Epidemiology of enterotoxaemia in livestock in the Kingdom of Saudi Arabia
⁎Corresponding author. obmkkwrc@yahoo.co.uk (Osama B. Mohammed)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
A cross sectional study was conducted during the period 2014–2015, to estimate the prevalence and assess contribution of some risk factors for the occurrence of enterotoxaemia in sheep, goats, cattle and camels in the Kingdom Saudi Arabia. A total of 1593 animals from 476 herds were investigated. ELISA tests were conducted using a commercial kit for the detection of different toxins produced by Clostridium perfringens causing the disease. The toxicogenic typing of C. perfringens in clinical samples by ELISA kit, revealed that the predominant types were C. perfringens type A (67.2%) followed by type D (16.4%), then type B (13.4%) and type C (3%). Further confirmation of representative C. perfringens isolated from enterotoxaemia positive animals was performed using polymerase chain reaction. The overall prevalence of enterotoxaemia disease at the animal and herd level was 27.2% and 26.47% respectively. There were significant differences in the prevalence of enterotoxaemia in animals between the different regions in KSA, animal species and months of the year. The highest prevalence was detected in Aljouf region (41.7%), followed by Hail region (40.9%), Qassim (37.8%), Jazan (31.1%), the eastern region (26.1%) and Riyadh region (10.4%). The prevalence was the highest cattle (64.3%), followed by goats (29.9%), camels (21.5%) and sheep (21.4%). Risk factors such as regions (χ2 = 89.65, p = 0.000), months of the year (χ2 = 76.65, p = 0.000), animal species (χ2 = 50.81, p = 0.000), disease presence by years (χ2 = 29.75, p = 0.000), herds prevalence (χ2 = 1443.6, p = 0.000) showed statistically significant association with enterotoxaemia disease. In the multivariate analysis, species of animals (OR = 4.88, p = 0.000) was found to be the most statistically significant risk factor associated with enterotoxaemia. Cattle were found to be approximately five times more likely to have infection with enterotoxaemia (OR = 4.88, CI = 2.55–9.37, p = 0.0001) compared to other animal species. High overall prevalence of enterotoxaemia cases in all animal species occurred during the period August–December of the year 2015 with the highest prevalence in September (50%).
Keywords
Enterotoxaemia
Epidemiology
Saudi Arabia
Prevalence
Clostridium perfrinegns
1 Introduction
Clostridium perfrinegens (formerly known as Bacillus aerogenes capsulatus, Bacillus perfringens, Bacillus welchii or Clostridium welchii), the causative agent of enterotoxaemia, is a gram positive bacilli and is commonly isolated from the environment and the gastrointestinal tract of mammals (livestock, humans and wildlife) as well as birds (Hein and Timms, 1972; Willis, 1984; Kiu and Hall, 2018). C. perfringens is classified into five types A to E based on the major toxins produced and these are alpha, beta, epsilon and iota (Petit et al., 1999). Type A strains produce only alpha toxin, type B strain produce alpha, beta and epsilon-toxin, type C strains produce alpha and beta-toxin, type D strains produce alpha and epsilon-toxin, and type E strains produce alpha and iota-toxin. The different toxin types cause different types of enteritis and enterotoxaemia in various hosts (Songer, 1996).
Enterotoxaemia in animals in the Kingdom of Saudi Arabia (KSA) is endemic. Exisiting reports from the Ministry of Environment, Water and Agriculture in KSA show that enterotoxaemia was diagnosed in different animal species from almost all regions of the kingdom, however information about the real situation of enterotoxaemia affecting different animal species and the types of C. perfringens involved in causation of the disease is scarce. Only few publications have been found regarding the occurrence of enterotoxaemia in domestic animals in the Kingdom of Saudi Arabia (Moussa and Hessan, 2011; Fayez et al., 2013a, 2013b).
Part of the problem is that no large-scale organized disease investigations in different regions of the Kingdom have been conducted before. The main objectives of this research work are to investigate the prevalence of enterotoxaemia in sheep, goats, camels and cattle and identify the most important epidemiological factors associated with disease occurrence in KSA.
2 Materials and methods
2.1 Field investigations
2.1.1 Study design:
This study involved a cross-sectional observation using a multistage sampling technique and targeting animal herds of sheep, goats, cattle and camels reared in open extensive systems or kept as backyard animals by small stakeholders in the traditional sector. Nine regions and one province namely: Riyadh, Eastern, Qassim, Makah, Jazan, ALjouf, Asseer, Hail and Baha, in addition to Bisha province were randomly selected from a total of 13 regions in KSA. Then from each region, two administration units were selected and five herds with previous history of enteric diseases showing mainly symptoms of diarrhoea were selected from each unit. Finally, from each herd, five animals out of all animals in the herd farm showing symptoms of diarrhoea were selected by using simple random sampling.
2.1.2 Sample size determination
The sample size from each region was calculated by the following formula (Martin et al., 1987):
Therefore, the sample size will be 173 animals according to the above equation.
The expected prevalence in this formula was set to be 87.1% according to a previous study done in central KSA (Moussa and Hessan, 2011).
2.1.3 Data recording
A pre-tested structured form with the primary objective of elucidating the multi factorial background of the disease problem was conducted in an interactive manner at every herd investigated. The form was filled out by asking the owner or animal attendant about the animal attributes, the farm attributes and the general management factors. Finally, five animals from each herd were examined and sampled as described previously.
2.1.4 Sample collection
Faecal samples were collected from animals showing symptoms of profuse diarrhoea. Recently dead animals or animals with severe diarrhoea and/or at the terminal stage of death were autopsied. Samples from autopsied animals included small intestines, kidneys, watery faeces, peritoneal fluids, if available. From all animal species a total number of 1593 of faecal specimens, 205 small intestines, 127 kidneys, and 87 peritoneal fluids were collected. Different types of specimens collected from the same animal were considered one sample representing one animal. All samples were collected, placed individually in clean sterile containers, numbered and sent cooled in ice, with the relevant data and information, to the nearest regional veterinary diagnostic laboratory or directly to the project research laboratories at King Saud university or Riyadh veterinary diagnostic laboratory within 24 h, otherwise, the samples were kept frozen at −20˚C and sent later for laboratory investigations.
2.2 Laboratory investigations
All laboratory investigations were done by members of the research team at King Saud University and Riyadh Veterinary Diagnostic laboratory.
2.2.1 Processing of clinical samples for ELISA assay
Intestinal contents, fecal material and chopped small pieces of the kidneys were diluted (1:1) with 1 X dilution buffer provided with the ELISA kit. The suspensions were transferred to narrow diameter sterile tubes and left to stand for 10–15 min at room temperature. Crud sediments were excluded by decantation of suspension supernatants into sterile cryotubes. Peritoneal fluids and C. perfringens culture supernatants were used undiluted in the ELISA test. Bacterial cultures of C. perfringens were obtained from C. perfringens isolates grown in Trypticase - yeast extract -glucose medium at 37 ˚C for a period of 4 h (for alpha toxin) or 8 h (for other toxins). Bacterial cultures were centrifuged at 5000 rpm for 10 min after which culture supernatants were collected. Furthermore, intestinal contents samples obtained from healthy enterotoxaemia negative sheep were processed with the same procedure and used later as negative controls. All processed samples were preserved frozen at −80 ˚C till the time of conducting the ELISA test.
2.2.2 Detection and typing of C. perferingens by ELISA assay
Previously processed intestinal contents, kidney tissue suspensions, faecal material, peritoneal fluids and C. perfringens cultures supernatants were used for detection of toxins and cellular antigen of C. perfringens employing a commercial enterotoxaemia ELISA kit (Bio-X Diagnostics, Belgium).
The kit contents and the frozen processed samples were brought to room temperature. The concentrated dilution buffer and washing solution were diluted 5 and 20 folds respectively in sterile double distilled water. The Elisa test was done according to the manufacturer's instructions
Briefly, in each ELISA microtiter plate, 100 µl from the positive and negative controls were added to each well in column 1 and 2 respectively. Similarly, 100 µl from each test sample were added to each well in columns 3–12. The plates were then incubated at room temperature for 1 h. After this first incubation the plates were washed 3 times using 1X washing solution and 100 µl quantities from the diluted (1:20) appropriate conjugate were added into each well of the appropriate raw in the plate (i.e.: anti -alpha toxin conjugate row A and B, anti-Beta toxin conjugate row C and D, anti-epsilon toxin conjugate row E and F, and finally anti C. perfringens conjugate row G and H. The plates were then incubated at room temperature for 1 h. After this incubation and washing of the plates, 100 µl of indicator solution (a mixture of chromogen and substrate) were added to each well. All the plates were then incubated at room temperature for 15 min. After this incubation, the reactions were stopped by adding 50 µl quantities of stop solution (1 M phosphoric acid) to each well containing the reactants. Finally, the optical densities (OD) were recorded at 450 nm using a micro plate reader (Multiscan).
The net OD for each sample was calculated by subtracting the OD of the corresponding negative control from the reading of each sample well. According to the manufacturer's QC data sheet, the limit of OD positivity for alpha (α-), beta (β-) and epsilon (ε-) toxins is 0.150; therefore, any sample that gave a difference in the OD ≥ 0.150 was considered positive for the toxins and cellular antigen tested. Conversely, any difference in OD ≤ 0.150 was considered negative for the toxins and cellular antigen tested.
Typing of C. perfringens into the different types A, B, C, D or E was done by comparing the types of toxins detected in the clinical material or culture supernatants using the ELISA test.
2.2.3 Microscopic examination of gram stained smears
Impression smears made from inflamed parts of the small intestines, kidneys and smears made from different types of bacterial isolates were air-dried, heat fixed, stained with gram stain and examined microscopically for detection of large sized gram-positive bacilli similar to the morphology of C. perfringens.
2.2.4 Bacterial isolation and identification by multiplex PCR
The types of media used for C. perfringens isolation from clinical samples obtained from animals positive for enterotoxaemia were Liquid cooked meat medium (Oxoid), Neomycin blood agar (Oxoid), C. perfringens agar with perfringens supplement (Oxoid), Reinforced Clostridium Agar (RCA) (Oxoid).
All inoculated media were incubated anaerobically for 24–48 h using anaerobic jars supplemented with gas generating kit for anaerobiosis (Oxoid) as described by Quinn et al. (1994). Suspected C. perfringens pure colonies were identified by using the API 20A (Biomereaux, France) according to the manufacturer’s instructions. Confirmation of identification for some representative isolates of C. perfringens was done using the polymerase chain reaction (PCR). DNA extraction and typing of C. perfringens isolates was achieved by conducting a multiplex PCR assay according to the protocol described by Baums et al. (2004)
2.3 Statistical analysis
All data collected were entered into Microsoft excel spreadsheet. For analysis of the data SPSS version 22 software was used (IBM Corp, 2013). Data were analyzed descriptively in the first step, using the frequency table and cross tabulation. Then the association of the different variables with the prevalence of enterotoxaemia at the animal level was analyzed using a Chi-square test (χ2). The level of significance was set at p < 0.25. For the investigation of the association between the probabilities of occurrence of enterotoxaemia in response to potential individual, management and hygienic risk factors, multivariate analysis was performed in which logistic regression model was used. The strength of association between the risk factors and the prevalence of enterotoxaemia was analyzed using the odds ratio and the level of significance was set at p < 0.05.
3 Results
Across sectional study was conducted during the period 2014–2015 to estimate the prevalence and assess contribution of some risk factors for the occurrence of enterotoxaemia disease in sheep, goats, cattle and camels in different regions of the Kingdom of Saudi Arabia (KSA).
A total of 1593 animals from different animal species from 476 herds were investigate. A commercial enterotoxaemia ELISA kit (Bio-X Diagnostics, Belgium) was used for the diagnosis of enterotoxaemia in the investigated animals. An animal is considered positive for enterotoxaemia, if any of the different types of specimens obtained from the same animal, was found positive for enterotoxaemia by ELISA test. Further confirmation of the types of representative C. perfringens bacterial isolates from enterotoxaemia positive animals during this study was conducted using the polymerase chain reaction (PCR) using specific primers.
ELISA results confirmed the presence of Clostridium perfringens and its toxins in the samples investigated. Toxinogenic typing of Clostridium perfringens done by ELISA test revealed that the predominant types were C. perfringens type A (67.2%) followed by type D (16.4%), type B (13.4%) and finally type C (3%).
Table 1 shows herds' prevalence of Enterotoxaemia in sheep, goats, camels and cattle in the Kingdom of Saudi Aarabia during the years 2014–2015. The overall percentage (%) of enterotoxaemia positive herds irrespective of the type of animal species was higher in the year 2014 (40.3%) compared to the year 2015 (19.6%). Likewise, higher numbers of enterotoxaemia positive animals from all animal species were detected in the year 2014 (32.8%) compared to the year 2015 (20.4%). The overall prevalence of enterotoxaemia disease during the two years at the animal and herd levels was 24.9% and 26.47% respectively.
| Year of investigation | Sheep | Goats | camels | Cattle | All species | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No of herds investigated | No of herds positive | % of herds Positive | No of herds investigated | No of herds Positive | % of herds Positive | No of herds investigated | No of herds Positive | % of herds positive | No of herds investigated | No of herds positive | % of herds positive | No of herds investigated | No of herds positive | % of herds positive | |
| 2014 | 81 | 32 | 39.5 | 56 | 25 | 44.6 | 15 | 5 | 33.3 | 7 | 2 | 28.6 | 159 | 64 | 40.3 |
| 2015 | 184 | 36 | 19.6 | 92 | 14 | 15.2 | 29 | 4 | 13.8 | 12 | 8 | 66.7 | 317 | 62 | 19.6 |
| 2014–2015 | 256 | 68 | 26.6 | 148 | 39 | 26.4 | 44 | 9 | 20.5 | 19 | 10 | 52.6 | 476 | 126 | 26.47 |
During the two years the highest enterotoxaemia herds' prevalence was detected in cattle (66.7%) followed by goats (44.6%), sheep (39.5%) and finally camels (33.3%). Table 2 shows the % of enterotoxaemia positive animals detected during the years 2014–2015. At the individual animal level 24.9% (398 /1593) of the animals were positive for enterotoxaemia. The highest prevalence was detected in cattle (64.3%), followed by goats (29.9%), camels (21.5%) and finally sheep (21.4%). The prevalence of enterotoxaemia at the herd level decreased significantly in the year 2015 compared to year 2014.
| Year of investigation | Sheep | Goats | camels | cattle | All species | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No Specimens analyzed | No Specimens positive | % Specimens positive | No Specimens analyzed | No Specimens positive | % Specimens positive | No Specimens analyzed | No Specimens positive | % Specimens positive | No Specimens analyzed | No Specimens positive | % Specimens positive | No Specimens analyzed | No Specimens positive | % Specimens positive | |
| 2014 | 310 | 87 | 28.1 | 228 | 90 | 39.5 | 33 | 12 | 36.4 | 17 | 4 | 23.5 | 588 | 193 | 32.8 |
| 2015 | 586 | 105 | 17.9 | 281 | 62 | 22.1 | 88 | 14 | 15.9 | 50 | 27 | 54 | 1005 | 205 | 20.4 |
| 2014–2015 | 896 | 192 | 21.42 | 509 | 152 | 29.9 | 121 | 26 | 21.5 | 67 | 31 | 64.3 | 1593 | 398 | 24.9 |
Tables 3 and 4 show, respectively, the prevalence of enterotoxaemia at the herd and individual animal levels in different regions of KSA during the year 2015. It was found that an overall prevalence of enterotoxaemia in all regions of KSA among herds suspected to have enterotoxaemia was (19.8%). The highest prevalence at the herd level was detected in Hail region (50%), followed by Jazan region (43.3%), Qassim (30.8%), the eastern region (25%) and finally Aljouf region (18.8%). At the individual animals' level an over prevalence of (20.4%) was detected in all regions. The highest prevalence was detected in Aljouf region (41.7%), followed by Hail region (40.9%), Qassim (37.8%), Jazan (31.1%), the eastern region (26.1%) and finally Riyadh region (10.4%).
| Sheep | Goats | Camels | Cattle | All species | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Region | Number of herds investigated | Number of herds ositive (%) | Number of herds investigated | Number of herds positive (%) | Number of herds investigated | Number of herds positive (%) | Number of herds investigated | Number of herds positive (%) | Number of herds investigated | Number of herds positive (%) |
| Riyadh | 60 | 6 (10%) | 29 | 2 (6.9%) | 10 | 0 (0%) | 4 | 3 (75%) | 103 | 11 (10.7%) |
| Eastern | 46 | 14 (30.4%) | 39 | 8 (20.6%) | 12 | 2 (16.7%) | 3 | 1 (33.3%) | 100 | 25 (25%) |
| Qassim | 20 | 6 (30%) | 6 | 2 (33.3%) | – | – | – | – | 26 | 8 (30.8%) |
| Makkah | 15 | 0 (0%) | 2 | 0 (0%) | – | – | – | – | 17 | 0 (0%) |
| Jazan | 14 | 5 (35.7%) | 5 | 1 (20%) | 6 | 2 (33.3%) | 5 | 5 (100%) | 30 | 13 (43.3%) |
| Aljouf | 16 | 3 (18.8%) | – | – | – | – | – | – | 16 | 3 (18.8%) |
| Asser | 6 | 0 (0%) | 5 | 0 (0%) | 1 | 0 (0%) | – | – | 12 | 0 (0%) |
| Hail | 2 | 2 (100%) | 4 | 1 (25%) | – | – | 6 | 3 (50%) | ||
| Baha | 4 | 0 (0%) | 1 | 0 (0%) | 1 | 0 (0%) | – | – | 6 | 0 (%) |
| Bisha | 1 | 0 (0%) | 1 | 0 (0%) | – | – | – | – | 2 | 0 (%) |
| Total | 184 | 36 (19.6%) | 92 | 14 (15.2%) | 29 | 4 (13.8%) | 12 | 8 (66.7%) | 318 | 63 (19.8%) |
| Sheep | Goats | Camels | Cattle | All species | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Region | Number of herds investigated | Number of herds positive (%) | Number of herds investigated | Number of herds positive (%) | Number of herds investigated | Number of herds positive (%) | Number of herds investigated | Number of herds positive (%) | Number of herds investigated | Number of herds positive (%) |
| Riyadh | 213 | 17 (7.9%) | 69 | 7 (10.1%) | 21 | 0 (0%) | 13 | 9 (69.2%) | 316 | 33 (10.4%) |
| Eastern | 157 | 39 (24.8%) | 129 | 39 (30.2%) | 40 | 8 (20%) | 11 | 2 (18.2%) | 337 | 88 (26.1%) |
| Qassim | 52 | 21 (40.4%) | 22 | 7 (31.8%) | – | – | – | – | 74 | 28 (37.8%) |
| Makkah Al-Mukaramah | 48 | 0 (0%) | 9 | 0 (0%) | – | – | – | – | 57 | 0 (0%) |
| Jazan | 52 | 12 (23.1. %) | 18 | 3 (16.7%) | 23 | 6 (26.1%) | 26 | 16 (61.5%) | 119 | 37 (31.1%) |
| Aljouf | 24 | 10 (41.7%) | 24 | 10 (41.7%) | ||||||
| Asseer | 14 | 0 (0%) | 12 | 0 (0%) | 2 | 0 (0%) | – | – | 28 | 0 (0%) |
| Hail | 8 | 6 (75%) | 14 | 3 (21.4%) | – | – | – | – | 22 | 9 (40.9%) |
| Baha | 14 | 0 (0%) | 3 | 0 (0%) | 2 | 0 (0%) | – | – | 19 | 0 (0%) |
| Bisha | 4 | 0 (0%) | 5 | 0 (0%) | – | – | – | – | 9 | 0 (0%) |
| Total | 586 | 105 (17.9%) | 281 | 62 (22.1%) | 88 | 14 (15.9%) | 50 | 27(54%) | 1005 | 205 (20.4%) |
The results of the univariate analysis using chi-square test are presented in Table 5. Statistical analysis showed that there were significant differences between the different regions, months of the years 2014 and 2015, prevalence at the herd level, prevalence at the individual animal level and different animal species (p ≤ 0.0001). Risk factors such as regions (χ2 = 89.65, p = 0.000), Months during the year (χ2 = 76.65, p = 0.000), species of animals (χ2 = 50.81, p = 0.000), disease prevalence by years (χ2 = 29.75, p = 0.000), and herds' prevalence (χ2 = 1443.6, p = 0.000) showed statistically significant association with enterotoxaemia disease. The results of the multivariate analysis showed that only one independent risk factor was statistically significant and made the greatest effect (Table 6). Cattle were found to be approximately five times more likely to be infected with enterotoxaemia (OR = 4.88, CI = 2.55–9.37, p = 0.0001) compared to other animal species. Fig. 1 shows the monthly distribution of enterotoxaemia cases in all regions of KSA throughout the year 2015. It was found that the highest overall prevalence of enterotoxaemia cases in all animal species occurred during the period August - December 2015 with the highest prevalence in September (50%).
| Potential risk factors | No. animals tested | No. positive (%) | χ2 | p- value |
|---|---|---|---|---|
| Regions: | 89.65 | 0.000 | ||
| Riyadh | 316 | 33 (10.4%) | ||
| Eastern | 337 | 88 (26.1%) | ||
| Qassim | 74 | 28 (37.8%) | ||
| Makah | 57 | 0 (0.0%) | ||
| Jazan | 119 | 37(31.1%) | ||
| ALjouf | 24 | 10 (41.7%) | ||
| Asseer | 28 | 0 (0.0%) | ||
| Hail | 22 | 9 (40.9%) | ||
| Baha | 19 | 0 (0.0%) | ||
| Bisha | 9 | 0 (0.0%) | ||
| Total | 1005 | 205 (20.4%) | ||
| Enterotoxaemia by Months (2015): | 76.426 | 0.000 | ||
| January | 130 | 23 (17.6%) | ||
| February | 129 | 19 (14.7%) | ||
| March | 136 | 25 (18.4%) | ||
| April | 74 | 15 (20.3%) | ||
| May | 65 | 12 (18.5%) | ||
| June | 118 | 22 (18.6%) | ||
| July | 56 | 13 (23.2%) | ||
| August | 98 | 27 (27.6%) | ||
| September | 54 | 16 (29.6%) | ||
| October | 48 | 13 (27.1%) | ||
| November | 55 | 13 (23.6%) | ||
| December | 42 | 7 (16.7%) | ||
| Total | 1005 | 205 (20.4%) | ||
| Species of animals: | 50.81 | 0.000 | ||
| Sheep | 896 | 192 (21.4%) | ||
| Goat | 509 | 185 (36.3%) | ||
| Camel | 121 | 26 (21.5%) | ||
| Cattle | 67 | 31 (46.3%) | ||
| Total | 1593 | 434 (27.2%) | ||
| disease presence by years: | ||||
| 2014 | 29.75 | 0.000 | ||
| 2015 | 540 | 193 (35.7%) | ||
| Total | 1053 | 241 (22.9%) | ||
| 1593 | 434 (27.2%) | |||
| Infection in herds: | 1443.6 | 0.000 | ||
| 2014 | 159 | 64 (40.3%) | ||
| 2015 | 317 | 62 (19.6%) | ||
| Total | 476 | 126 (26.47%) |
| Variable | No. tested | + ve (%) | OR | Cl 95% | P-value |
|---|---|---|---|---|---|
| Species of animals: | |||||
| Sheep | 896 | 192 (21.4%) | Ref. | ||
| Goat | 509 | 185 (36.3%) | 1.04 | 0.707–1.53 | 0.837 |
| Camel | 121 | 26 (21.5%) | 0.75 | 0.396–1.42 | 0.379 |
| Cattle | 67 | 31 (46.3%) | 4.88 | 2.55–9.37 | 0.000 |
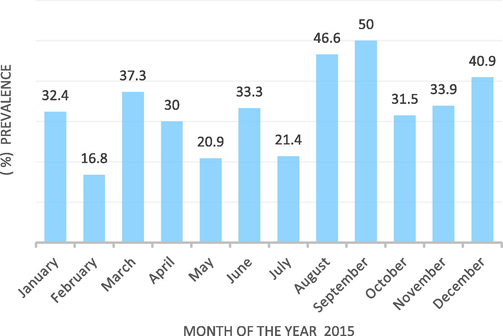
- Monthly distribution of enterotoxaemia in animals in the Kingdom of Saudi Arabia in the year 2015.
4 Discussion
Clostridium perfringens enterotoxaemia is a fatal enteric disease, which affects sheep, lambs, calves, piglets, and occasionally foals (Quinn et al., 1994). Camels (Fayez et al., 2013b) and goats (Uzal and Kelly, 1996) are susceptible. Other wildlife may be affected (Radostitis et al., 1994).
In this study we used different types of specimens for laboratory diagnosis of enterotoxaemia. The majority of samples were fecal samples obtained from diarrhoeic animals. Faecal specimens were used by several authors for detection of C. perfringens toxins (Cassutto and Cook, 2002; Moussa and Hessan, 2011; Mudassar, 2016; Maqbool et al., 2017).
The microscopic examination of gram stained impression smears from enterotoxaemia suspected animals was used as a preliminary screening diagnostic tool. The presence of Large numbers of gram-positive bacilli similar in morphology to C. perfringens strengthen the possibility of finding such animals enterotoxaemia positive.
Enterotoxaemia is caused by the major exotoxins of Clostridium perfringens types A, B, C, D and E. In this study, the C. perfringens types detected by antigen capture ELISA tests were types A, B, C and D. Our results are to some extent similar to the results of Moussa and Hessan (2011) who reported that out of 27 C. perfringens isolates from different animal species in central KSA 20 (74.1%) of them were identified as type A by classical methods. One strain (3.7%) was identified as type C and 3 strains (11.1%) as type D. Moreover, PCR results confirmed the traditional methods in typing one strain as type B (3.7%). Nazki et al. (2017) reported the occurrence of C. perfingens types A, D and absence of other types in sheep and goats in Kashmir, India. Tutuncu et al. (2018) also reported that C. perfringens types A and D were the dominant types in sheep and goats in Samsun province, northern Turkey. In this study, the highest prevalence of enterotoxaemia was detected in cattle (64.3%), followed by goats (29.9%), camels (21.5%) and finally sheep (21.4%). Rahaman et al. (2013), reported a prevalence of (32.1%) cases of C. perfringens in sheep and goats in Khuzestan province whereas Maqbool et al. (2017) reported an overall prevalence of (26%) cases of C. perfringens in small ruminants in Pakistan. In this study, enterotoxaemia outbreaks occurred in six out of the ten regions which were investigated. The highest prevalence of enterotoxaemia during the year 2015 ,irrespective of the animal species involved and types of the C. perfringens, was detected in Aljouf region (41.7%), followed by Hail region (40.9%), Qassim region (37.8%), Jazan (31.1%), the eastern region (26.1%) and finally Riyadh region (10.4%). No samples from Bisha, Baha, Aseer and Makkha regions were found positive for C. perfingens. This is probably due to the relatively small numbers of herds, samples or the few animal species, which were examined from these regions. Fayez et al. (2013a), reported 30.4% prevalence of C. perfringens from the intestinal samples of sheep in Al-Ahsa province, in the eastern region of KSA. The geographical variation in the distribution of enterotoxaemia may be attributed to many factors. Enterotoxaemia caused by the different types of C. perfringens are often precipitated by some husbandry and environmental factors. Lamb dysentery is more prevalent during cold weather where lambs are kept closely confined in small yards or in the field for lambing hence cross contamination of the surroundings with the bacteria is more likely to occur in these circumstances (Radostitis et al., 1994). Persistence of C. perfringens in the environment is a result of previous cases of enterotoxaemia in the same area (Smith and Sherman, 1994). The ability of the bacterium to survive in the environment under unsuitable conditions is another important factor, which helps to initiate disease outbreaks. In goats, cold weather, stress and concomitant infestation with coccidia were suggested to be possible predisposing factors for caprine enterotoxaemia (Uzal et al., 1994). In this study, the highest prevalence of enterotoxaemia was found in Aljouf (41.7%) followed by Hail (40.9%). These two regions are located in the northern part of KSA, which normally have colder weather than the other regions included in the study. The relatively cold weather prevailing in these two regions may have contributed to the high prevalence of enterotoxaemia in these two regions compared to other regions in KSA.
In this study, it was found that the morbidity, mortality and case fatality rates in animal herds from different animal species associated with diarrhoea and symptoms suggestive of enterotoxaemia varied greatly.
The morbidity, mortality and case fatality rates in sheep were found to range between 0.33 and 28.5 % (8.0 ± 9.6%), 0.33–28.5% (7.1 ± 8.8%); and 58–100% (87.2 ± 19.3%) respectively. The morbidity, mortality and case fatality rates in goats were found to range between 5 and 26.7 % (12.5 ± 7.7%), 5–25.3% (10.3 ± 7.7%) and 36.4–100% (84.2 ± 24.6%) respectively. The morbidity, mortality and case fatality rates in camels were found to range between 5 and 40 % (22.5 ± 24.7%), 1.3–32% (16.6 ± 21.7%) and 25–80% (52.3 ± 38%) respectively. The morbidity, mortality and case fatality rates in cattle were found to be 1.3%, 1% and 75% respectively. The morbidity, mortality and case fatality rates varied greatly among the different herds and the different animal species however, the case fatality rate was on the average greater than 80% in sheep and goats, 75% in cattle and 52.3% in camels. Hussain et al. (2014) reported 100% case fatality due to enterotoxaemia in Chinkara deer (Gazella bennettii) kept under desert conditions in Pakistan. Ershaduzzaman et al. (2007) observed that nearly 64% of black Bengal goat kids with symptoms of diarrhea in Bangladesh died due to enterotoxaemia. The greater values of some standard deviations compared to the mean values may be explained by the fact that the range of values for morbidity and mortality for some animal species was so wide. Mortality occurred in animals of all age groups. Young animals (newborn to 3 months old) represented 33.3% of the dead animals. Sudden change of feed was associated to 12.5% of the mortalities among affected animals
According to Seifert (1996) enterotoxaemia in animals is associated with sudden dietary changes to feedstuff rich in protein and carbohydrate sources and to over eating. Reduction of the intestinal transit due to over eating lead to retention of the bacteria and their toxins and to an observed in affected animals, were closely similar to those described by Radostitis et al. (1994). In young animals which were autopsied, hemorrhagic enteritis with ulcerations of the mucosa were the major lesions in all animals' species. The contents of the small intestine were tinged with blood according to the severity of the infection. Some animals showed excessive fluid in the intestine. In older animals, lesions were less severe compared to young animals. Hemorrhagic areas in the intestine were often observed. Pulmonary edema was rarely observed. In this study, it was found that enterotoxaemia occurred in animal species all around the year. The highest overall prevalence of enterotoxaemia cases in all animal species occurred during the period August–December 2015 with the highest prevalence in September (50%). This is probably due to the relatively moderate to cold weather and the delivery and weaning of newborn animals during these months of the year. Ershaduzzaman et al. (2007) reported a higher mortality rate of young goats' kids in Bangladesh due to enterotoxaemia during the October- March season (69.6%) when compared to the period April–September season (50%). In conclusion, it is obvious that enterotoxaemia is endemic in KSA. It affects sheep, goats, camels and cattle of all ages throughout the year with particular higher prevalence rates during the relatively moderate to cold months of the year. In this study it was found that out of all potential risk factors which were investigated, the animal species represent the most significant risk factors associated with enterotoxaemia in KSA. Cattle were found to be approximately five times more likely to have infection with enterotoxaemia (OR = 4.88, CI = 2.55–9.37, p = 0.0001) compared to other animal species. The high susceptibility of cattle to enterotoxaemia may be explained by the fact that vaccination against enterotoxaemia is performed usually and more regularly for sheep and goats compared to cattle in addition to cattle are usually confined to small areas pens and are not allowed to graze around in the nearby natural pasture unlike sheep, goats and camels which make cattle more susceptible to infection from the heavily contaminated environment with C. perfringens organisms.
5 Conclusions
Enterotoxaemia caused by Clostridium perfringens has been detected in different livestock species (cattle, camel, sheep, and goats) from different regions in Saudi Arabia. C. perfringens types A, B, C and D have been reported in the present study and their identities have been determined using ELIS A and further confirmed by the polymerase chain reaction. Cattle were found to be the highly susceptible animal species and the highest record of enterotoxaemia was reported from the Aljouf region in the northern Saudi Arabia. The highest prevalence of enterotoxaemia among animals was found to be during the period August to December of the year.
Acknowledgement
This project was funded by the National Plan for Science, Technology and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia, Studies on enterotoxaemia in livestock in the Kingdom of Saudi Arabia, Award Number 11-BIO-1855-02.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Diagnostic multiplex PCR for toxin genotyping of Clostridium perfringens isolates. Vet. Microbiol.. 2004;100:11-16.
- [Google Scholar]
- An epidemiological survey of Clostridium perfringens-associated enterotoxemia at an army veterinary treatment facility. Mil. Med.. 2002;167:219-222.
- [Google Scholar]
- Studies on the diseases and mortality patterns of goats under farm conditions and some factors affecting mortality and survival rates in Black Bengal kids. Bang. J. Vet. Med.. 2007;5:71-76.
- [Google Scholar]
- Prevalence and toxinotyping of the toxigenic Clostridium perfringens in sheep with suspected enterotoxaemia. Nat. Sci.. 2013;11:15-21.
- [Google Scholar]
- Clostridium perfringens enterotoxaemia In camel (Camelus dromedarius) calves. Inter. J. Adv. Res.. 2013;1:239-247.
- [Google Scholar]
- Bacterial flora in the alimentary tract of chickens infected with Eimeria brunetti and in chickens immunized with Eimeria maxima and cross-infected with Eimeria brunetti. Exp. Parasitol.. 1972;31:188-193.
- [Google Scholar]
- Clinicopathologic findings of enterotoxaemia in Chinkara deer (Gazella bennettii) under desert conditions in Pakistan. Pak. Vet. J.. 2014;34:400-402.
- [Google Scholar]
- IBM Corp. Released, 2013. IBM SPSS Statistics for Windows, Version 22.0, IBM Corp, Armonk, NY.
- An update on the human and animal enteric pathogen Clostridium perfringens. Emerg. Microbes Infect.. 2018;7:1-15.
- [Google Scholar]
- Prevalence and chemotherapy of enterotoxaemia (Clostridium perfringens) in diarrheic sheep and goats. J. Innov. Biomed. Res.. 2017;1:30-35.
- [Google Scholar]
- Veterinary Epidemiology: Principles and Methods (Ames 1st ed.). Iowa State University Press; 1987. p. :265p.
- Molecular typing of Clostridium perfringens toxins recovered from Central Saudi Arabia. Saudi Med. J.. 2011;32:669-674.
- [Google Scholar]
- Mudassar, M., 2016. Molecular epidemiology of Clostridium perfringens isolated from small ruminants. PhD thesis. Department of Basic Medical Sciences, Al-Nafees Medical College and Hospital, Isra University, Pakistan.
- Isolation, molecular characterization and prevalence of Clostridium perfringens in sheep and goats of Kashmir Himalayas, India. Vet. World. 2017;10:1501-1507.
- [Google Scholar]
- Clostridium perfringens: toxinotype and genotype. Trends Microbiol.. 1999;7:104-110.
- [Google Scholar]
- Quinn, P.J., Carter, M.E., Markey, B., Carter, G.R., 1994. Clostridium species. In: Clinical Veterinary Microbiology, Wolfe Publishing, London, pp. 191–208.
- Veterinary Medicine, a Text Book of the Disease of Cattle, Sheep, Goats, Pigs and Horses (8th ed.). London: Bailliere Tindall; 1994.
- Isolation, identification and characterization of Clostridium perfringens from lamb dysentery in Dinajpur district of Bangladesh. Sci. J. Microbiol.. 2013;2:83-88.
- [Google Scholar]
- Seifert, H.S.H., 1996. Enterotoxaemia complex. In: Tropical Animal Health, Kluwer Academic Press, Netherland, pp. 304–310.
- Enterotoxaemia. In: Smith M.C., Sherman D.M., eds. Goat Medicine. Pennsylvania: Lea and Febiger; 1994. p. :289-305.
- [Google Scholar]
- Clostridial enteric diseases of domestic animals. Clin. Microbiol. Rev.. 1996;9:216-234.
- [Google Scholar]
- Prevalence and toxin typing of Clostridium perfringens enterotoxins in small ruminants of Samsun Province, Northern Turkey. J. Anim. Plant Sci.. 2018;28:1204-1207.
- [Google Scholar]
- Treatment of anaerobic infections. Scand. J. Gastroenterol.. 1984;90(Suppl.):53-64.
- [Google Scholar]







