Translate this page into:
Erythroid induction activity of Aquilegia fragrans and Aquilegia pubiflora and identification of compounds using liquid chromatography-tandem mass spectrometry
⁎Corresponding authors at: H.E.J. Research Institute of Chemistry, International Center for Chemical and Biological Sciences, University of Karachi, Karachi-75270, Pakistan (Syed Ghulam Musharraf). Pharmacognosy Group, Department of Pharmaceutical Biosciences, BMC, Uppsala University, SE-751 23 Uppsala, Sweden (Hesham R. El-Seedi). hesham.el-seedi@farmbio.uu.se (Hesham R. El-Seedi), musharraf1977@yahoo.com (Syed Ghulam Musharraf)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Aquilegia fragrans (AF) and Aquilegia pubiflora (AP) are the two medicinally important species of genus Aquilegia used for the treatment of various diseases and infections. This paper describes the potential of fetal hemoglobin induction activity of the methanolic extracts of AF and AP in K562 cell line. AF and AP have shown 27.147 ± 1.376 and 32.786 ± 1.048 percent erythroid induction, respectively at 15.625 (µg/mL) concentration which suggested that both plants can be the source of potential fetal hemoglobin inducers and may be used for the treatment of β-thalassemia. Phytochemical analyses of both species were also evaluated by using high-resolution LC-ESI-QTOF-MS/MS techniques. A Total of thirty compounds were identified using positive and negative ionization modes. The identification was based on the matching of high-resolution masses, isotopic pattern, and MS/MS fragmentation. Several statistical analyses were performed to evaluate the distribution of compounds in both species. Identified compounds belong to various classes including flavonoids, steroids, lignans, terpenoids, benzofuran and coumarins. The established chemical fingerprints will be helpful in standardization and quality control of plant extracts.
Keywords
Aquilegia pubiflora
Aquilegia fragrans
β-thalassemia
Fetal hemoglobin
1 Introduction
β-thalassemia, an inborn genetic disorder, is characterized by total absence or insufficient synthesis of β-globin chain (Thein, 2018). Due to reduced synthesis or total absence of β-globin chains, excessive α-globin chains, get accumulated in cells and form inclusion bodies that affect the membrane integrity and cause the premature death of erythroid cells (Sankaran and Orkin, 2013). This leads to ineffective erythropoiesis, which results in chronic anemia associated with β-thalassemia (Nienhuis and Nathan, 2012).
Current treatment options for β-thalassemia are mostly symptomatic, including blood transfusion and iron chelation therapy. Bone marrow transplantation and gene therapy could be promising, but these methods have limitations due to the high cost and availability to common man, especially in developing countries (Cavazzana-Calvo et al., 2010). Therefore, current research focuses on a cost-effective and safe therapeutic approach to treat β-thalassemia, which is fetal hemoglobin (HbF) induction. Fetal hemoglobin, a predominant form of hemoglobin during fetal stage of development, and about one year after birth, it is gradually replaced with adult hemoglobin as predominant form. This developmental suppression of fetal hemoglobin can be reversed by using inducers to ameliorate the clinical symptoms of thalassemia (Rees et al., 1999). Several potential HbF inducers, like hydroxyurea, butyrates, erythropoietin, and 5-azacytidine have been discovered, but their therapeutic usage is limited due to their cytotoxicity and carcinogenic properties (Osti et al., 1997). Therefore, there is a strong need to search for potential HbF inducers which are affordable and associated with minimal side effects.
Plants have been the source of lead molecules that are being used for the treatment of various diseases. Aquilegia (common name columbine) is one of the phytochemically important, phenotypically diverse and experimentally a tractable genus that belongs to family Ranunculaceae. Aquilegia fragrans is a perennial herb that mostly grows in northern regions of Pakistan, north-west Himalaya, eastern Afghanistan, north-western India and highly distributed at higher regions of Kashmir valley (Hussain et al., 2011). A. fragrans is commonly called as sweet-scented columbine and commonly called as Jangli kuth and Kalumb. The roots of A. fragrans are used to treat wounds and various inflammatory diseases like, gout, cystitis, eczema, psoriasis, blood sugar, kidney stones, and veterinary diseases such as bovine mastitis (Ganie et al., 2019). Dried flower paste is used for the relief of headache while seeds and the whole plant are used to cure jaundice and pneumonia, respectively (Khan et al., 2018). Aquilegia pubiflora is another medicinally important herb widespread in the Himalaya in India, Afghanistan, and northern Pakistan. It is commonly known as Hairy-flowered columbine while locally called as Domba and Thandi buti. This species is reported to have many medicinal values including anti-asthmatic, astringent, stimulant, cardio-tonic, febrifuge, jaundice, and dyspepsia. Dried roots are used to treat toothache, eye diseases, snakebite and in homeopathy, especially for the nervous system (Dhar and Samant, 1993; Hazrat et al., 2011).
Previous phytochemical studies on Aquilegia species, including A. vulgaris L., A. panicii, A. oxysepala, A. canadensis, A. glandulosa, A. excimia were reported based on various extraction, isolation and analytical methods. These studies have shown identification of flavonoids (Refaat et al., 2015), alkaloids, cyanogenic constituents, cycloartane-type glycosides (Yoshimitsu et al., 1999); butenolides (Guerriero and Pietra, 1984); terpenoids, tannins and unusual unsaturated fatty acids (Bylka and Matlawska, 1999). Until now, reported protocols for phytochemical analysis of Aquilegia species by mass spectrometric techniques were either low-resolution or restricted to a nonpolar class of compounds. Previously, no descriptive literature has been published on A. fragrans and A. pubiflora related to their phytochemistry.
This research aims to screen the potential of A. fragrans and A. pubiflora as fetal hemoglobin induction for β-thalassemia treatment and the identification of their secondary metabolites by chemical profiling using LC-ESI-QTOF tandem mass spectrometry.
2 Experimental
2.1 Sample collections
A. fragrans was gathered from village Lawat of Athmaqam tehsil in Neelum district (34°41′18.5 ∼ N 73°58′12.3 ∼ E′) while A. pubiflora was obtained from Makra village, Patekha tehsil in Muzaffarabad district (34°27′14.1 ∼ N 73°32′56.2 ∼ E′) of Azad Kashmir, Pakistan. Plants were identified by the Taxonomist from the Herbarium, University of Karachi, Pakistan and voucher numbers were assigned after deposit of plant material. Voucher no. 95,314 was given for A. fragrans while voucher no. 95314B was given for A. pubiflora.
2.2 Reagents and chemicals
For the mobile phase, methanol (HPLC grade) was acquired from “Merck” KGaA, (Darmstadt, Germany). Formic acid was bought from “DaeJung Chemical and Metals”, (Korea) and type I water was obtained from Barnstead™ GenPure (USA) water purification assembly. Reagents for erythroid induction activity are Fetal Bovine Serum (Gibco), RPMI-1640 (Gibco), Pen/Strip (Gibco), DMSO (Sigma Aldrich, France), Benzidine hydrochloride (Perkin Chemicals, China), Hydrogen Peroxide-30% (Sigma Aldrich, Germany), Glacial acetic acid (Lab Scan), Trypan Blue 4% (Sigma Aldrich, UK), Hydroxyurea (Sigma Aldrich USA).
2.3 Sample preparation
Shade dried whole plants were ground using liquid nitrogen into homogenized powder. Exactly 1 g of each sample was weighed and extracted with 10 mL methanol in a 15 mL falcon tube using a ultra-sonicator for 30 min which were then centrifugated for 15 min at room temperature at 12,000 RPM in order to remove solid particles. 1 mL of upper layer from each sample was filtered through a PTFE syringe-driven filter (0.22 µm) and transferred to 1.5 mL centrifuge tubes for LC-MS analysis. Filtered sample extracts were further diluted by taking 50 µL of sample and diluted with 950 µL (20 times) of methanol in HPLC vial.
2.4 Erythroid induction activity
K562 cells (CCL243, ATCC,), were cultured in RPMI-1460 complete medium containing 10% FBS, and 1% penicillin/streptomycin solution, in a 5% CO2 at 37 °C under humid conditions. 10 mg/mL stock solutions of methanolic extracts of each plant were prepared in sterile deionized water as a solvent to prepare a stock solution. To determine the erythroid induction, the cells were seeded at density of 4x104 cells/mL in culture and treated in concentrated depend manner range from 250 µg to 15.625 µg of each extract. The cells were then placed in 5% CO2 incubator at 37 °C for five days.
For erythroid induction benzidine-hydrogen peroxide was used. Briefly, 0.2% benzidine solution was prepared in 0.5 M acetic acid, and 20 µL of hydrogen peroxide was added per 1 mL of ice-chilled 0.2% benzidine solution. On day five, the cells were washed with phosphate buffer saline (PBS) and resuspended in it. Equal volume of benzidine-hydrogen peroxide solution was added to cell suspension in each well and plate was placed in the dark for about 3 min at room temperature (Theodorou et al., 2016). Then the cells were observed under an inverted compound microscope to determine percent benzidine positive cells.
2.5 Analysis of cell viability
The cell viability was determined by using alamarBlue fluorescence assay. This assay utilizes the non-toxic resazurin dye, in healthy cells resazurin undergoes reduction by mitochondrial enzymes and produces a highly fluorescent pink color. This fluorescence intensity of resorufin has been reported as a direct indication of the number of healthy living cells in the medium (Rampersad, 2012).
To determine the viability, cells were seeded (1 × 104 cells/mL) in 96 wells plate and treated with extracts in a dose-dependent manner and incubated for periods of up to 48 h at 37 °C. After incubation, 10 µL alamarBlue dye (Sigma, USA) (25 mg/100 mL PBS) was added to each well and was incubated in dark for 4 h. Cell viability and growth kinetics were determined by measuring the fluorescence intensity at 560 nm (excitation wavelength) and 570 nm (emission wavelength) with SpectraMax M5e Multi-Mode Microplate Reader (Molecular Devices, USA) using SoftMax Pro software (Molecular Devices USA). The cell viability was calculated using the following formula. where Fb, Fc, and Ft describes for fluorescence of blank (only medium), untreated cells, and treated cells, respectively. The data were analyzed and presented as mean ± SEM (Error for Mean) using Graph Pad Prism 5.
2.6 LC-ESI-QTOF-MS/MS analysis
The samples were analyzed using LC-ESI-QTOF-MS/MS mass spectrometer (Bruker MaXis-II, Bremen, Germany), coupled to Thermo Fisher Scientific™ Ultimate™ 3000 series UPLC system, (USA), attached with an autosampler, solvent degasser, column thermostat. Chromatography was optimized using five different columns including columns Zorbax (Eclipse XDB-C18), Zorbax (Eclipse XDB-phenyl), Zorbax (Eclipse XDB-CN), Poroshell (120 EC-C18), Nucleodur Gravity column (RP-C18). For efficient chromatographic separation, the Macherey-Nagel RP-C18 Nucleodur Gravity column (3.0 × 100.0 mm, 1.8 µm particle size) was used, at 45 °C. Mobile phase A was an aqueous solution (0.1% formic acid) while methanol (0.1% formic acid) was used as mobile phase B with 0.70 mL/min flow rate. The solvent gradient system was started with 20% B, raised to 70% from 0.0 to 3.0 mins, and kept constant for 3.00 min and then again raised to 90% from 6.0 to 8.0 mins then lowered back to 20% at 9.0 min with 1 min equilibrium.
Tandem mass spectrometric analysis was performed using electrospray ionization through positive and negative ionization modes separately. The ion source parameter were set as voltage at 4500 V, drying gas at 270 °C temperature and 12.0 L/min flow rate, nebulizer gas with 45.0 psi pressure, mass range at 100 to 2000 m/z while scan speed at 5 Hz for MS, and 12 Hz for MS/MS analysis. Acquisition software, ‘Bruker Compass TargetAnalysis’ was used for quick screening of metabolites.
2.7 Identification of compounds
Compounds identification was based on various features including exact mass matching, error > 5 ppm, MS/MS spectral data was compared with available ESI-MS libraries. An in-house library was prepared from Dictionary of Natural Products on DVD (DNP ver. 26.2) based on previously identified compounds of Aquilegia species. Then these identified compounds were screened against high-resolution mass spectra using Target Analysis version 1.3 (Bruker Daltonics, Bremen Germany). MS/MS-based identification was performed using different available ESI mass spectral libraries including MassBank of North America, MassBank of Europe, mzCloud and NIST-MS/MS library through comparison of exact masses, isotopic pattern and MS/MS fragmentation pattern.
3 Results and discussion
3.1 Erythroid induction assay
Both, A. fragrans and A. pubiflora, investigated in this study showed erythroid induction activity in a concentration-dependent manner. The results of Erythroid induction are given in Table 1 and Fig. 1. Both extracts have shown substantial erythroid induction activity at indicated concentration as compared to untreated control group. The extracts have shown more erythroid induction at lower concentrations which decreases with the increase in extract concentration. However, the A. fragrans extract was found more potent at all the concentrations as compared to A. pubiflora. The maximum activity was observed as 27.15 ± 5.1 and 31.30 ± 1.0 percent benzidine positive cells by A. fragrans and A. pubiflora extracts, respectively at 15.625 (µg/mL) concentration.
Drug Conc: (µg/mL)
AP Extract
AF Extract
Control
Hydroxy Urea
250
15.33 ± 1.4
17.31 ± 3.9
–
–
125
18.71 ± 0.9
18.25 ± 3.1
–
–
62.25
21.2 ± 1.3
25.77 ± 5.6
–
–
31.25
25.22 ± 2.0
27.13 ± 1.2
–
–
15.625
27.15 ± 5.1
31.30 ± 10
–
–
Control
–
–
10.84 ± 2.1
–
Hydroxy Urea
–
–
–
37.06 ± 0.1
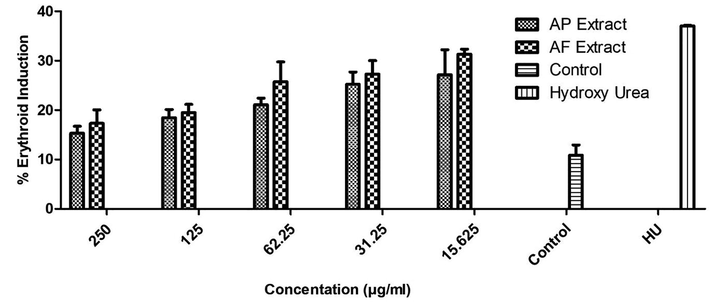
Erythroid induction activity of Aquilegia pubiflora and Aquilegia fragrans compared with standard drug hydroxy urea and control. The results are represented as mean %benzidine positive cells at different concentrations.
3.2 Evaluation of cell viability
In the cell viability experiment, AF and AP extracts have shown concentration-dependent response. Cell viabilities were represented as percent of control and data is expressed as mean ± SEM for experiments (n = 3). The gradual increase in cell viability was observed with a decrease in the concentration of treatment along. AP extract showed 6.3% cell viability (93.7% cytotoxicity) and 57.6% cell viability (42.6% cytotoxicity) at 250 µg/ml and 125 µg/ml concentrations respectively, but no cytotoxic effects were observed at concentrations ≤ 62.25 µg/ml against K562 cell line. Similarly, 94.5% cell viability (5.5% cytotoxicity) was observed at 250 µg/ml AF extract, but it had no cytotoxic effects at concentrations ≤ 125 µg/ml. Many bioactive plants are toxic on higher concentrations and their dose determines their therapeutic significance (Deshpande, 2002). Both AP and AF extracts showed pro-proliferative effects on K562 cells at lower concentrations (Fig. 2). Our findings suggest that the potent erythroid differentiation of K562 cells by methanolic extracts of A. fragrans and A. pubiflora, signify these plants as potential sources of HbF inducers for the treatment and efficient management of β-thalassemia.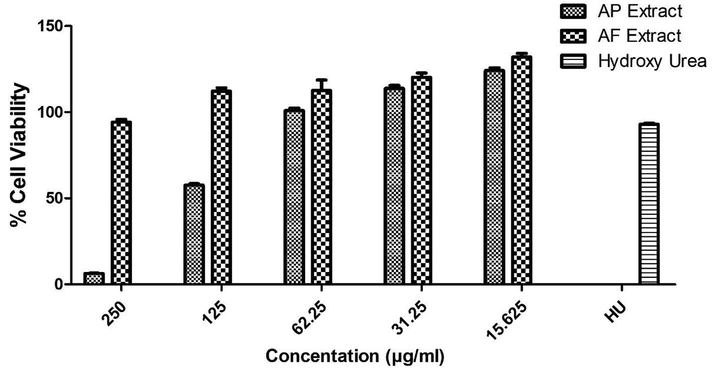
Cell viability of K562 cells against AP, AF and hydroxy urea at different concentration levels, represented as percent viability of control.
3.3 Chemical fingerprinting of Aquilegia samples through identification of compounds
Plant samples were analyzed using high-resolution LC-ESI-QTOF tandem mass spectrometry. LC-MS method was optimized using five columns with different chemistry and dimensions. The best-resolved chromatogram (Fig. 3) was observed using RP-C18, column (3.0 × 100.0 mm, 1.8 µm) and thus selected for LC-MS analysis. Further optimizations of run-time, mobile phase gradient and flowrate were performed on the selected column. Based on exact masses and MS/MS matching, total thirty chemical compounds were identified including nineteen compounds through positive ionization mode and thirteen through negative ionization mode. For identification of compounds, every m/z values were checked for mass error (less than 5 ppm) and mSigma values (less than 50 units) which shows the matching of observed isotopic pattern with a calculated isotopic pattern of that molecular formula. The list of identified compounds through both positive and negative ionization modes are given in Table 2. Fig. 3 shows the Base Peak Chromatograms of both species through negative and positive modes of ionization. These chromatograms have peaks labeled with the codes of identified compounds which were found in each of these peaks. Except three compounds, all the compounds were identified in the retention time between 2 min and 4 min. a-common in both species, b-only in AF, c-only in AP.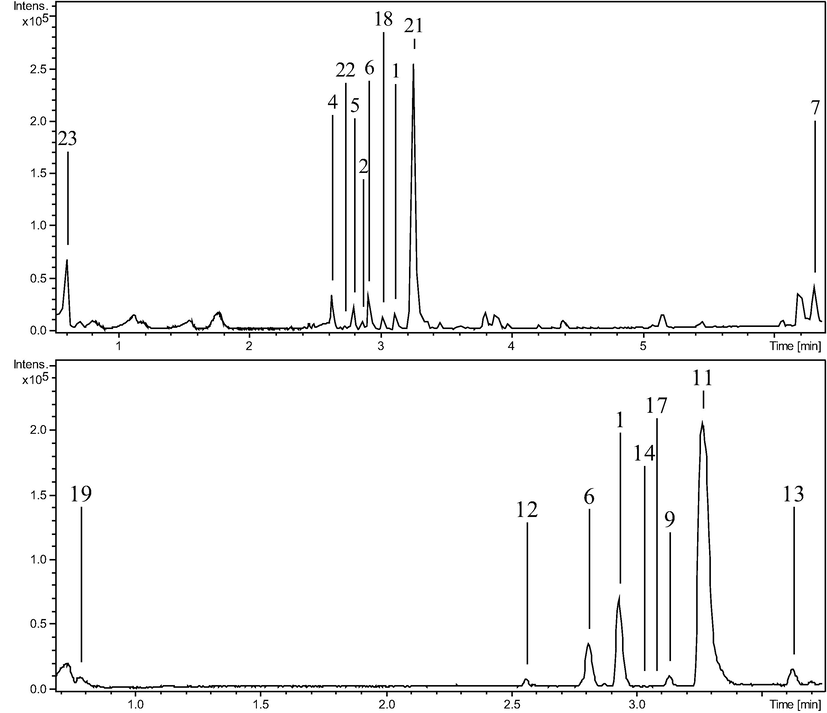
Base Peak Chromatograms of: AF in positive mode, AF in negative mode, AP in positive mode and AP in negative mode, respectively. Peaks are labeled with the codes of compounds identified in it.
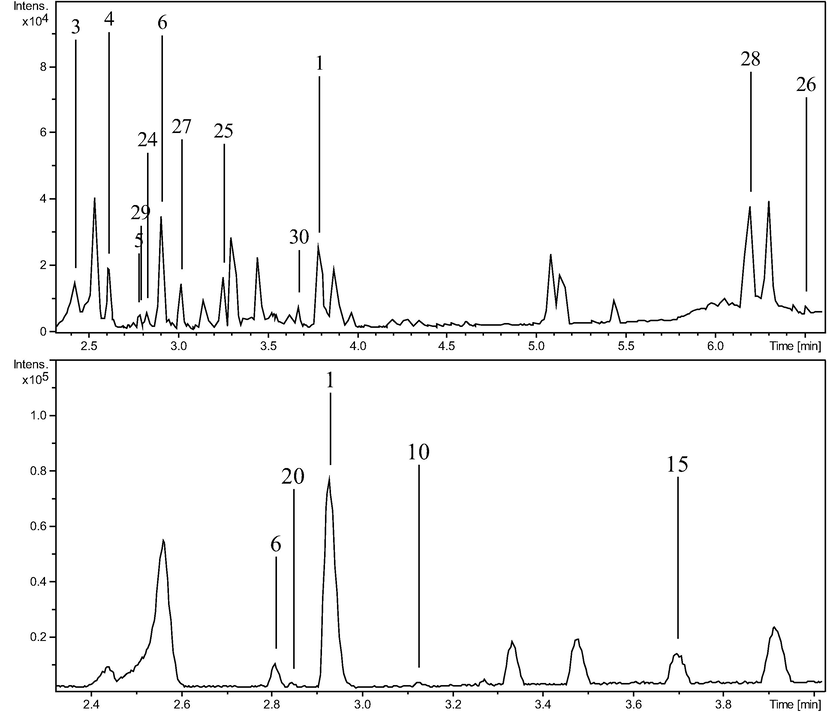
Base Peak Chromatograms of: AF in positive mode, AF in negative mode, AP in positive mode and AP in negative mode, respectively. Peaks are labeled with the codes of compounds identified in it.
S#
Name
Formula
RT (min)
Observed Precursor mass (m/z)
Ion type
Calculated Precursor mass (m/z)
Error (ppm)
Fragments
Biological activities
1
Rhoifolina
C27H30O14
3.0
579.1702
[M + H]
579.1708
1.1
271.060
Antioxidant, anti-inflammatory, anti-microbial, hepatoprotective and anticancer agent, inhibitor of α-glucosidase and α-amylase (Refaat et al., 2015).
577.1574
[M-H]−
577.1563
1.9
269.044
2
Apiinb
C26H28O14
2.8
565.1545
[M + H]
565.1552
1.2
325.071, 379.081, 4099.091, 511.124
Antiviral agent, anti-inflammatory, α-exo sialidase inhibitor (Liu et al., 2008a)
3
Apigeniin-6-C-glucoside-7-O-glucosidec
C27H30O15
2.4
595.1673
[M + H]
595.1657
2.7
337.069, 397.091, 283.058, 313.070, 415.101, 577.153
Antifungal and anti-cancer (Smiljkovic et al., 2017)
4
3-O-Neohesperidoside kaempferola
C27H30O15
2.6
595.1650
[M + H]
595.1657
1.3
397.092, 415.103, 445.113, 475.124, 499.124, 541.135, 559.1045, 577.153
Anticancer (Wannes et al., 2017)
5
Luteolinosidea
C21H20O11
2.8
449.1076
[M + H]
449.1078
0.5
313.071, 397.092, 415.103, 475.124, 529.135, 559.145, 541.135, 577.165, 595.165
Antioxidant
6
Vitexina
C21H20O10
2.9
433.1126
[M + H]
433.1120
1.4
283.059, 313.070, 337.070, 397.091, 379.081, 367.0809, 284.063
Antiplatelet, an α-glucosidase inhibitor, an antineoplastic (Can et al., 2013; Afifi and Abu-Dahab, 2012)
431.0978
[M-H]−
431.0971
1.6
311.055, 312.059, 341.065, 323.050, 342.069, 353.065, 413.082
7
Sclareolb
C20H36O2
6.3
273.2576
[M + H-2H2O]
273.2565
4.0
163.148, 191.179, 149.132, 203.179, 177.162
Antimicrobial and anti-fungal (Noori et al., 2013)
8
Lawsonaringinc
C20H19O5Na
6.2
363.1111
[M + Na]
363.1110
0.3
204.979, 217.054, 235.063, 261.043, 279.053
Anticancer, immunocytochemistry, apoptosis (Anwar et al., 2018)
9
Sissotrinb
C22H22O10
3.6
445.1134
[M-H]
445.114
1.3
325.068
Estrogenic (Shajib et al., 2012)
10
Kaempferol-7-O-glucosidea
C21H20O11
3.1
447.0936
[M-H]
447.0926
2.2
285.039
Antioxidant (Elbarbry and Shoker, 2007)
11
3,6,2′,4′-Tetrahydroxyflavoneb
C15H10O6
3.4
285.0405
[M-H]
285.0400
1.8
255.030
Not found
12
Saponarinb
C27H30O15
2.6
593.1535
[M-H]
593.1512
3.9
473.108, 474.110, 503.120, 594.154
Antioxidant (Simeonova et al., 2014)
13
3′-Methoyxy-4′,5,7-trihydroxy flavonolb
C16H12O7
3.7
315.0515
[M-H]
315.0510
1.6
300.027
prevents endothelial dysfunction, and reduce overexpression induced by angiotensin II (Sheu et al., 2004; Kanadaswami et al., 2005).
14
Peonidin-3-O-β-glucosideb
C22H22O11
3.0
461.1086
[M-H]
461.1086
0.0
298.050, 341.050, 371.084
Antioxidant, and α-glucosidase inhibitor (Nile and Antioxidant, 2014)
15
Secoisolariciresinolc
C20H26O6
3.7
361.1656
[M-H]
361.1656
0.0
346.104
Antidepressant, anti-cancer, phytoestrogen (Wang et al., 2013)
16
Orientina
C21H20O11
2.8
447.0925
[M-H]
447.0927
0.4
227.050, 328.055, 357.062, 358.064
Antioxidant, antiaging, antiviral, antibacterial, antiinflammation, vasodilatation and cardioprotective, radioprotective, neuroprotective, antidepressant, antinociceptive (Lam et al., 2016).
17
Astragalinb
C21H20O11
3.1
447.0932
[M-H]
447.0932
0.0
384.032, 285.039
anti-inflammatory, antioxidant, neuroprotective, cardioprotective, antiobesity, antiosteoporotic, anticancer, antiulcer, and antidiabetic (Bahadır Acikara et al., 2016).
18
Biochanin Ab
C25H23O13
3.1
532.1211
[M-H]+
532.1201
2.0
297.032, 327.123, 351.123, 381.009, 411.031, 429.123
Tyrosine kinase inhibitor, phytoestrogen, antineoplastic and anticancer (Bhardwaj et al., 2014; Ying et al., 2001; Holbech et al., 2013)
19
N-Fructosyl
pyroglutamateb
C11H17NO8
0.7
290.0872
[M-H]
290.0876
1.4
201.059, 212.056, 170.045, 200.056
Not found
20
4,5-Dihydroxy-6-(hydroxymethyl)-3-
[3,4,5-trihydroxy-6-(hyroxymethyl)oxan-2-yl]oxyoxan-2-yl]-5,7-dihydroxy-2-(4-hydroxyphenyl)chromen-4onea
C27H30O15
2.9
593.1527
[M-H]
593.1512
2.5
293.046, 413.087
Not found
21
Swertisinb
C22H22O10
3.3
447.1286
[M-H]+
447.128
1.3
285.112, 297.021, 309.198, 327.123, 337.022, 351.109, 375.081, 381.100, 393.198, 411.099
Anticancer, anti-inflammatory antioxidant, radioprotective, and hypoglycaemic (34)
22
7-Amino-4-methylcoumarinb
C10H9NO2
2.7
176.0706
[M + H]
176.0706
0.0
173.060
Antifungal and antibacterial (Liu et al., 2008b)
23
Elmycin Bb
C19H18O6
0.6
365.1003
[M + Na]
365.0995
2.2
366.108, 347.094
Angucyclinone antibiotic (Fotso et al., 2008)
24
2-Cyclohexen-1-one, 4-[(1E)-3-(β-D-glucopyranosyl-oxy)-1-buten-1-yl]-4-hydroxy-3,5,5-trimethyl-, (4S)c
C19H30O8
2.8
409.1824
[M + H]
409.1830
1.5
203.052, 410.187, 411.198
Not found
25
β-D-Xylopyranoside, 1,3,4,6,7,7,7,8,9,10,11,13,13,15,15 -pentadecahydro-13-hydroxy-11-(1hydroxy-1-methylethyl)-1,1,7,8,13-pentamethyl-10,12-epoxy-2H,5H -cyclopropa[1′,8′a] naphth[2′,1′:4,5]indeno[2,1]oxepin-2-yl]c
C35H54O9
3.2
641.3660
[M + Na]
641.3599
9.5
491.297
Not found
26
Mycophenolic acidc
C17H20O6
6.5
321.1369
[M + H]
321.1333
11
303.122
Immunosuppressant, antibiotic, antineoplastic and antimicrobial (Elbarbry and Shoker, 2007; Dzidic et al., 2006)
27
(3Z)-3-Hexen-1-yl 6-O-alpha-L-arabinopyranosyl-β-D-glucopyranosidec
C17H30O10
3.0
417.1734
[M + H]
417.1731
0.7
418.176
Not found
28
2-Acetoxy-4-pentadecyl-benzoic acidc
C24H38O4
6.2
413.2519
[M + Na]
413.2565
11
310.140
Not found
29
2-[2-Hydroxy-5-[(1E)3-methoxyphenyl]-3-(4-hydroxy-3-methoxyphenyl) propylhexopyranosidec
C26H34O11
2.8
545.1993
[M + H]
545.1993
0.0
383.146, 546.293, 547.203
Not found
30
(3S,3R,4S,4R,7R,8R,9R)-3,4,8-Trimethyl-2,5-dioxo-2,3,3,4,4,5,7, 8,9,9-decahydroazuleno [6,5–b] furan-4-yl (2Z)-2-methyl-2-butenoatec
C20H26O5
3.7
369.1671
[M + Na]
369.1672
0.3
287.124, 370.171
Not found
The reported biological activities of identified compounds were also tabulated such as cardioprotective, prevent endothelial dysfunction, reduce overexpression, inhibition of α-glucosidase enzyme, antidiabetic, antidepressant, anti-inflammatory, antimicrobial, antiviral, antifungal and protozoal, antineoplastic, antiplatelet and anticancer activity (Table 2).
3.4 Chemometric analysis of identified compounds
To visualize the distribution of identified compounds between both species, chemometric analysis were performed. Perseus software (version 1.6.2.1) was utilized to generate heatmap clusters. Heatmap from compounds identified using positive ionization mode revealed that compound 6 was found with high intensities in both plants and could be responsible for erythroid induction activity of these plants. Compounds 1 and 5 were found in higher intensity in AF sample while lower intensity in AP. Similarly, compound 4 exhibited lower intensity in AF than AP. Compounds 9, 12 and 13 were found with good intensities in AF while completely absent in AP. Compounds 8, 14, 16 and 19 were absent in AF sample but found in AP with low intensity. Compound 10 showed the highest intensity compared to all the compounds but present in AF only. Heatmap from the positive mode of ionization is given in Fig. 4.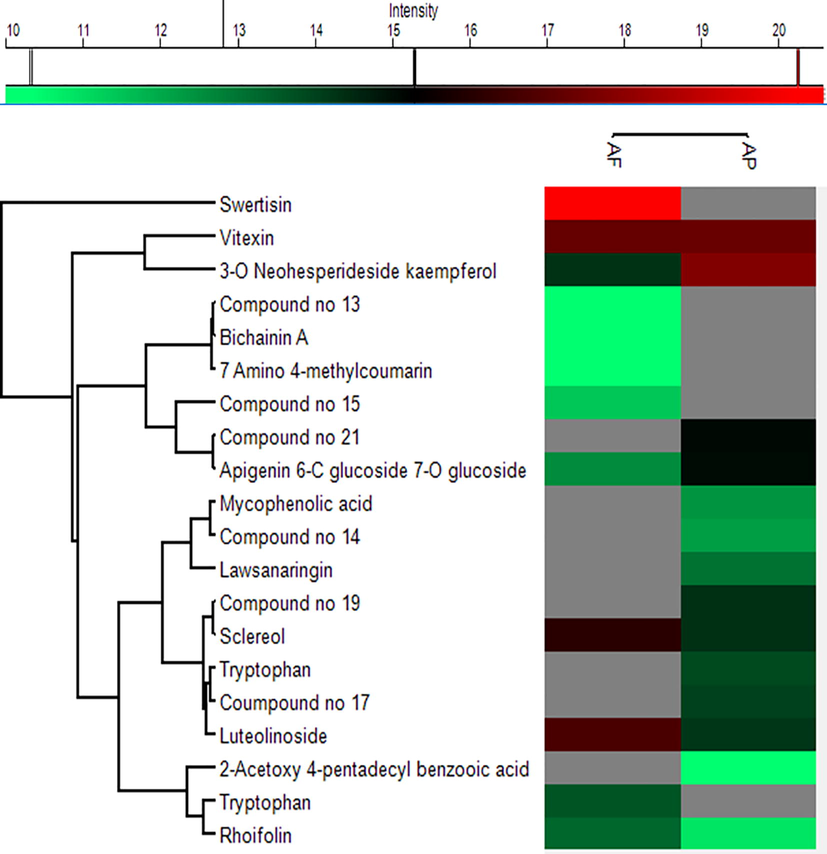
Heatmap and hierarchical clustering based on peak areas of identified compounds in A. pubiflora and A. fragrans through positive ion mode.
Through negative ionization mode, compounds 2, 6, 9, 10, 12 and 14 were present in both species. Compound 1 was found with higher intensity in AF, while compound 9 was at a lower intensity in AP. Compound 14 exhibited higher intensity in AP and lower intensity in AF. Compound 6 was present with higher intensity in AP while lower in AF. Compound 12 exhibited a very high intensity in AP as compared to AF sample. Compounds 1, 3, 4, 5, 7, 11, 13 were not found in AP while compounds 1 and 3 were present with higher intensities in AF. Heatmap from the negative mode of ionization is illustrated in Fig. 5.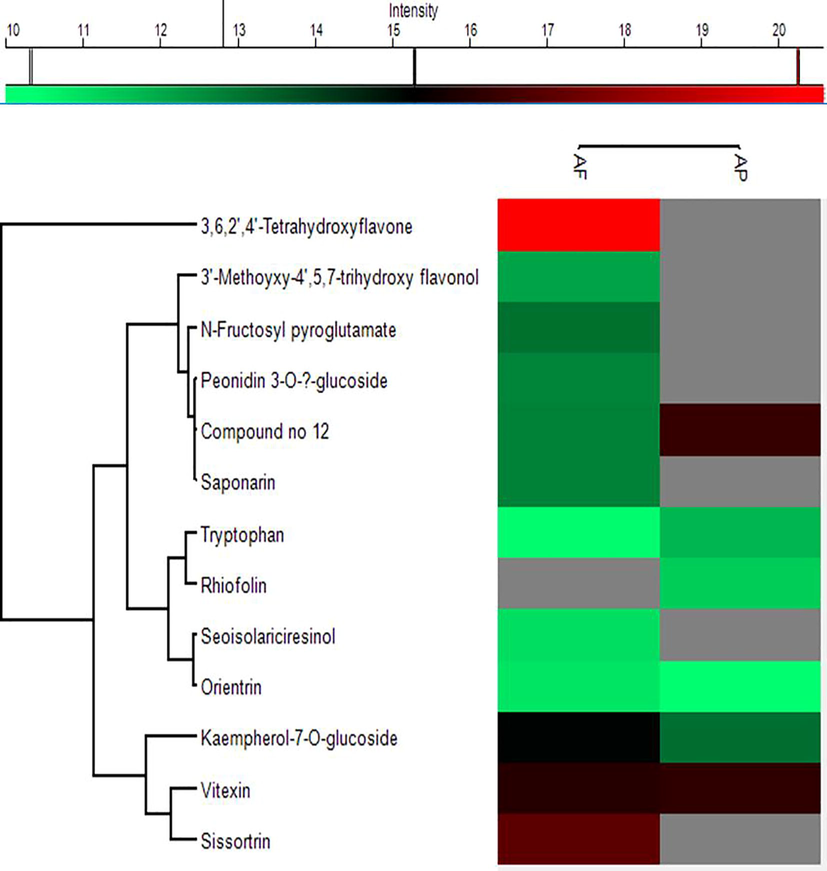
Heatmap and hierarchical clustering based on peak areas of identified compounds in A. pubiflora and A. fragrans through negative ion mode.
Comparative studies of both plant species showed that in positive ion mode out of 19 identified compounds, 16 compounds were confined to one species while 5 compounds are similar in both species. Similarly, in negative ion mode, total thirteen compounds were identified among these 8 compounds were different and 6 compounds were similar in both species (Table 2). The previous phytochemical studies have reported cycloartane glycosides from Aquilegia fragrans while no phytochemical studies were performed on Aquilegia pubiflora. However, these compounds were not seen in the present study. The compounds identified here are mainly belonged to flavonoids class of natural products while some steroids, lignans, terpenoids, benzofuran and coumarins were also found. None of these identified compounds were previously reported to have erythroid induction activity.
4 Conclusion
Methanolic extract of Aquilegia fragrans and Aquilegia pubiflora have shown erythroid induction activity at lower concentrations, suggesting that the plants can be the potential source of fetal hemoglobin inducing compounds to treat β-thalassemia and other β-hemoglobinopathies. Moreover, a rapid and simple method was also developed for the chemical profiling and identification of compounds in selected Aquilegia species, using high-resolution LC-ESI-QTOF tandem mass spectrometry. Total nineteen compounds were identified through positive ion mode and thirteen through negative ion mode of mass spectrometer. Additionally, chemometric analysis was also performed to understand the distribution of identified compounds in both species. Identified compounds may be responsible for the aforementioned activity but require screening in purified form for further confirmation.
Acknowledgment
The authors would like to acknowledge the contribution of Mr. Junaid and Mr. Arsalan in LC-MS/MS analysis and Mr. Shabbir Ijaz for sample collection.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Molecular basis of β thalassemia and potential therapeutic targets. Blood Cells Mol. Dis.. 2018;70:54-65.
- [Google Scholar]
- The switch from fetal to adult hemoglobin. Cold Spring Harbor Perspect. Med.. 2013;3(1):a011643
- [Google Scholar]
- Pathophysiology and clinical manifestations of the β-thalassemias. Cold Spring Harbor Perspect. Med.. 2012;2(12):a011726
- [Google Scholar]
- Transfusion independence and HMGA2 activation after gene therapy of human β-thalassaemia. Nature. 2010;467(7313):318.
- [Google Scholar]
- Why are hemoglobin F levels increased in HbE/β thalassemia? Blood. 1999;94(9):3199-3204.
- [Google Scholar]
- Human leukemia K562 cells: induction to erythroid differentiation by guanine, guanosine and guanine nucleotides. Haematologica. 1997;82(4):395-401.
- [Google Scholar]
- Traditional drug therapies from various medicinal plants of central Karakoram National Park, Gilgit-Baltistan, Pakistan. Pakistan Journal of Botany.. 2011;43:79-84.
- [Google Scholar]
- Impact assessment of anthropogenic threats to high-valued medicinal plants of Kashmir Himalaya, India. J. Nat. Conserv.. 2019;50:125715
- [Google Scholar]
- Exploration and local utilization of medicinal vegetation naturally grown in the Deusai plateau of Gilgit, Pakistan. Saudi J. Biol. Sci.. 2018;25(2):326-331.
- [Google Scholar]
- Endemic plant diversity in the Indian Himalaya I. Ranunculaceae and Paeoniaceae. J. Biogeogr. 1993:659-668.
- [Google Scholar]
- Ethnobotanical Study of Some Elite Plants Belonging to Dir, Kohistan Valley, Khyber Pukhtunkhwa, Pakistan. Pakistan J. Botany.. 2011;43(2):787-795.
- [Google Scholar]
- Rhoifolin: A review of sources and bilogical activities. Int. J. Pharmacogn.. 2015;2:102-109.
- [Google Scholar]
- Cycloartane-type glycosides from Aquilegia flabellata. Phytochemistry. 1999;51(3):449-452.
- [Google Scholar]
- A Butenolide Atypical of the Ranunculaceae - Aquilegiolide from Aquilegia-Atrata (Var Atroviolacea) Phytochemistry. 1984;23(10):2394-2396.
- [Google Scholar]
- The investigation of resveratrol and analogs as potential inducers of fetal hemoglobin. Blood Cells Mol. Dis.. 2016;58:6-12.
- [Google Scholar]
- Multiple applications of Alamar Blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors. 2012;12(9):12347-12360.
- [Google Scholar]
- Toxic metals, radionuclides, and food packaging contaminants. New York, USA: Deshpande SS editör Hand Book of Food Toxicology Marcel Dekker Inc; 2002. p. :783-810.
- Anti-influenza virus activities of flavonoids from the medicinal plant Elsholtzia rugulosa. Planta Med.. 2008;74(8):847-851.
- [Google Scholar]
- Apigenin-7-O-Glucoside Versus Apigenin: Insight into the Modes of Anticandidal and Cytotoxic Actions. Excli. J.. 2017;16:795-807.
- [Google Scholar]
- A review of Tunisian medicinal plants with anticancer activity. J. Complementary Integr. Med.. 2017;15(1)
- [Google Scholar]
- Anti-depressant-like effect of vitexin in BALB/c mice and evidence for the involvement of monoaminergic mechanisms. Eur. J. Pharmacol.. 2013;699(1–3):250-257.
- [Google Scholar]
- Phytochemical screening and biological activities of Eminium spiculatum (Blume) Kuntze (family Araceae) Nat. Prod Res.. 2012;26(9):878-882.
- [Google Scholar]
- Sclareol reduces CD4+CD25+FoxP3+T-reg cells in a breast cancer model in vivo. Iran. J. Immunol.. 2013;10(1):10-21.
- [Google Scholar]
- A natural flavonoid lawsonaringenin induces cell cycle arrest and apoptosis in HT-29 colorectal cancer cells by targeting multiple signalling pathways. Mol. Biol. Rep.. 2018;45(5):1339-1348.
- [Google Scholar]
- Phytotoxic effect, uptake, and transformation of biochanin A in selected weed species. J. Agric. Food Chem.. 2012;60(43):10715-10722.
- [Google Scholar]
- Therapeutic drug measurement of mycophenolic acid derivatives in transplant patients. Clin. Biochem.. 2007;40(11):752-764.
- [Google Scholar]
- Protective effects of the apigenin-O/C-diglucoside saponarin from Gypsophila trichotoma on carbone tetrachloride-induced hepatotoxicity in vitro/in vivo in rats. Phytomedicine. 2014;21(2):148-154.
- [Google Scholar]
- Mechanisms involved in the antiplatelet activity of rutin, a glycoside of the flavonol quercetin, in human platelets. J. Agric. Food Chem.. 2004;52(14):4414-4418.
- [Google Scholar]
- α-glucosidase and xanthine oxidase inhibitory activity of bioactive compounds from maize (Zea mays L.) Chem. Biol. Drug Des.. 2014;83(1):119-125.
- [Google Scholar]
- The antidepressant effect of secoisolariciresinol, a lignan-type phytoestrogen constituent of flaxseed, on ovariectomized mice. J. Nat. Med.. 2013;67(1):222-227.
- [Google Scholar]
- A review on medicinal properties of orientin. Adv. Pharmacol. Sci.. 2016;2016:4104595.
- [Google Scholar]
- Turkish Scorzonera species extracts attenuate cytokine secretion via inhibition of NF-κB activation, showing anti-inflammatory effect in vitro. Molecules. 2016;21(1):43.
- [Google Scholar]
- Biochanin A reduces pancreatic cancer survival and progression. Anticancer Drugs. 2014;25(3):296-302.
- [Google Scholar]
- Growth inhibition of human endothelial cells by the phyto-oestrogen biochanin A, a metabolite of genistein. Br. J. Nutr.. 2001;85(5):615-620.
- [Google Scholar]
- Estrogenic effect of the phytoestrogen biochanin A in zebrafish, Danio rerio, and brown trout, Salmo trutta. Aquat. Toxicol.. 2013;144–145:19-25.
- [Google Scholar]
- Antimicrobial activity of an endophytic Xylaria sp.YX-28 and identification of its antimicrobial compound 7-amino-4-methylcoumarin. Appl. Microbiol. Biotechnol.. 2008;78(2):241-247.
- [Google Scholar]
- Angucyclinones from an Indonesian Streptomyces sp. J. Nat. Prod.. 2008;71(1):61-65.
- [Google Scholar]
- Effects of mycophenolic acid on inosine monophosphate dehydrogenase I and II mRNA expression in white blood cells and various tissues in sheep. J. Vet. Med. Ser. a-Physiol. Pathol. Clin. Med.. 2006;53(4):163-169.
- [Google Scholar]







