyllene oxide
30.841
0.38
27.
α-Zingiberene
24.061
17.94
(−)-Campherenone
32.666
4.10
28.
β-Bisabolene
24.381
7.59
(−)-Drimenol
34.778
0.51
29.
(−)-α Panasinsene
24.690
0.27
Capnellane-5 α -ol-8-one
35.161
0.83
30.
β-Sesquiphellandrene
24.879
7.69
Cis–Geranylacetone
49.844
0.42
31.
Trans. γ -Bisabolene
25.068
0.27
32.
Elemol
25.583
0.29
33.
β-Germacrene
25.829
0.47
34.
L. Nerolidol
25.966
0.61
35.
Viridiflorol
26.744
0.36
36.
(+)-β-Cedrene
27.900
1.12
37.
β-Eudesmol
28.478
0.25
38.
(+)-α-Bisabolol
28.987
0.29
39.
Thujopsene
29.508
0.86
40.
β-Cedrenol
29.697
0.60
41.
Neral
30.155
0.41
42.
(−)-Campherenone
32.655
1.23
43.
(Z,E)- Farnesal
49.850
0.24
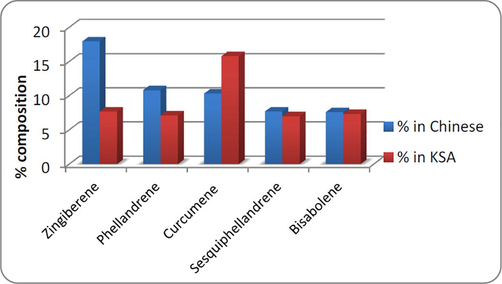
Five major volatile constituents identified in the Chinese and Saudi ginger rhizome varieties.
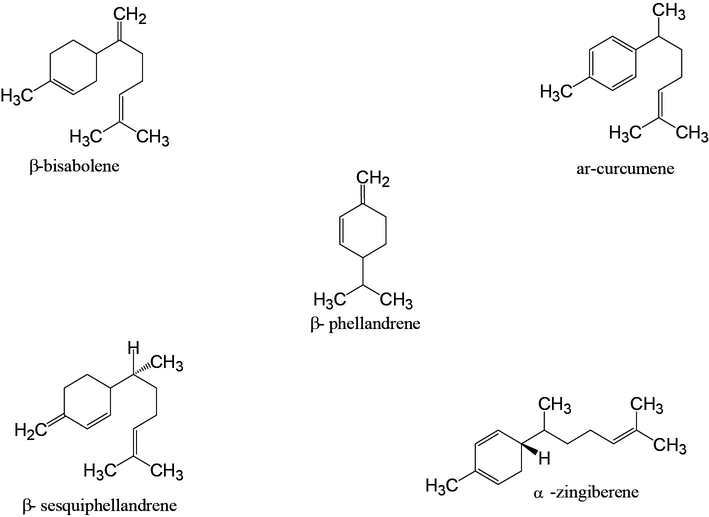
Chemical structure of five major volatile constituents found in Chinese and Saudi ginger oil.
Results of the previous studies carried out on ginger from the Chinese, Guinean and Sub Himalayan region have reported that variation in chemical compositions of different ginger cultivars are influenced by geographic origin, climatic conditions, maturity at harvest, age of plant, time of harvest and method of extraction, etc (Toure and Xiaoming, 2007; Nampoothir, 2012; Onyenekwe and Hashimoto, 1999). Several studies demonstrated α–zingiberene to be the major constituent in the ginger rhizomes from the Ghana, Thailand, Poland, Nigeria, Australia and India whereas the oil from the Cuba and Brazil contained ar-curcumene as the major component (Menon et al., 2007; Nogueira de Melo et al., 2011).
3.3 Antibacterial activity of ginger essential oil
The results of the antibacterial effect at different concentrations (2.5, 5.0 and 10 µL) of ginger oil against Gram positive bacteria (S. aureus) and Gram negative (E. coli) by using well diffusion method are presented in Table 2 (Al-Aamri et al., 2018). Ginger oil showed the dose dependant zone of inhibition against both the Gram positive and Gram negative bacteria. Results indicate that ginger oil failed to inhibit the growth of Gram negative bacteria at the lowest dose of 2.5 µL. In general, ginger volatile oil was found to exhibit better activity against Gram positive bacteria (9–13 mm zone of inhibition) in comparison to Gram negative strain (7–10 mm zone of inhibition). This difference in activity could be because of the presence of the lipopolysaccharide layer in the structure of Gram negative bacteria which reduces the activity of the ginger oil. Though, the oil differs in their chemical composition but interestingly no difference in their antibacterial spectrum against Gram positive bacteria was observed. Both the oils at the dose of 10 µL exhibited 13 mm of zone of inhibition against gram positive bacteria. Saudi ginger oil exerted better effect than the Chinese oil against E. coli (8 and 10 mm respectively). The results of ginger oil antibacterial activity are comparable to the standard drug Ampicillin (25 µg). Our study also proves that the Chinese and Saudi ginger oil exhibits slightly better antibacterial activity than the Vietnamese ginger oil (Stoyanova et al., 2006). It could be proposed that the antibacterial activity of the oil is related to its chemical composition, especially sesquiterpenes. It can be stated that the major constituent does play a role in antibacterial activity but minor components also help in exerting the synergistic action.
Sample
Dose (µL or µg)
Zone of inhibition (mm)
S. aureus
E. coli
Chinese oil
2.5
9
–
5.0
11
7
10.0
13
8
Saudi oil
2.5
9
–
5.0
12
7
10.0
13
10
Ampicillin disc
25.0
19.3
19
DMSO
10.0
–
–
3.4 In silico prediction of bioactivities and molecular docking studies
Prediction of activity spectra for substances (PASS) was used to predict the antimicrobial, antineoplastic/chemopreventive and anti-inflammatory activities of the five major volatile constituents of the ginger volatile oil. The PASS software predicts the bioactivity of molecules based on the structural similarity to the large data base of known active substances. Pa and Pi values (bioactivity score) predict the probability of compound to be active. A compound with a bioactivity score of 0.5 or above has higher chances to be active. The Pa values for predicted bioactivities lies in between 0.856 and 0.369 (Table 3). The predicted score for antineoplastic/chemopreventive activity (0.856–0.644) was found in the following order; β–bisabolene > β-phellandrene > β-sesquiphellandrene > ar–curcumene > α–zingiberene. All the five compounds are predicted to possess antifungal activity (0.638 – 0.491) while only β–bisabolene is observed to have anti-inflammatory activity (0.723). Only four constituents are found to possess antibacterial activity (0.369–0.441).
S. No
Compound
Predicted activity
Pa
Pi
1.
α – Zingiberene
Antineoplastic
0.644
0.036
Antifungal
0.607
0.018
Antimycobacterial
0.467
0.024
Antibacterial
0.416
0.026
2.
β-Phellandrene
Antineoplastic
0.830
0.009
Antifungal
0.491
0.032
Antibacterial
0.369
0.038
3.
ar–Curcumene
Antiviral (Rhinovirus)
0.700
0.003
Anti-inflammatory
0.688
0.016
Chemoprotective
0.534
0.008
Antifungal
0.521
0.027
4.
β-Sesquiphellandrene
Antineoplastic
0.827
0.009
Antiviral (Rhinovirus)
0.665
0.004
Antifungal
0.638
0.015
Antibacterial
0.441
0.023
5.
β-Bisabolene
Antineoplastic
0.856
0.006
Anti-inflammatory
0.723
0.013
Antineoplastic (breast cancer)
0.708
0.005
Antiviral (Rhinovirus)
0.676
0.003
Antifungal
0.585
0.020
Antibacterial
0.413
0.027
Molecular docking studies were also performed using mycobacterial acyl carrier protein reductase enzyme (PDB ID: 4TZK) to corroborate the experimental results of antibacterial activity. Enoyl acyl carrier protein reductase is considered as a potential target to inhibit bacterial growth (Alsaraf et al., 2020). The binding energies of the major constituents of ginger oils were found to be in the range −8.4 to −6.9 kcal/mol and indicate moderate to good inhibition of the enzyme (Table 4). Ar-curcumene and α–zingiberene were observed to fit and bind strongly in the enzyme pocket. The binding energy of the major volatile constituents was found in the following order; ar-curcumene < α–zingiberene < β–bisabolene < β–sesquiphellandrene < β–phellandrene. Interactions of ar-curcumene and α–zingiberene with the protein chain, the polar receptor surface and their 2D interactions are illustrated in Figs. 5 and 6. 2D interaction diagram of ar-curcumene with the protein showed only π - σ (PHE-41, ILE-95), π -alkyl (ILE-122, VAL-65) and alkyl (ILE-16) hydrophobic interactions with the protein residues (Fig. 5c). The two endocyclic double bonds of α–zingiberene form π -alkyl interactions with ILE-95, ILE-122 and VAL-65 while the exocylic double bond in the side chain forms the similar bonding with ILE-16. Docking pose of α–zingiberene also revealed π-π and π-σ interactions with the protein residues (Fig. 6c).
S. No
Name of compound (Ligand)
Binding energy (Kcal/mol)
1.
ar-Curcumene
−8.4
2.
α –Zingiberene
−8.3
3.
β -Bisabolene
−8.0
4.
β-Sesquiphellandrene
−7.5
5.
β-Phellandrene
−6.9
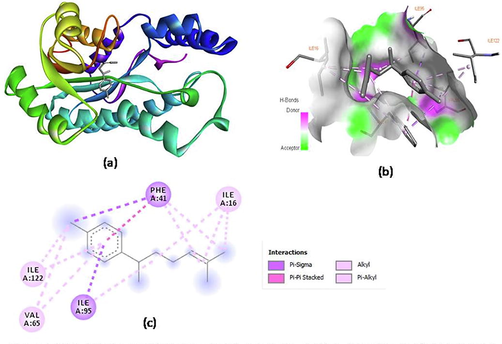
Molecular docking of ar-curcumene with enoyl acyl carrier protein reductase PDB:4TZK) (a) ar-curcumene in the pocket of protein (b) Hydrogen bond receptor surface of enzyme interacting with ar-curcumene and (c) 2D interactions of ar-curcumene with enzyme.
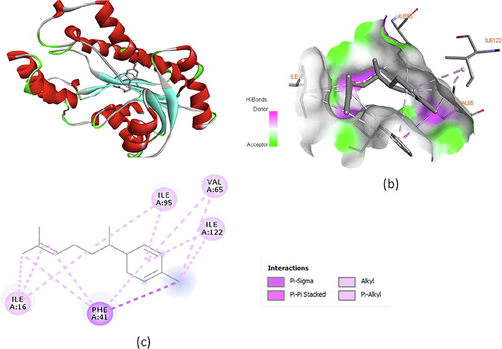
Molecular docking of α–zingiberene with enoyl acyl carrier protein reductase PDB:4TZK) (a) α–zingiberene in the pocket of protein (b) Hydrogen bond receptor surface of enzyme interacting with α–zingiberene and (c) 2D interactions of α–zingiberene with the enzyme.
4 Conclusion
Ginger is one of the most commonly used aromatic spices across the world. The volatile oil from Chinese and Saudi ginger variety showed variation in the % yield and chemical composition. The ginger essential oils displayed significant antibacterial activity and showed pronounced effect against Gram positive bacteria in comparison to the Gram negative microbe. This antimicrobial activity of ginger oil could be attributed to the presence of the biologically active volatile compounds like α–zingiberene, ar-curcumene and β-sesquiphellandrene making ginger essential oil the potential source for the therapeutic agent(s) in the treatment of microbial diseases and as an additive/preservative in the food industry. Molecular docking studies further showed that the antibacterial actions of ginger oil could be because of the presence of higher content of ar-curcumene and α–zingiberene. Ar-curcumene and α–zingiberene showed much lower binding energy and high affinity towards the bacterial protein. However, further studies are warranted to explore the therapeutic potential of ar-curcumene and α–zingiberene.
Acknowledgement
Authors extend their appreciation to the Researchers supporting project number (RSP-2020/185) King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Essential oil composition of different rhizomes of ginger (Zingiber officinale Rosc.) Flavour Fragrance J.. 2002;7–8:39-41.
- [Google Scholar]
- Al-Aamri, M.S., Al-Abousi, N,M., Al-Jabri, S.S., Alam, T., Khan, S.A., 2018. Chemical composition and in-vitro antioxidant and antimicrobial activity of the essential oil of Citrus aurantifolia L. leaves grown in Eastern Oman. J. Taibah Uni. Med. Sci.13 (2), 108-112.
- Comparative GC-MS analysis, in-vitro antioxidant and antimicrobial activities of the essential oils isolated from the peel of Omani lime. Chiang Mai J. Sci.. 2018;45(4):1782-1795.
- [Google Scholar]
- Essential oils used in aromatherapy: A systemic review. Asian Pac. J. Trop. Biomed.. 2013;5(8):601-611.
- [Google Scholar]
- Chemical composition, in vitro antibacterial and antioxidant potential of Omani Thyme essential oil along with in silico studies of its major constituent. J. King Saud Univ. Sci.. 2020;32(1):1021-1028.
- [Google Scholar]
- Identification of organic compounds by gas chromatography. Nature. 1960;188:1021-1022.
- [Google Scholar]
- Study on comprehensive utilization and deep processing of ginger extract. Food Indus. Technol.. 2000;21(4):76-78.
- [Google Scholar]
- Complete fatty acid analysis data of flaxseed oil using GC-FID method. Data Brief. 2019;23:103845
- [Google Scholar]
- The practice of ethnomedicine in the Northern and Southern provinces of Oman. Oman Med. J.. 2016;31(4):245-252.
- [Google Scholar]
- Fresh organically grown ginger (Zingiber officinale): Composition and effects on LPS-induced PGE2 production. Phytochemistry. 2004;65:1937-1954.
- [Google Scholar]
- Liu, Y., Liu, J., Zhang, Y., 2019. Research progress on chemical constituents of Zingiber officinale Roscoe. BioMed Res. International. 2019, Article ID 5370823, 21 pages.
- Effects of processing on the flavour compounds of India fresh ginger (Zingiber officinale Roscoe) J. Essent. Oil Res.. 2007;19(2):105-109.
- [Google Scholar]
- Influence of two extraction methods on essential oils of some Apiaceae family plants, Egypt. Pharmaceut. J.. 2019;18:160-164.
- [Google Scholar]
- GC and GC/MS analysis of essential oil composition of the endemic Soqotraen Leucas virgata Balf.f. and its antimicrobial and antioxidant activities. Inter. J. Mol. Sci.. 2013;14(11):23129-23139.
- [Google Scholar]
- Comparison of essential oil composition of three ginger cultivars from sub Himalayan region. Asian Pac. J. Trop. Biomed.. 2012;22:11-14.
- [Google Scholar]
- Principles and instrumentation of gas chromatography-mass spectrometry. In: Curr. Practice Gas Chromatogr.-Mass Spectrometry Chromatogr. Sci. Series. Vol 86. New York: Marcel Dekker; 2001. p. :1-30.
- [Google Scholar]
- Inhibitory effects of ginger (Zingiber officinale Roscoe) essential oil on leukocyte migration in vivo and in vitro. J. Nat. Med.. 2011;65(1):241-246.
- [Google Scholar]
- The composition of essential oil of dried Nigerian ginger (Zingiber officinale Roscoe) J. Euro. Food Res. Technol.. 1999;209:407-410.
- [Google Scholar]
- Chemical composition and antioxidant and antibacterial activities of an essential oil extracted from an edible seaweed, Laminaria japonica L. Molecules (Basel, Switzerland).. 2015;20(7):12093-12113.
- [Google Scholar]
- Cancer preventive properties of ginger: A brief review. Food Chem. Toxicol.. 2007;45(5):683-690.
- [Google Scholar]
- Composition and antimicrobial activity of ginger essential oil from Vietnam. J. Essent. Oil Bearing Plants.. 2006;9:93-98.
- [Google Scholar]
- Chemical analysis of essential oil of ginger (Zingiber officinale) Pak. J. Bio. Sci.. 2005;8(11):1576-1578.
- [Google Scholar]
- Gas chromatographic analysis of volatile components of Guinean and Chinese ginger oils (Zingiber officinale) extracted by steam distillation. J. Agro.. 2007;6:350-355.
- [Google Scholar]
- Indian spices for healthy heart – An overview. Curr. Cardiol. Rev.. 2010;6(4):274-279.
- [Google Scholar]
- Essential oil composition of diploid and tetraploid clones of ginger (Zingiber officinale Roscose) grown in Australia. J. Agric. Food Chem.. 2006;54(4):1414-1419.
- [Google Scholar]







