Translate this page into:
Escin induces apoptosis in ovarian cancer cell line by triggering S-phase cell cycle arrest and p38 MAPK/ERK pathway inhibition
⁎Corresponding author. bwsj1116@sina.com (Jian Shen)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background
Ovarian cancer (OC), is a common malignant tumors in the female reproductive system with the increased mortality rate. The occurrence of OC is elevating rapidly in recent decades. Escin is a triterpene saponin reported to possess the significant biological activities.
Objective
The current investigation focused to address the in vitro anticancer actions of escin against the OC A2780 cells through the inhibition of p38 MAPK/ERK pathway.
Methodology
The in vitro antioxidant potential of escin was examined using different free radicals scavenging assays like reducing power, DPPH, and superoxide radicals. Escin treated Vero and A2780 cell’s viability was investigated by MTT assay. The lipid peroxidation, antioxidants SOD and GSH was quantified in the escin treated A2780 cells were by standard methods. The effect of escin on the ROS accumulation, MMP, and apoptotic cell death in A2780 cells was scrutinized by respective fluorescence staining assays. The cell cycle transition was studied by flow cytometry and the expressions of p38 MAPK/ERK molecules were investigated using RT-PCR analysis.
Results
Escin possessed the appreciable reducing power and scavenged the DPPH and superoxide radicals, which proves the antioxidant capacity of escin. The viability of A2780 cells was remarkably suppressed by the escin and it did not possessed toxicity to the normal Vero cells. Escin improved the lipid peroxidation and suppressed the SOD and GSH levels in the A2780 cells. The status of MMP was substantially decreased and the ROS and apoptotic cells were drastically elevated in the escin administered A2780 cells. Escin treatment notably suppressed the p38 MAPK/ERK signaling axis in the A2780 cells.
Conclusion
The findings of this investigation have revealed that the escin has demonstrated the potent in vitro anticancer actions against the OC A2780 cells.
Keywords
Ovarian cancer
Escin
Apoptosis
P38 MAPK/ERK pathway
Cell cycle arrest
1 Introduction
Cancer signifies the global public health burden as it ranked on second major cause of mortalities around the world (Bray et al., 2018). Ovarian cancer (OC), is a common malignant tumors in the female reproductive system, holds a top rank in all malignant gynecological cancers with the increased mortality rate (Yang et al., 2018). The occurrence of OC is elevating rapidly in recent decades. Though, OC has numerous concealed initial signs, and victims have already formed a distant metastasis earlier to the arise of visible signs. On this stage, nearly 70% of victims are diagnosed at their developed stage only (Zheng et al., 2013). The chemotherapy and surgery could ameliorate the signs nonetheless, these approaches can not fundamentally eradicate the disease, and most developed victims experience the reversion within 2–3 years, ultimately resulting to death due to the resistance towards chemotherapy (Helleman et al., 2010).
Reactive oxygen species (ROS) are said to be a crucial player in the numerous cellular events. The excessive ROS accumulation could cause oxidative stress and thereby abolish cellular homeostasis (Richardson et al., 2015). Oxidative stress could trigger the mitogen-activated protein kinase (MAPK) signaling axis (Guignet et al., 2019). MAPK modules are the crucial players of intracellular signaling along with vital functions in diverse physiological mechanisms (Ki et al., 2013). There are three major subfamilies of the MAPK signaling pathway including c-Jun N-terminal kinases (JNKs), p38-MAPKs, and extracellular responsive kinases (ERKs) that can be triggered by oxidative stress (McCain, 2013). Stimulation of p38, JNK, and ERK in response to oxidative stress via the accumulation of ROS has dual roles in survival and proapoptotic events. MAPK/ERK signaling cascade is imperative signaling axis in regulating the cell multiplication and migration. The triggering of ERK/MAPK signaling regularly noted in the various human cancers including OC (Sun et al., 2020). ERK is a crucial signaling cassettes of the MAPK signaling that participates in the regulation of mitosis and meiosis in differentiated cells. ERK family is the crucial players in the regulation of cell growth, endurance, and differentiation under normal and pathological situations (Guo et al., 2020).
Apoptosis is a natural cellular process that consecutively controls the turnover of cytoplasmic organelles and whole cells via stress-regulated autophagy (Marino et al., 2014). It is distinguished by cell morphological alterations like membrane blebbing, shrinkage, and nuclear fragmentation and condensation. The benefits of triggering apoptosis in cancer cells have been experientially recognized. Apoptosis plays an essential roles in the chemotherapy for numerous cancers. The increased levels of apoptosis triggering in cancer cells are indispensable for the development of anticancer agents (Maji et al., 2018; Lin et al., 2019). The few of the currently used anticancer drugs to OC are reported to be highly toxic to other normal cells and incompetent. These unwanted adverse effects and incapability is a major problems to the efficacious cancer chemotherapy (Maggioni et al., 2015). Hence, the need for the exploration novel agents with effective anticancer actions and without adverse effects was emerged greatly.
In recent years, therapeutic agents from the natural origin have been explored extensively to treat array of ailments (Halberstein, 2005). These natural compounds are safer to use than the synthetic chemical drugs and have the potential to become substitutes to existing clinical therapies with synthetic drugs (Bishayee and Sethi, 2016). Escin is a triterpene saponin isolated from the Aesculus hippocastanum plant. Escin was well-known to demonstrate the anti-inflammatory properties (Wang et al., 2011). Escin effectively ameliorated the lung injury through suppression of inflammation (Xin et al., 2011), suppressed the lipopolysaccharides-provoked inflammation (Cheng et al., 2015), and attenuated the brain injury (Bo et al., 2018). Escin was extensively investigated for its therapeutic roles against chronic venous insufficiency (Dudek-Makuch and Studzińska-Sroka, 2015), anti-edematous activity (Sirtori, 2001), anticancer activity (Rimmon et al., 2013; Lee et al., 2014; Ciftci et al., 2015), and anti-inflammatory (Patlolla and Rao, 2015). Unfortunately the anticancer action of escin against the OC A2780 cells was not investigated yet. Hence, the current exploration focused to address the in vitro anticancer actions of escin against the OC A2780 cells through the cell cycle arrest and down-regulation of p38 MAPK/ERK pathway.
2 Materials and methods
2.1 Chemicals
Escin, doxorubicin (DOX), 2,4,6-tripyridyl s-triazine (TPTZ), HEPES buffer, Dulbecco's modified Eagle's medium (DMEM), and other chemicals were procured from the Sigma-Aldrich, USA. All the assay kits were acquired from the Abcam, USA, and Biocompare, USA, respectively.
2.2 In vitro antioxidant assays
2.2.1 Ferric reducing antioxidant power (FRAP) assay
FRAP activity of escin was evaluated by the technique of Benzie and Strain, (1996). For this, the 1 ml of various doses of escin (5–100 µM) was mixed with the 1 ml of FRAP reagent, with 300 mM of acetate buffer (pH 3.6), 10 mM of TPTZ solution and 20 mM ferric chloride. For these solutions, 200 µl of the reaction mixture was pipetted out and placed in a 96-well plate and incubated for 10 min. later then, absorbance of the colored product i.e. ferrous tripyridyltriazine complex was detected at 593 nm against the blank with the aid of microplate reader.
2.2.2 DPPH radical scavenging activity
The DPPH free radical scavenging capacity of the escin was investigated by the technique of Shimomura et al. (1998). 150 µl of DPPH in ethanol (0.25 mM) was mixed to the different doses of (5–100 µM) of the escin. Then this reaction solution was sustained for 30 min at 37 °C in dark. Lastly, absorbance was taken at 515 nm against an ethanol as blank. The DPPH scavenging ability of escin was depicted as an equivalent of Trolox. The outcomes were determined and depicted as mM.
2.2.3 Superoxide radical (O2•−) scavenging activity
The O2•− scavenging by the escin was studied using Shimada et al. (1992) approach. The reaction solution consists of 10 μl of chemiluminescence (CL) reagent, different doses of (5–100 µM) of the escin, and 80 μl of xanthine oxidase enzyme solution. The control was prepared with HEPES buffer instead of the escin. Then the reaction mixture was injected into the luminometer subsequently 200 µl of hypoxanthine substrate solution (0.72 mM). CL was detected for 10 min at 10 s intervals with the aid of luminometer. Test was conducted in triplicate and the outcomes were depicted as relative luminescence.
2.2.4 Oxygen radical antioxidant capacity (ORAC) assay
The ORAC activity of the escin was investigated to determine the peroxyl radical (ROO•) scavenging ability of escin using the assay kit. The experiment was conducted as per the protocols given by kit’s manufacturer (Abcam, USA). The outcomes were attained subsequent to one hour of average reading at 485 nm excitation and 520 nm emission.
2.2.5 Cell culture collection and maintenance
Human OC A2780 cells and normal Vero cells were procured from the ATCC, USA. Cells were sustained in DMEM along with supplementation of 10% of FBS and 1% of penicillin/streptomycin mixtures at 37 °C in a moistened CO2 (5%) chamber.
2.2.6 Cell viability assay
To assess the effect of escin on the viability of A2780 cells, cells were loaded on the 96-well plate and incubated with diverse doses of escin (5–100 μM) for 24 h at 37 °C. Then MTT reagent was mixed to every well then incubated for additional 4 h at 37 °C. The formed blue formazan crystals were liquefied with the addition of 100 μl of DMSO. Lastly, plates were read at 570 nm with the help of microplate reader. The assay was conducted in triplicate and the outcomes depicted as relative viability percentage. Cell viability was determined by O.D. of escin treated cells/O.D. of control cells × 100(%).
2.2.7 Determination of lipid peroxidation (LPO) and antioxidants level
The ecin administered A2780 cells were trypsinized and gathered then homogenated using buffered saline. The homogenate was utilized for the examination LPO and antioxidants status. The LPO level was assayed via measuring the status of LPO product thiobarbituric acid reactive substances (TBARS) in the homogenate through the approach of Ohkawa et al. (1979). The superoxide dismutase (SOD) enzyme activity was investigated as per the protocol defined by the Marklund, (1985). The level of glutathione (GSH) in the escin administered A2780 cells was quantified using Aykac et al. (1985) approach.
2.2.8 Measurement of ROS level
The ROS status in the escin treated A2780 cells was studied by the DCFH-DA staining technique. A2780 cells were placed onto a 24-well plate at the 1 × 106 cell population per well with DMEM and maintained for 24 h at 37 °C. Cells were then administered with the 25 µM of escin and 2 μg of standard drug DOX and sustained for 24 h. Later, 10 μl of DCFH-DA stain was added to each well and maintained for another 1 h and finally, ROS accumulation status was detected using fluorescence microscope.
2.2.9 Mitochondrial membrane potential (MMP)
Effect of escin on the MMP of OC A2780 cells was investigated using Rhodamine-123 (Rh-123) staining assay. For this, A2780 cells were placed in the 24-well plate at 1 × 106 cell population with the DMEM medium and administered with the 7.5 μM of escin and 2 μg of DOX and maintained for 24 h at 37 °C. Then 10 μg/ml of Rh-123 was added to each well and stained for 30 min. Finally, the MMP status of treated A2780 cells was investigated using fluorescence microscope.
2.2.10 Dual staining
The early and late apoptotic cell death in the escin administered A2780 cells was studied by using dual staining. The A2780 cells were placed onto the 24-well plate at 5 × 105 cell density in a DMEM medium and administered with 25 μM of escin and 2 μg of DOX for 24 h at 37 °C. Then the 100 μg/ml of AO/EB dye at 1:1 ratio was added for 5 min at 37 °C. Finally the early and late apoptotic cell death was investigated using fluorescence microscope.
2.2.11 Determination of apoptosis and cell cycle transition
For the detection of apoptosis and cell cycle transition of escin treated cells, A2780 cells were loaded onto the 24-well plate and sustained with the of escin (25 μM) and DOX as standard drug for 24 h at 37 °C. Then A2780 cells were gently fixed with 70% ethanol at 4 °C and then the buffered saline with 40 μg/ml of PI dye, 0.1 mg/ml of RNase and 0.1% Triton X-100 was added and cells were maintained for another 30 min at 37 °C. The apoptosis and cell cycle transition was then examined using flow cytometry.
2.2.12 RT-PCR analysis
The total RNA from the treated A2780 cells was extracted using the Trizol kit as per the protocols given with kit’s manufacturer (Biocompare, USA). The isolated RNA was suspended in the distilled water and the purity and concentration of RNA was investigated through measuring absorbance at 260 nm. The cDNA was constructed using the isolated RNA using respective kit (Abcam, USA). The primers for JNK1/2 upstream- 5′-AAAGAAUGUCCUACCUUC-3′, downstream- 5′-AGAAGGUAGGACAUUCUUU-3′; ERK1/2- upstream 5′-TCAAGCCTTCCAACCTC-3′, downstream 5′-GCAGCCCACAGACCAAA-3′; p38- upstream 5′-GTGCCCGAGCGTTACCAGACC-3′, downstream 5′-CTGTAAGCTTCTGACATTTC-3′; AKT- upstream 5′-TCTATGGCGCTGAGATTGTG-3′, downstream 5′-CTTAATGTGCCCGTCCTTGT-3′. The GAPDH was utilized as control and utilized to standardize the outcomes. The 2−ΔΔCt method was applied to determine the fold change.
2.2.13 Statistical analysis
The data were displayed as mean ± SD of obtained triplicate values. Statistical variations between groups were determined using one way ANOVA subsequently Tukey’s post hoc assay using SPSS software and the level of significance was fixed at p < 0.05.
3 Results
3.1 In vitro antioxidant activity of escin
The in vitro antioxidant role of escin was investigated using different free radicals scavenging assays like FRAP assay, DPPH, O2•−, and ROO• radicals scavenging assays and the outcomes were illustrated in the Fig. 1(A–D). The escin was demonstrated the potent in vitro antioxidant action through the effective scavenging of free radicals. Escin has the notable ferric reducing ability (Fig. 1A) at the dosage reliant manner. Escin potentially scavenged the DPPH (Fig. 1B), O2•− (Fig. 1C), and ROO• (Fig. 1D) radicals. With the increased dosage of escin (5–100 µM) the free radicals were effectively scavenged. The status of DPPH, O2•−, and ROO• radicals were effectively suppressed by the escin treatment at the dose dependant manner. These outcomes evidenced the in vitro antioxidant actions of escin, which is identified through the different free radicals scavenging ability.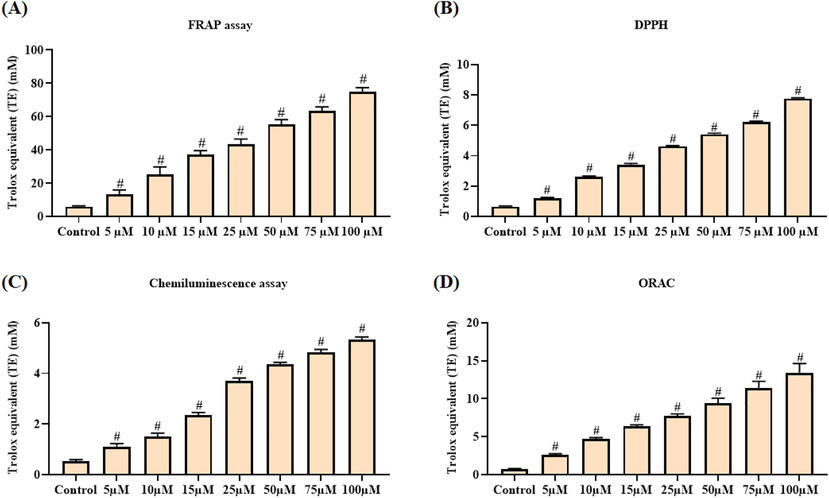
In vitro antioxidant activity of escin. Escin demonstrated the potent ferric reducing activity (A), scavenged the DPPH (B), superoxide (C) and peroxide radicals (D). Data were portrayed as mean ± SD of triplicate assays examined by one way ANOVA and Tukey’s post hoc assay using SPSS software. ‘#’ indicates data are significantly differ at p < 0.05 from control.
3.2 Effect of escin on the cell viability of Vero and A2780 cells
The cytotoxic effects of escin against the OC A2780 cells and normal Vero cells were studied through MTT assay and outcomes were depicted in Fig. 2(A&B). Escin treatment appreciably restrained the viability of A2780 cells at dose reliant manner. The cell viability of the A2780 cells was remarkably suppressed by the escin treatment (Fig. 2A), which proved the cytotoxic nature of escin against the OC A2780 cells. The IC50 dose of escin was found at the 25 µM against the A2780 cells. Interestingly, escin did not affected the viability of normal Vero cells (Fig. 2B). At the same dose, escin prevented the A2780 cell’s growth and did not affected the Vero cell’s growth. This outcomes evidenced that the escin possessed the selective cytotoxicity to the A2780 cells.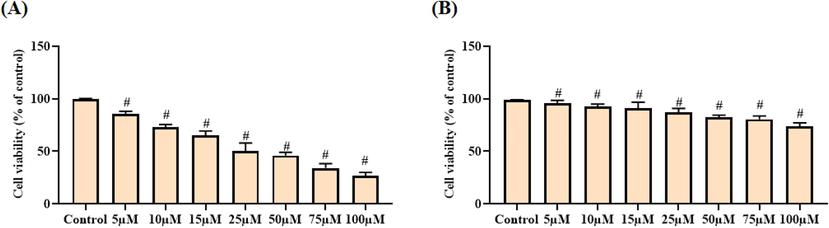
Effect of escin on the cell viability of A2780 and Vero cells. Escin treatment notably suppressed the A2780 cells viability (A) and did not affected the normal Vero cells viability (B). Data were portrayed as mean ± SD of triplicate assays examined by one way ANOVA and Tukey’s post hoc assay using SPSS software. ‘#’ indicates data are significantly differ at p < 0.05 from control.
3.3 Effect of escin on the levels of LPO and antioxidants SOD and GSH in the A2780 cells
The intracellular status of LPO, SOD, and GSH in the escin treated A2780 cells were investigated and the outcomes were depicted in the Fig. 3(A–C). Escin treatment effectively improved the intracellular LPO status when compared with control (Fig. 3A). Escin treatment also suppressed the SOD activity (Fig. 3B), and GSH status (Fig. 3C) in the A2780 cells than the control. The 25 and 50 µM of escin treatment appreciably elevated the LPO status and diminished the SOD activity, and GSH status in the A2780 cells. These outcomes evidently proved that the escin provoked LPO and diminished antioxidants status in the A2780 cells.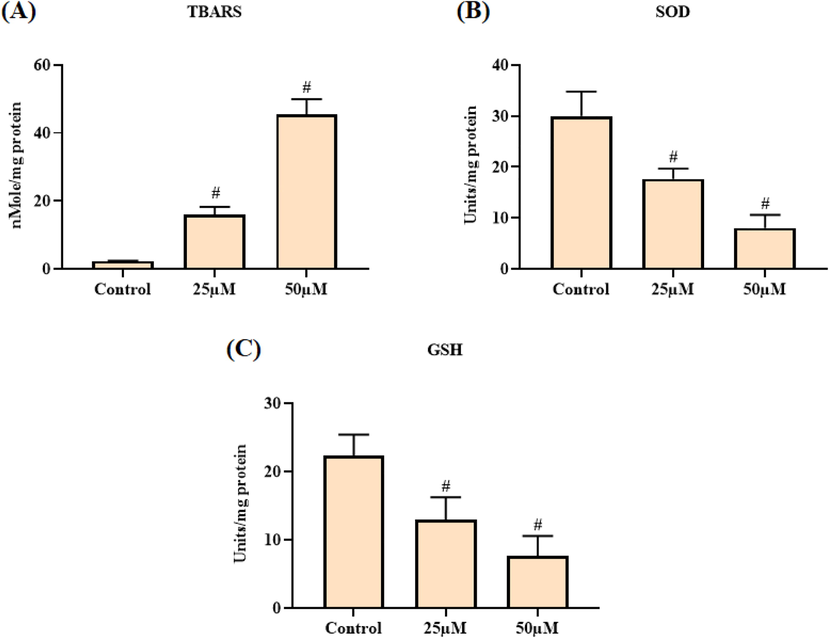
Effect of escin on the levels of LPO and antioxidants SOD and GSH in the A2780 cells. Escin treatment improved the TBARS status (A) and suppressed the SOD activity (B) and GSH (C) level in the A2780 cells. Data were portrayed as mean ± SD of triplicate assays examined by one way ANOVA and Tukey’s post hoc assay using SPSS software. ‘#’ indicates data are significantly differ at p < 0.05 from control.
3.4 Effect of escin on the intracellular ROS, MMP, and apoptotic cell death levels in the A2780 cells
The intracellular ROS accumulation, MMP status, and apoptotic cell death in the escin treated A2780 cells were investigated using different fluorescence staining techniques and the outcomes were displayed in the Fig. 4. The outcomes of DCFH-DA staining was evidenced that the ROS accretion was notably increased by the escin to the A2780 cells, which is evidenced by the elevated green fluorescence than the control cells (Fig. 4A). The Rh-123 staining of escin treated A2780 cells revealed the decreased green fluorescence that evidencing the diminished MMP status than the control cells (Fig. 4B). The AO/EB staining images revealed the marked yellow and orange fluorescence in the escin supplemented cells that proved the incidence of both early and late apoptotic cell death in the escin administered A2780 cells (Fig. 4C). The PI staining also evidenced the increased apoptotic cell death in the escin supplemented A2780 cells (Fig. 4D). The escin and DOX was displayed the similar kind of outcomes.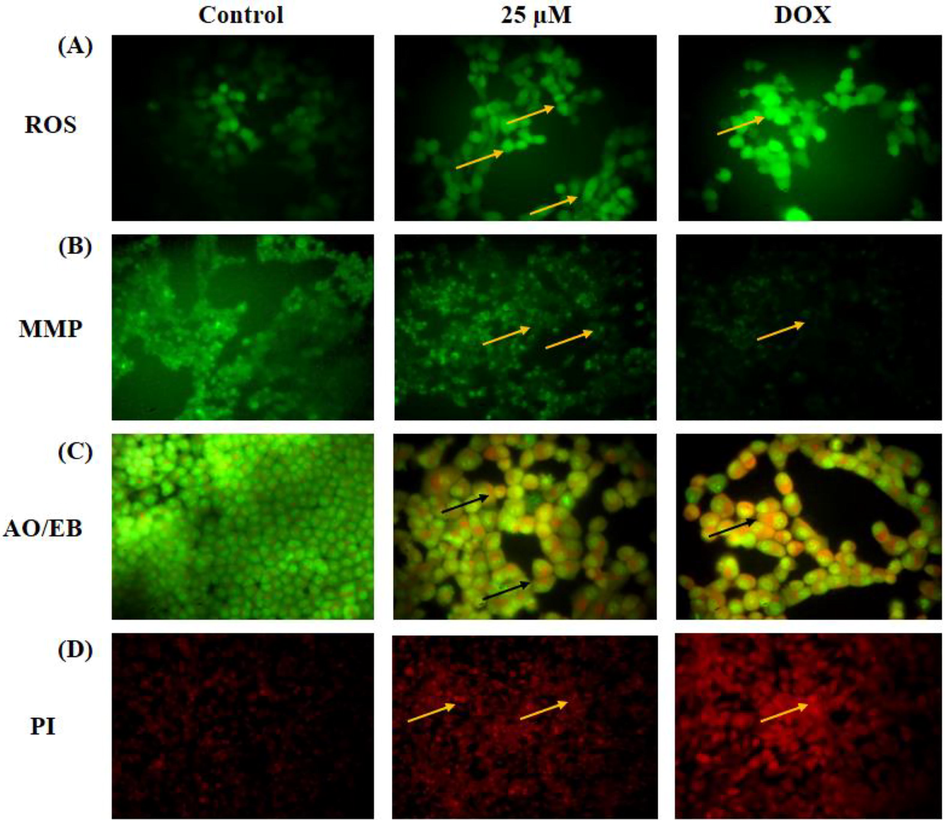
Effect of escin on the intracellular ROS, MMP, and apoptotic cell death levels in the A2780 cells. The escin administered A2780 cells exhibited the improved green fluorescence (yellow arrows) that denotes the high ROS levels (A). The MMP of A2780 cells were appreciably decreased (yellow arrows) by the escin treatment (B). The AO/EB reveals the higher number of yellow and orange fluorescence (black arrows) (C) and PI staining shows increased red fluorescence (yellow arrows) (D) that proves the higher number of apoptotic cells death.
3.5 Effect of escin on the cell cycle transition in the A2780 cells
The level of cell cycle arrest in the escin treated A2780 cells was studied through the flow cytometry and the outcomes were illustrated in the Fig. 5. The escin treatment improved the cell proportion in the sub G0/G1 phase and G1 phase. However, the escin treatment effectively arrested the cell cycle on the S phase and G2/M phase of A2780 cells. This outcome evidenced that the escin treatment appreciably arrested the S phase and G2/M phase of cell cycle in the A2780 cells (Fig. 5). The outcomes of escin and DOX treatment found similar with each other.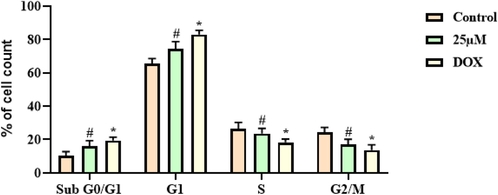
Effect of escin on the cell cycle transition in the A2780 cells. Escin effectively arrested the cell cycle of A2780 cells at S and G2/M phase. Data were portrayed as mean ± SD of triplicate assays examined by one way ANOVA and Tukey’s post hoc assay using SPSS software. ‘#’ & ‘*’ indicates data are significantly differ at p < 0.05 from control.
3.6 Effect of escin on the mRNA expression of p38 MAPK/ERK signaling molecules in the A2780 cells
The effect of escin on the mRNA expression level of ERK1/2, AKT, p38, and JNK1/2 in the A2780 cells were studied through the RT-PCR and the outcomes were depicted in the Fig. 6. The findings of this assay revealed that the treatment with the 25 µM of escin appreciably down-regulated the mRNA expressions of ERK1/2, p38, and JNK1/2 in the A2780 cells. This outcome evidently proved that the escin treatment was appreciably restrained the p38 MAPK/ERK signaling cascades in the OC A2780 cells (Fig. 6). The escin and DOX treated A2780 cells demonstrated the analogous outcomes, which is similar with each other.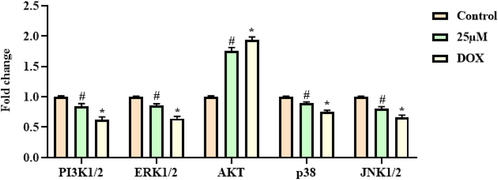
Effect of escin on the mRNA expression of p38 MAPK/ERK signaling molecules in the A2780 cells. The p38 MAPK/ERK signaling pathway in the A2780 cells was effectively inhibited by the escin treatment. Data were portrayed as mean ± SD of triplicate assays examined by one way ANOVA and Tukey’s post hoc assay using SPSS software. ‘#’ & ‘*’ indicates data are significantly differ at p < 0.05 from control.
4 Discussion
OC is a primary malignant gynecological tumor with elevated mortality rates along with less 5-year survival rate. The prior diagnosis of OC is more difficult and the recurrence and metastasis are frequently noted. Besides to surgical procedure, chemotherapy, radiotherapy, and molecular targeted approaches play a vital function in the OC treatment. Though, the overall survival rates of OC patients still continues <50–60% (Siegel et al., 2019). The pathological progression of OCs has the multifactorial events. The surplus of lifestyle and numerous environmental factors were recognized as a major risk factors of OCs (Li et al., 2020). OC has identified as a very hostile tumors and most of the victims demonstrates the loco-regionally developed phase during diagnosis, for that situation requires a multimodality therapies. The elevated mortalities of OC tightly connected with the high metastasis, invasion, and regional recurrence. Additionally, even at the advanced stage, OC are highly aggressive and has the high affinity to metastasis and invasion. Solid malignant tumors like OC have the ability for unrestrained and rapid proliferation because of their resistance to apoptosis. The avoidance of apoptosis permits tumor cells to endure longer with increased multiplication (Xia and Wang, 2019). Apoptosis is an imperative event in the cell death provoked by anticancer agents and participates into the removing of needless and annoying cells. Apoptosis primarily connected with the distinctive biochemical as well as morphological events (Ouyang et al., 2012). Likely, our findings from AO/EB and PI staining have disclosed that the escin effectively triggered the apoptotic cell death. Hence, it was clear that the escin has the ability to trigger the apoptotic cell death in OC A2780 cells.
ROS is a vital player in the numerous cellular signaling cascades in normal as well as tumor cells. The upholding of ROS homeostasis is essential for cell survival (Panieri and Santoro, 2016). The increasing of ROS accumulation in a continuous manner assists carcinogenesis. Remarkably, once the exceeding of toxic threshold, the accumulation of ROS could provoke the oxidative stress that results in the cell death (Sosa et al., 2013). Tumors cells are different and unique from the normal cells. Tumor cells have established the well-organized antioxidant systems to equalize the over generation of ROS (Moloney and Cotter, 2018). In this exploration, our findings disclosed that the escin treatment appreciably triggered the elevated LPO status and suppressed the SOD activity, and GSH status in the A2780 cells. These outcomes evidenced that the escin could diminish antioxidant system and provoked the LPO in the A2780 cells. In the biological system, the ROS was accumulated and removed continuously as a typical cellular process. ROS are the crucial players of array of regular biochemical activities and atypical pathological events. Over accumulation of ROS in the cells is known to provoke the apoptosis (Sastre et al., 2000). Our findings revealed that the escin treatment noticeably provoked the over intracellular ROS accumulation in the A2780 cells.
G2/M cell-cycle arrest is one of the most conspicuous barriers of numerous anticancer drugs that can provoke hindering of proliferation and apoptosis via diminishing the exclusion of injured chromosomes during mitosis (Park et al., 2000). In this exploration, we found that the escin exposure of A2780 cells to arrested the cell growth at G1, S, and G2/M phase. MMP is vital for the proton incline across the mitochondrial membrane and is lost because of the opening of the mitochondrial penetrability transition pore. The depolarization of the mitochondrial membranes could result in the serious consequences that include reduction on the ATP synthesis and the redeployment of pro-apoptotic mitochondrial molecules (Kroemer et al., 2007). In this exploration, we noticed that the escin effectively diminished the MMP status in the OC A2780 cells.
ROS play a crucial function in the stimulation of the p38 and JNK cascades, and subsequently result in the elevation of other pro-apoptotic molecule levels in the cells (Zhang et al., 2016). The remarkable elevation in the ROS status could cause the oxidative stress and can provoke the cells death via triggering autophagy, necrosis, and apoptosis (Kamogashira et al., 2015). During the cells exposed to ROS-triggered stress, the MAPK signaling cascade was successively stimulated, primarily including the ERKs, JNKs and p38 MAPKs (Darling and Cook, 2014). It was already disclosed that the apoptosis provoked by ROS is primarily regulated via p38 and JNK stimulation. MAPKs are the imperative players in the mediation of diverse cellular activities like cell multiplication, differentiation and apoptosis. Numerous investigations disclosed that the various signaling molecules like MAPKs are tightly connected with the apoptosis and autophagy (Wang et al., 2017; Guo et al., 2016). There are three major cassettes of MAPKs was reported, namely, JNK, ERK1/2, and p38 MAPK that mediate the array of cellular mechanisms like autophagy and apoptosis (Burotto et al., 2014; Jalmi and Sinha, 2015). In this exploration, we disclosed that the escin treatment remarkably suppressed the ERK1/2, p38, and JNK1/2 expressions in the A2780 cells. Hence, it was clear that the escin could inhibit the p38 MAPK/ERK signaling axis in the OC A2780 cells.
5 Conclusion
In overall, our findings from this investigation revealed the in vitro anticancer potential of escin against the OC A2780 cells. Escin also possessed the potent in vitro antioxidant actions via effective scavenging of various free radicals. Escin treatment appreciably reduced the cell viability of A2780 cells without affecting the normal Vero cell’s viability. Escin remarkably suppressed the MMP and provoked the apoptotic cell death in the A2780 cells. The p38 MAPK/ERK signaling axis in the A2780 cells effectively inhibited by the escin treatment. These findings proved the anticancer action of escin against the OC A2780 cells.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- The effect of chronic ethanol ingestion on hepatic lipid peroxide, glutathione, glutathione peroxidase and glutathione transferase in rats. Toxicology. 1985;36(1):71-76.
- [Google Scholar]
- The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem.. 1996;239(1):70-76.
- [Google Scholar]
- Bioactive natural products in cancer prevention and therapy: progress and promise. Semin. Cancer Biol.. 2016;40–41:1-3.
- [Google Scholar]
- Effects of sodium aescinate freeze-dried powder on MMP-9 and AQP4 in brain tissue of hemorrhagic brain edema rats. Chin. J. Tradit. Chin. Med.. 2018;33:1060-1062.
- [Google Scholar]
- Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: Cancer J Clin. 2018;68(6):394-424.
- [Google Scholar]
- The MAPK pathway across different malignancies: A new perspective. Cancer. 2014;120(22):3446-3456.
- [Google Scholar]
- Escin increases the survival rate of LPS-induced septic mice through inhibition of HMGB1 release from macrophages. Cell. Physiol. Biochem.. 2015;36(4):1577-1586.
- [Google Scholar]
- Escin reduces cell proliferation and induces apoptosis on glioma and lung adenocarcinoma cell lines. Cytotechnology. 2015;67(5):893-904.
- [Google Scholar]
- The role of MAPK signalling pathways in the response to endoplasmic reticulum stress. Biochim. Biophys. Acta. 2014;1843(10):2150-2163.
- [Google Scholar]
- Horse chestnut – efficacy and safety in chronic venous insufficiency: an overview. Rev. Bras. Farmacogn.. 2015;25(5):533-541.
- [Google Scholar]
- Persistent behavior deficits, neuroinflammation, and oxidative stress in a rat model of acute organophosphate intoxication. Neurobiol. Dis.. 2019;133:104431
- [Google Scholar]
- ERK/MAPK signalling pathway and tumorigenesis. Exp. Therap. Med.. 2020;19:1997-2007.
- [Google Scholar]
- Amorphous silica nanoparticles trigger vascular endothelial cell injury through apoptosis and autophagy via reactive oxygen species-mediated MAPK/Bcl-2 and PI3K/Akt/mTOR signaling. Int. J. Nanomed.. 2016;11:5257-5276.
- [Google Scholar]
- Medicinal plants: historical and cross-cultural usage patterns. Ann. Epidemiol.. 2005;15(9):686-699.
- [Google Scholar]
- Integrated genomics of chemotherapy resistant ovarian cancer: a role for extracellular matrix, TGFbeta and regulating microRNAs. Int. J. Biochem. Cell Biol.. 2010;42(1):25-30.
- [Google Scholar]
- ROS mediated MAPK signaling in abiotic and biotic stress- striking similarities and differences. Front. Plant Sci.. 2015;6:7692015.
- [Google Scholar]
- Reactive oxygen species, apoptosis, and mitochondrial dysfunction in hearing loss. BioMed Res. Int.. 2015;2015:1-7.
- [Google Scholar]
- JNK and p38 MAPK regulate oxidative stress and the inflammatory response in chlorpyrifos-induced apoptosis. Toxicol. Lett.. 2013;218:235-245.
- [Google Scholar]
- Mitochondrial membrane permeabilization in cell death. Physiol. Rev.. 2007;87(1):99-163.
- [Google Scholar]
- Escin suppresses migration and invasion involving the alteration of CXCL16/CXCR6 axis in human gastric adenocarcinoma AGS cells. Nutr. Cancer. 2014;66(6):938-945.
- [Google Scholar]
- Prognostic significance and related mechanisms of hexokinase 1 in ovarian cancer. Oncol. Targets Ther.. 2020;13:11583-11594.
- [Google Scholar]
- Lin, XM., Li, S., Zhou, C., Li, RZ., Wang, H., Luo, W., Huang, YS., Chen, LK., Cai, JL., Wang, TX., Zhang, QH., Cao, H., Wu, XP., 2019. Cisplatin induces chemoresistance through the PTGS2-mediated anti-apoptosis in gastric cancer. Int. J. Biochem. Cell Biol. 116 105610-105610.
- Flavonoids in oral cancer prevention and therapy. Eur. J. Cancer Prev.. 2015;24:517-528.
- [Google Scholar]
- Bcl-2 antiapoptotic family proteins and chemoresistance in cancer. Adv. Cancer Res.. 2018;137:37-75.
- [Google Scholar]
- Self-consumption: the interplay of autophagy and apoptosis. Nat. Rev. Mol. Cell Biol.. 2014;15(2):81-94.
- [Google Scholar]
- Product of extracellular-superoxide dismutase catalysis. FEBS Lett.. 1985;184:237-239.
- [Google Scholar]
- The MAPK (ERK) pathway: investigational combinations for the treatment Of BRAF-mutated metastatic melanoma. Pharm. Ther.. 2013;38:96-108.
- [Google Scholar]
- Ohkawa H, Ohishi N, Yagi K., 1979, Assay for lipid peroxides in animal tissue by thiobarbituric acid reaction. Anal. Biochem. 95: 351-8.
- Programmed cell death pathways in cancer: A review of apoptosis, autophagy and programmed necrosis. Cell Prolif.. 2012;45(6):487-498.
- [Google Scholar]
- Panieri, E., Santoro, M.M., 2016. ROS homeostasis and metabolism: a dangerous liason in cancer cells. Cell Death Dis. 7 (6) e2253-e2253.
- Constitutive activation of cyclin B1-associated cdc2 kinase overrides p53-mediated G2-M arrest. Cancer Res.. 2000;60:542-545.
- [Google Scholar]
- Anti-inflammatory and anti-cancer properties of β-Escin, a Triterpene Saponin. Curr. Pharmacol. Reports. 2015;1(3):170-178.
- [Google Scholar]
- Oxidative stress, bone marrow failure, and genome instability in hematopoietic stem cells. Int. J. Mol. Sci.. 2015;16(2):2366-2385.
- [Google Scholar]
- Escin chemosensitizes human pancreatic cancer cells and inhibits the nuclear factor-kappaB signaling pathway. Biochem. Res. Int.. 2013;2013:1-9.
- [Google Scholar]
- Mitochondrial oxidative stress plays a key role in aging and apoptosis. IUBMB Life. 2000;49:427-435.
- [Google Scholar]
- Antioxidative properties of xanthone on the auto oxidation of soybean in cylcodextrin emulsion. J. Agric. Food Chem.. 1992;40:945-948.
- [Google Scholar]
- Evaluation of five imidazopyrazinonetype chemiluminescent superoxide probes and their application to the measurement of superoxide anion generated by Listeria monocytogenes. Anal. Biochem.. 1998;258(2):230-235.
- [Google Scholar]
- Aescin: pharmacology, pharmacokinetics and therapeutic profile. Pharmacol. Res.. 2001;44:183-193.
- [Google Scholar]
- SRT2183 impairs ovarian cancer by facilitating autophagy. Aging (Albany NY). 2020;12(23):24208-24218.
- [Google Scholar]
- Escin attenuates cerebral edema induced by acute omethoate poisoning. Toxicol. Mech. Methods. 2011;21(5):400-405.
- [Google Scholar]
- Bardoxolone methyl (CDDO-Me or RTA402) induces cell cycle arrest, apoptosis and autophagy via PI3K/Akt/mTOR and p38 MAPK/Erk1/2 signaling pathways in K562 cells. Am. J. Transl. Res.. 2017;9(10):4652-4672.
- [Google Scholar]
- Effects of adenosine on apoptosis of ovarian cancer A2780 cells via ROS and caspase pathways. Oncol. Targets Ther.. 2019;12:9473-9480.
- [Google Scholar]
- Escin attenuates acute lung injury induced by endotoxin in mice. Eur. J. Pharm. Sci.. 2011;42(1-2):73-80.
- [Google Scholar]
- Epigenetics in ovarian cancer: premise, properties, and perspectives. Mol. Cancer. 2018;17(1)
- [CrossRef] [Google Scholar]
- Zhang J, Wang X, Vikash V, Ye Q, Wu D, Liu Y and Dong W: ROS and ROS-mediated cellular signaling. Oxid. Med. Cell Longev. 2016: 43509652016.
- Advances in circulating microRNAs as diagnostic and prognostic markers for ovarian cancer. Cancer. Biol Med.. 2013;10(3):123-130.
- [Google Scholar]







