Environmentally friendly production, characterization, and evaluation of ZnO NPs from Bixa orellana leaf extract and assessment of its antimicrobial activity
⁎Corresponding authors at: Department of General Science, Ibn Sina National College for Medical Studies, Al Mahajar Street: 31906, Jeddah 21418, Saudi Arabia (S.M. Shakeel Iqubal), KLE Technological University, Hubballi, Karnataka 580031, India (U.M. Muddapur). muddapur@kletech.ac.in (Uday M. Muddapur), shakeeliqubal@ibnsina.edu.sa (S.M. Shakeel Iqubal) shakeeliqubal@gmail.com (S.M. Shakeel Iqubal)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Zinc oxide nanoparticles (ZnO NPs) are establishing themselves as an important class of nanomaterials due to their exceptional physicochemical properties and wide range of applications. Due to their affordability, lack of toxicity, and strong biocompatibility, ZnO NPs find extensive use in the field of biomedicine. ZnO NPs are promising in biomedicine, especially for their ability as anticancer and antimicrobial agents. The ecologically sustainable preparation of metallic NPs using different plant extracts is a viable alternative to more conventional synthesis methods. The present study investigates the effects of changing the physical conditions on ZnO NPs synthesis from Bixa orellana (B. orellana) extract using the precipitation method. Confirmation and characterization of the ZnO NPs were achieved by analytical techniques. EDS results verified that highly pure ZnO NPs were synthesized. X-ray diffraction analysis verified the crystal nature of the synthesized NPs and their crystalline particle size of 82.66 nm. The XRD graphs strongly indicate the formation of wurtzite ZnO due to the presence of the (1 0 0), (0 0 2), and (1 0 1) planes. The antibacterial activity was assessed through the utilization of agar disc diffusion. The findings revealed that ZnO NPs exhibited significant efficacy in inhibiting the growth of both Gram-positive and Gram-negative bacteria. The zone of inhibition with the greatest diameter (22 mm) was reported for the bacterial strain B. cereus. The present investigation provides evidence that B. orellana leaves extract is capable of producing ZnO NPs, which play a crucial role in its antibacterial action. Additional investigation is necessary to validate the role of diverse phytochemicals in the synthesis of ZnO NPs and their applications in diverse fields such as agriculture, cosmetics, food, and healthcare.
Keywords
Antimicrobial
Bixa orellana
Zinc oxide nanoparticles
Characterization
Green synthesis
1 Introduction
Growing antibiotic resistance is a major problem that people face around the world. Infectious diseases are a major public health concern worldwide. The growing resistance of bacteria to pharmaceuticals, coupled with the drawbacks of medications such as reduced safety, increased costs, and decreased effectiveness, has created a pressing demand for the development of alternative treatments to replace antibiotics. Due to the aforementioned issue, the world is shifting toward plant-based medicines that have less side effects and are more effective for treating diseases such as cancer, diabetes, and microbiological infections (Aditya et al., 2019, Vidhya et al., 2013, Poornima et al., 2021, Muhsinah et al., 2022). The WHO reports that each year there are 14 million cases of cancer and over 9.6 million cancer-related mortality (Poornima et al., 2021, Thilagam et al., 2013, Rathnasamy et al., 2017). Nanoparticles are considered to have a potential applications as antimicrobial and cytotoxic agents due to their various advantages such as the less contaminations, good adhesion, and colonization (Thilagam et al., 2013, Rathnasamy et al., 2017, Gnanasangeetha and Sarala, 2014, Akintelu and Folorunso, 2020).
Nanoparticles (NPs) are extremely small particles, ranging in size from 1 to 100 nm. NPs have distinctive chemical and physical properties owing to their nanoscale dimensions and extensive surface area. The unique dimensions, configuration, and composition of a material also exert an influence on its responsiveness, resilience, and additional attributes. The aforementioned attributes render them suitable contenders for a diverse array of industrial and domestic applications, encompassing environmental, imaging, medical, energy-based research, as well as catalysis and medical applications (Poornima et al., 2021, Thilagam et al., 2013, Rathnasamy et al., 2017, Gnanasangeetha and Sarala, 2014, Iqbal et al., 2021, Muddapur et al., 2022, Akintelu and Folorunso, 2020). The green synthesis methods for the production of NPs has garnered considerable interest in recent years, emerging as a pioneering field within the realm of nanotechnology (Gnanasangeetha and Sarala, 2014, Wang et al., 2017, Gharpure et al., 2022, Jayachandran et al., 2021). Green nanotechnology means a clean method used for the production of NPs to eliminate or to reduce hazardous materials. The mechanism by which NPs exert their effects involves direct interaction with the bacterial cell wall, eliminating the requirement for penetration. Therefore, the majority of processes that confer antibiotic resistance are not adaptable to nanoparticles. This suggests that nanoparticles may have a lower propensity to induce bacterial resistance compared to conventional antibiotics. As a result, focus has shifted to novel and enticing NP-based compounds with antimicrobial and cytotoxic properties (Thilagam et al., 2013, Rathnasamy et al., 2017, Gnanasangeetha and Sarala, 2014, Iqbal et al., 2021, Muddapur et al., 2022, Uday et al., 2022, Wang et al., 2017, Gharpure et al., 2022, Jayachandran et al., 2021). Nanocrystalline cellulose (NCC) assumes a prominent role in the field of drug delivery and release owing to its inherent benign characteristics. Biosensors incorporating graphene, as well as a composite of nanocellulose with gold and silver nanoparticles, have the capacity to act as a diagnostic tool for several illnesses and viral infections (Kadir et al., 2022).
Due to wide characteristics, metallic oxide NP’s, specifically ZnO NP’s, have gained huge importance in diabetics cancer and antimicrobial treatment, due to which these NP’s have been utilized in medicine, food science and agriculture areas. ZnO NPs possess non-toxicity, eco-friendliness, and the ability to enable large-scale production. (Thilagam et al., 2013, Rathnasamy et al., 2017). However, physical and chemical approaches for synthesis of NP’s have some drawbacks, such as the need for precise instruments, increased costs, and hazardous chemicals, which play a role in restrictions in many fields (Gnanasangeetha and Sarala, 2014, Wang et al., 2017). Nanoparticles produced using green technology are excellent than those produced using chemical and physical approaches in a variety of ways. Green techniques, for example, avoid the use of expensive chemicals, utilize less energy, are more sustainable, and provide ecologically friendly products. Synthesis of several ZnO NPs has been reported from different natural products such as leaves of Agathosma betulina, bulbs of Petroselium crispum, and milk of Carica papaya (Gnanasangeetha and Sarala, 2014, Wang et al., 2017).
B. orellana is native to and widely grown in Amazon, it is commonly cultivated in tropical nations for its dye and food coloring applications. It is typically a small tree with a broad stem. The most eye catching part of the plant is its fruit and has deep red seeds in it (Wang et al., 2017, Thilagam et al., 2013, Muddapur et al., 2023, Gharpure et al., 2022), known as urucum (synonyms: annatoo, achiote), its seeds contain geranylgeraniol and tocotrienols (Rajendran et al., 2021, Islam et al., 2019). Its extract is used to cure ulcers, high fever, asthma, and skin diseases, also has antioxidant, anticancer, and anti-inflammatory activity (Wang et al., 2017, Rajendran et al., 2021). In previous studies, qualitative analysis of phytochemicals confirmed the availability of steroids, carbohydrates, saponins, tannins, and proteins (Islam et al., 2019, Gunalan et al., 2012). These phytochemicals have significant bioactivities to act as stabilizing, reducing agents during the NPs synthesis (Rajendran et al., 2021, Islam et al., 2019, Gunalan et al., 2012, Al Awadh et al., 2022). Similarly, steroidal chlorohydrins presents a promising avenue for potential therapeutic interventions in neurodegenerative and other disorders (Hannan et al., 2020).
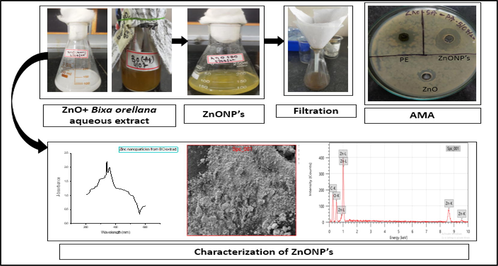
This study aimed to examine the process of green synthesis of ZnO NPs using the leaf extract of B. orellana. Additionally, the antibacterial properties of the synthesized ZnO NPs were evaluated against various bacterial strains. The characterisation of the ZnO NPs was conducted using powdered X-ray diffraction (XRD), scanning electron microscopy (SEM), and ultraviolet (UV) spectrum analysis.
2 Materials and methods
2.1 Materials
Bixa orellana leaves sample were collected from University of Agricultural Science (UAS) Dharwad. Zinc acetate (ZnC₄H₆O₄), and other chemicals and media components used in this work were procured from HI media, India. The microorganisms E. coli, Bacillus cereus, S. aureus, P. aeruginosa, & Z. mobilis were procured from NCIM, Pune, India; and B. Nakamuria from the Laboratory strain (Table 1). A water bath and magnetic stirrer were used for ZnO NPs synthesis. For concentration of ZnO NPs, a freeze dryer was used. Further characterization by powdered XRD and EDS was performed by Sophisticated Analytical Instrument Facility, Dharwad.
| S/N | Bacteria | Strain number | Gram strain |
|---|---|---|---|
| 1 | E. coli | ATCC 25922 | −ve |
| 2 | B. cereus | ATCC 10876 | +ve |
| 3 | P. aeruginosa | ATCC 27853 | −ve |
| 4 | S. aureus | ATCC 25923 | +ve |
| 5 | Z. mobilis | ATCC 31821 | −ve |
| 6 | B. nakamuria | Lab strain | +ve |
ATCC (American Type Culture Collection).
2.2 Selection and Collection of plant materials
B. orellana leaves (100 g) of were collected from UAS, Dharwad, India, rinsed with distilled water, shade-dried for approximately 25 days, and then powdered for use in an aqueous extraction process.
2.3 Preparation of aqueous extract (cold method)
In 50 ml of distilled water, 15 g of dried leaves were added, heated 1 hr in water bath at 60 °C, further the extract was filtered to get a clear extract. For next studies the final filtration was stored in a cool dry place (Poornima et al., 2019, Thilagam et al., 2013).
2.4 Preparation of zinc acetate solution for synthesis of ZnO NPs
0.1 M of Zinc acetate (Zn(O2CCH3)2(H2O)2) was prepared using distilled water, 18.348 g of Zinc acetate in 1000 ml of distilled water (183.48 g/L).
2.5 Synthesis of ZnO NPs
50 ml of 0.1 M (Zn(O2CCH3)2(H2O)2) solution was added to 20 ml of plant extract under constant stirring and heating, the process was continued till 1 hr to get zinc hydroxide, after the process, mixture becomes yellowish-cream color precipitate (Dolcet et al., 2012, Medina-Flores et al., 2016, Carofiglio et al., 2020, Al Awadh et al., 2022).
2.6 Antibacterial activity of ZnO NPs
The researchers tested the effectiveness of ZnO NPs against six different pathogens. These pathogens, including E. coli and S. aureus, are known to cause harmful effects such as food contamination, damage to the intestinal lining, and skin damage. The researchers specifically tested ZnO NPs derived from B. Orellana as a means of combating these infections.
2.6.1 Preparation of bacterial inoculum
Each bacterial strain was subcultured in nutrient broth (13 g/L) and was incubated in shaker incubator at 37 °C for 24 hrs. Further, the absorbance of bacterial culture was determined by UV visible spectrophotometry at 600 nm, and the optical density was adjusted to 0.5–0.6 (viable cell count in broth) (Rathnasamy et al., 2017, Thilagam et al., 2013).
2.6.2 Agar well diffusion assay
Preparing nutrient agar (add 2 % agar powder in nutrient broth) and autoclave at 121 °C & at 15 psi, considering sterile petri plates, pour 1/3rd of petri plate with nutrient agar, and let them to solidify for 30 mins, and then add 0.1 ml of bacterial inoculum and spread it by using spreader, then make three 8 mm holes in agar plate by puncher, for ZnO NPs, plant extract, and for negative control under sterile conditions (Rathnasamy et al., 2017, Thilagam et al., 2013).
Add 0.1 ml of samples to respective holes in agar plates, then keep in −4 °C freezer for diffusion for 3 hrs, and then keep the plates in bacteriological incubator at 37 °C for 24 hrs.
Following the incubation period, the area of inhibition was measured by using scale in mm, and comparative conclusion can be made by this, the accepted results will have no zone of inhibition by zinc acetate (negative control). Higher zone of inhibition by ZnO NPs indicates the ability to inhibit the activity of bacterial pathogens (Poornima et al., 2019, Rathnasamy et al., 2017, Al Awadh et al., 2022).
2.7 Concentration
Concentration of ZnO NPs by lyophilization or freeze drying for removal of water content to get powdered form of ZnO NPs for further characterization, freeze dryer helps to reduce water content and to reduce loose of activity. Concentration also helps to reduce the unwanted agents present in extract (Rathnasamy et al., 2017).
2.8 Characterization
The ZnO NPs can be characterized by using UV spectrum analysis, powdered XRD, and EDS analysis as previously described (Al Awadh et al., 2022, Poornima et al., 2021). These techniques help to understand the presence of Zinc nanoparticles.
2.8.1 UV absorption of ZnO NPs
UV spectrum analysis is done by using UV visible spectrophotometer (M&A Instruments Inc V-5000 VIS Spectrophotometer), the spectrum was taken from 200 to 600 nm, this data helps to verify the presence of ZnO NPs.
2.8.2 Powdered XRD (X ray Diffraction)
Powdered XRD analysis is used for XRD measurements and was performed as previously described (Al Awadh et al., 2022) at 20 °C–80 °C range for 2 mins (Model-Rigaku Smart lab SE).
2.8.3 Energy-dispersive X-ray spectroscopy (EDS) analysis
The composition present in the ZnO NPs were confirmed by EDS analysis, to determine and to validate zinc and oxygen. The provided knowledge possesses potential utility in the character analysis of the qualities of ZnO NPs and in comprehending their behavior within diverse applications, including the advancement of antibacterial medications. The size was determined using scanning electron microscopy (SEM) with the JSM-IT500 model manufactured by JEOL, USA, as previously reported. (Al Awadh et al., 2022).
3 Results and discussion
3.1 Production and characterization of ZnO NPs
Upon addition of the extract and ammonia to a solution containing 0.05 M zinc acetate, a precipitate range was observed. The physicochemical properties of a zinc acetate solution were altered by an addition of extracts. The incorporation of plant extracts resulted in a prominent alteration in physicochemical properties, primarily manifested as a rapid chromatic transition that transpired within a few minutes.
3.1.1 Characterization by UV-spectrum
Plants with secondary metabolites convert zinc ions to zinc oxide in the presence of water. In addition to its reducing effects, the plant extract also has stabilizing properties. The UV–visible spectrum investigation from 200 nm to 600 nm corroborated this. In the spectra, ZnO NPs were clearly visible as a peak at 360 nm. The absorbance peak of ZnO NPs has been found to occur between 310 nm and 380 nm (Dolcet et al., 2012) (Fig. 1). It has been found that ZnO NPs s have an absorption maximum between 320 and 390 nm, according to previous research. A characteristic of ZnO NPs is at 370 nm and a band energy of 3.35 eV has been observed, verifying its synthesis as reported by various research (Dolcet et al., 2012).
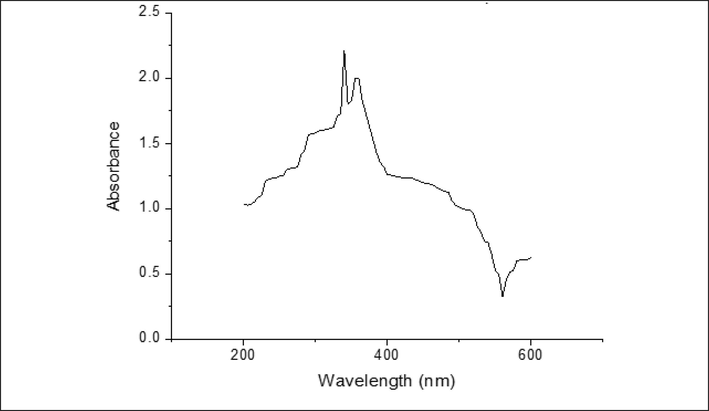
- UV analysis of B. orellana ZnO NPs.
3.1.2 Powdered XRD analysis of ZnO NPs
This is a prevalent methodology for evaluating the crystalline nature and configuration of solid specimens. B. orellana XRD pattern was studied, which revealed a Bragg reflection with X values of 2. Fig. 2 indicate ZnO NPs presence in the sample. The XRD patterns of the nanoparticles reveal information about the phases, structures, and crystalline (sharp peaks) orientations of the particles. Spectra of diffraction at 31.830, 34.480, 36.310, 47.400, 56.650, 62.910, 66.430, 68.000, and 69.140. They were connected to the lattice in a series of planes: (100, 002, 101, 102, 110, 103, 200, 112 and 201) (Medina-Flores et al., 2016, Al Awadh et al., 2022, Poornima et al., 2019).
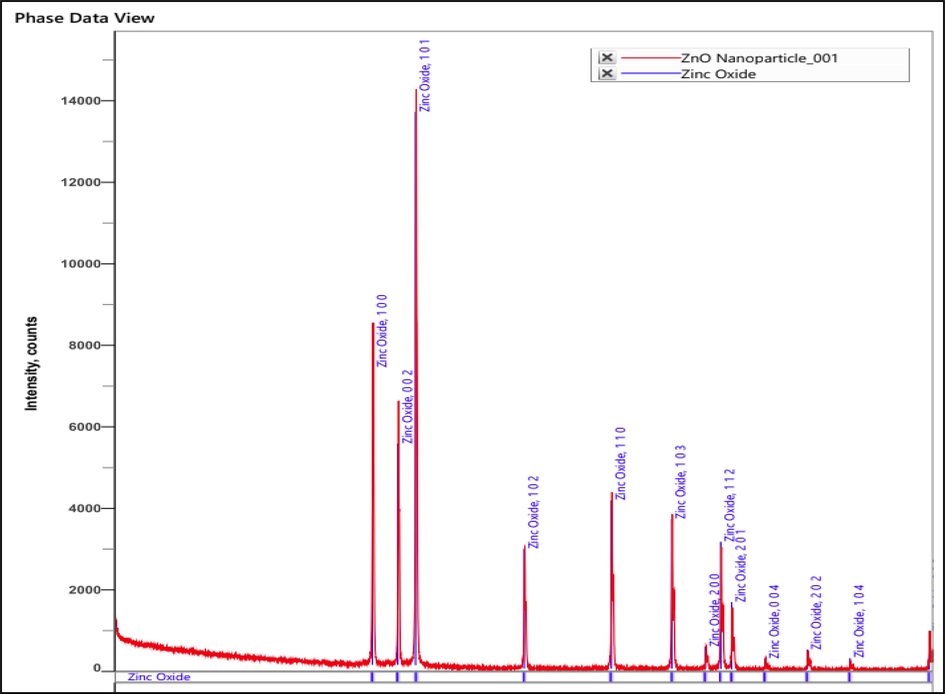
- XRD analysis of B. orellana ZnO NPs.
Using Debye-Scherrer equation:
The crystalline size of the generated nanoparticle ranges from 76.92 nm up to 84.41 nm. The NPs average crystalline size was measured as 82.66 nm.
3.1.3 EDS analysis of ZnO NPs
The presence of zinc acetate during the production of NPs was verified through the observation of distinct peaks corresponding to zinc, carbon, and oxygen in the energy-dispersive X-ray spectroscopy spectra, as depicted in Figs. 3 and 4. The absorbance maxima observed in this study can be attributed to the surface plasmon resonance of the ZnO nanoparticles. The synthesis of ZnO nanoparticles is characterized by the presence of oxygen in relatively low quantities (Francis et al., 2018; Shaikh et al., 2022; Kokate et al., 2011; Gupta et al., 2018). This observation implies that phytochemicals play a significant role in decreasing, capping, and stabilizing the nanoparticles throughout the synthesis process.
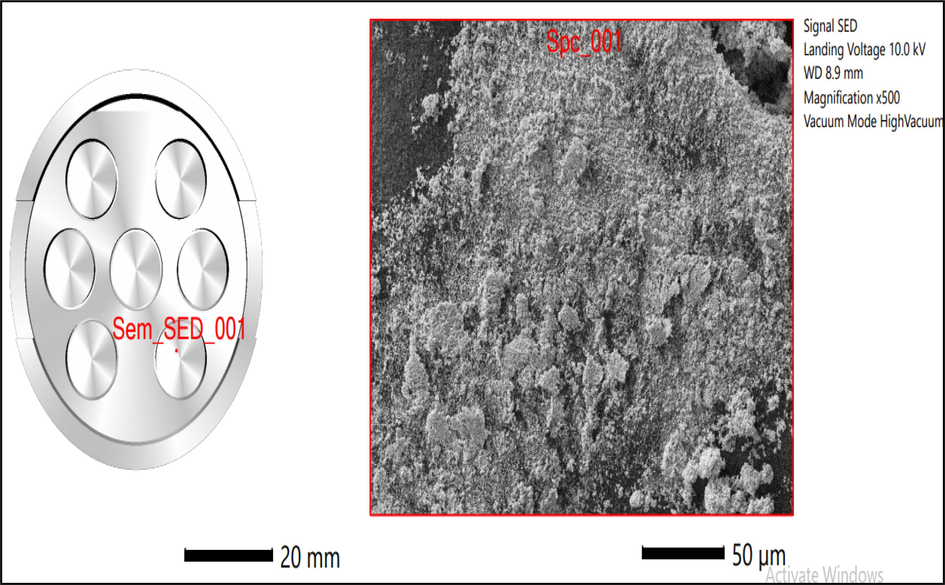
- SEM-EDS analysis of B. orellana ZnO NPs.
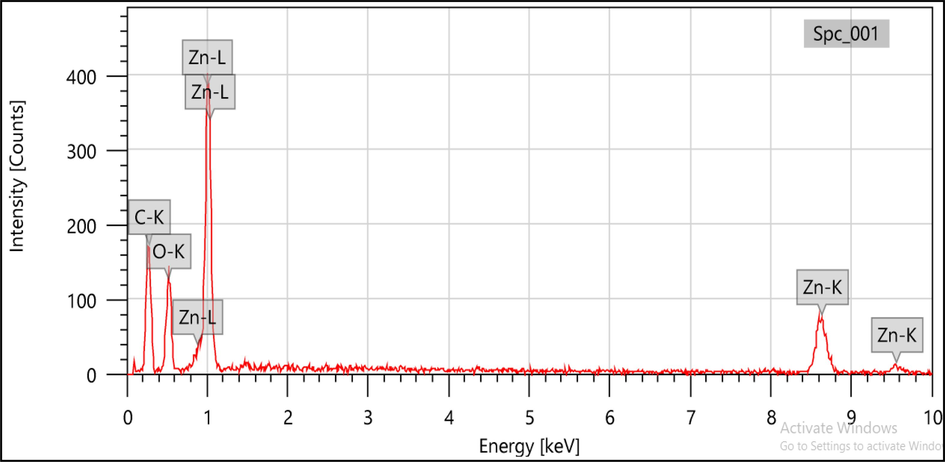
- EDS analysis of B. orellana ZnO NPs.
3.2 Antibacterial activity
Using agar disc diffusion, the antibacterial property of the ZnO NPs generated from B. orellana aqueous extract against diverse microorganisms, including S. aureus, E. coli, and P. aeruginosa. All the results indicated that ZnO NPs are highly effective against all types of tested bacteria. Fig. 5 displays that the largest inhibitory zone (22 mm) observed for B. cereus (Table 2).

- Antibacterial activity of ZnO NPs synthesized from B. Orellana.
| S.No | Bacteria | Zone of Inhibition (mm) |
|---|---|---|
| 1. | E. coli | 12 ± 0.9 |
| 2. | P. aeruginosa | 13 ± 1 |
| 3. | Z. mobilis | 16 ± 1 |
| 4. | B. cereus | 22 ± 1 |
| 5. | S. aureus | 14 ± 1 |
| 6. | B. nakamuria | 10 ± 1 |
Linezolid (control) Zone of inhibition was 22 ± 2 mm.
According to a previous report (Aldalbahi et al., 2020), the susceptibility of P. aeruginosa to ZnO NPs is comparatively higher than that of S. aureus. This difference in sensitivity may be because S. aureus has a thicker layer of peptidoglycan in its cell walls, which provides resistance to ZnO NPs. However, E. coli showed greater resistance than S. aureus in this case. The varying cell membrane polarity is responsible for this phenomenon. In contrast to E. coli, S. aureus membrane has a lesser negative charge, which allows for greater penetration of highly oxidizing free radicals and, ultimately, death (Reddy et al., 2007, Padmavathy and Vijayaraghavan, 2008, Sonohara et al., 1995).
ZnO NPs are able to penetrate microbial cell membranes thanks to the electrostatic interactions formed at the contact, which then leads to cell damage through the generation of reactive oxygen species (ROS) within the bacteria. ZnO surfaces generate H2O2 that is diffused throughout the cell, killing any resident bacteria or fungi (Sharma et al., 2009, Vidhya et al., 2013).
ZnO NPs have been shown to have antibacterial effects against a wide range of microbes, as reported in a number of published research (Aditya et al., 2019, (Carofiglio et al., 2020, (Barua and Mitragotri, 2014, Uresh et al., 2018). Numerous studies have demonstrated the antibacterial efficacy of ZnO NPs against both Gram-positive and Gram-negative bacteria. However, the precise mechanism behind these features remains incompletely understood. ZnO NPs antimicrobial effects stem mostly from the fact that they damage cell membranes, produce ROS, and liberate the metallic ion Zn2+. ZnO NPs are associated with bacterial cell membranes thanks to electrostatic interactions. When the nanoparticles come into touch with bacteria, they cause membrane disruption and leakage. The release of Zn2+ has negative effects on bacterial growth by impeding active transport, slowing amino acid metabolism, and inactivating different enzymes. Moreover, increased ROS production disrupts essential cellular functions, leading to cell death. ZnO NPs antibacterial capabilities can be explained in part by the fact that they trigger a variety of different pathways that ultimately lead to cell death. Similar antibacterial activity has been reported earlier, who reported remarkably substantial inhibition of growth against the tested bacterial strains, indicating a strong and statistically significant effect (Alqahtani et al., 2022; Mohmad et al., 2022; Shaikh et al., 2022; Awadh et al., 2022).
The study unveils the remarkable potential of B. orellana leaf extract in synthesizing ZnO NPs, thereby introducing a novel dimension to its antibacterial capabilities. These nanoparticles hold immense promise as advanced medical agents, particularly in the realms of anticancer and antibacterial interventions. Nevertheless, further exploration is warranted to elucidate the precise roles played by various biomolecules in the intricate synthesis process of ZnO NPs. The potential biological applications of these nanoparticles are manifold, making them an exciting area for continued investigation in the pursuit of enhanced medical therapies.
4 Conclusion
ZnO NPs are widely recognized for their antibacterial efficacy against a multitude of pathogens. This has resulted in their utilization across diverse domains, including but not limited to food, cosmetology, agriculture, healthcare, and pharmaceuticals. The precise mechanism underlying these effects is yet to be fully elucidated; nonetheless, the generation of reactive oxygen species significantly contributes to its antimicrobial efficacy. Further research is required to investigate the involvement of various biomolecules in the process of synthesizing zinc oxide nanoparticles and to study their potential applications in biological settings. This study presents empirical evidence that the extract of B. orellana leaves has the ability to generate ZnO NPs, which are integral to its antibacterial properties. To sum up, the research presented here underscores the transformational potential of B. orellana leaf extract-synthesized ZnO NPs in the field of biomedicine. The findings not only contribute to the advancement of nanoparticle synthesis techniques but also pave the way for innovative medical interventions that harness the remarkable properties of these nanoparticles for improved healthcare outcomes.
Acknowledgement
The authors are thankful to the Deanship of Scientific Research at Najran University, Saudi Arabia for funding this work under the General Research Funding Program grant code (NU/DRP/MRC/12/23). The work was supported by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP 2023R171), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia, as well as those affiliated with AlMaarefa University in Riyadh, Saudi Arabia, and KLE Technological University, BVB, Hubballi, Karnataka, India.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- ZnO nanoparticles modified with an amphipathic peptide show improved photoprotection in skin. ACS Appl. Mater. Interfaces. 2019;11:56-72.
- [CrossRef] [Google Scholar]
- A review on green synthesis of zinc oxide nanoparticles using plant extracts and its biomedical applications. Bionanoscience. 2020;10:848-863.
- [CrossRef] [Google Scholar]
- Sustainable synthesis and characterization of zinc Oxide Nanoparticles using Raphanus sativus extract and its biomedical applications. Crystals (basel). 2022;12:1142.
- [CrossRef] [Google Scholar]
- Greener synthesis of zinc oxide nanoparticles: Characterization and multifaceted applications. Molecules. 2020;25:4198.
- [CrossRef] [Google Scholar]
- In vitro antibacterial activity of green synthesized silver nanoparticles using Mangifera indica aqueous leaf extract against multidrug-resistant pathogens. Antibiotics. 2022;11(11):1503.
- [CrossRef] [Google Scholar]
- Sustainable synthesis and characterization of zinc oxide nanoparticles using Raphanus sativus extract and its biomedical applications. Crystals. 2022;12
- [Google Scholar]
- Challenges associated with penetration of nanoparticles across cell and tissue barriers: a review of current status and future prospects. Nano Today. 2014;9:223-243.
- [CrossRef] [Google Scholar]
- Doped zinc oxide nanoparticles: synthesis, characterization and potential use in nanomedicine. Appl. Sci. (basel). 2020;10:5194.
- [CrossRef] [Google Scholar]
- Miniemulsions as chemical nanoreactors for the room temperature synthesis of inorganic crystalline nanostructures: ZnO colloids. J. Mater. Chem.. 2012;22:1620-1626.
- [CrossRef] [Google Scholar]
- Microwave assisted green synthesis of silver nanoparticles using leaf extract of elephantopus scaber and its environmental and biological applications. Artif. Cells Nanomed. Biotechnol.. 2018;46:795-804.
- [CrossRef] [Google Scholar]
- Non-antimicrobial and non-anticancer properties of ZnO nanoparticles biosynthesized using different plant parts of Bixa orellana. ACS Omega. 2022;7:1914-1933.
- [CrossRef] [Google Scholar]
- Facile and eco-friendly method for the synthesis of zinc oxide nanoparticles using Azadirachta and Emblica. Int. J. Pharm. Sci. Res. IJPSR 2014:2866-2873.
- [Google Scholar]
- Green synthesized ZnO nanoparticles against bacterial and fungal pathogens. Prog. Nat. Sci.. 2012;22:693-700.
- [CrossRef] [Google Scholar]
- Effective antimicrobial activity of green ZnO nano particles of Catharanthus roseus. Front. Microbiol.. 2018;9:2030.
- [CrossRef] [Google Scholar]
- P.771 Deciphering pharmacological mechanism of neurotrophic activity of steroidal chlorohydrin by proteomic, immunocytochemical and computational approach. Eur. Neuropsychopharmacol.. 2020;40:S436-S437.
- [CrossRef] [Google Scholar]
- Effect of CTABr (surfactant) on the kinetics of formation of silver nanoparticles by amla extract. J. Mol. Liq.. 2021;329:115537
- [CrossRef] [Google Scholar]
- Sucrose-mediated fast synthesis of zinc oxide nanoparticles for the photocatalytic degradation of organic pollutants in water. ACS Omega. 2019;4:6560-6572.
- [CrossRef] [Google Scholar]
- Green synthesis and characterization of zinc oxide nanoparticles using Cayratia pedata leaf extract. Biochem. Biophys. Rep.. 2021;26:100995
- [CrossRef] [Google Scholar]
- Utilization of nanocellulose fibers, nanocrystalline cellulose and bacterial cellulose in biomedical and pharmaceutical applications. Nanotechnol. Paper Wood Eng. 2022:409-470.
- [Google Scholar]
- A text book of Pharmacognosy (47th ed.). Pune, India: Nirali Prakashan Publication; 2011.
- Antibacterial activity of Bixa orellana L. (achiote) against Streptococcus mutans and Streptococcus sanguinis. Asian Pac. J. Trop. Biomed.. 2016;6:400-403.
- [CrossRef] [Google Scholar]
- Radical scavenging capacity, antibacterial activity, and quantum chemical aspects of the spectrophotometrically investigated iridium (III) complex with benzopyran derivative. Front. Pharmacol.. 2022;13:945323
- [CrossRef] [Google Scholar]
- Plant-based synthesis of gold nanoparticles and theranostic applications: a review. Molecules. 2022;27
- [CrossRef] [Google Scholar]
- Phytochemical screening of Bixa orellana and preliminary antidiabetic, antibacterial, antifibrinolytic, anthelmintic, antioxidant, and cytotoxic activity against lung cancer (A549) cell lines. J. King Saud Univ.-Sci.. 2023;35
- [Google Scholar]
- Antibacterial activity of Illicium verum essential oil against MRSA clinical isolates and determination of its phyto-chemical components. J. King Saud Univ. Sci.. 2022;34:101800
- [CrossRef] [Google Scholar]
- Enhanced bioactivity of ZnO nanoparticles-an antimicrobial study. Sci. Technol. Adv. Mater.. 2008;9:035004
- [CrossRef] [Google Scholar]
- Sunlight induced rapid and efficient biogenic synthesis and characterization of silver nanoparticles from leaf and dye extract of Bixa orellana L. A natural food dye plant. Pharma Innovation. 2019;8:73-80.
- [Google Scholar]
- Analysis of intraspecific diversity in two green capsuled accessions of Bixa orellana L., a FoodDye plant by single sequence repeat (SSR) markers. J. Sci. Res.. 2021;65:136-145.
- [CrossRef] [Google Scholar]
- Synthesis of zinc oxide nanoparticles using Rubus fairholmianus root extract and their activity against pathogenic bacteria. Molecules. 2021;26:3029.
- [CrossRef] [Google Scholar]
- Green synthesis of ZnO nanoparticles using Carica papaya leaf extracts for photocatalytic and photovoltaic applications. J. Mater. Sci.: Mater. Electron.. 2017;28:10374-10381.
- [CrossRef] [Google Scholar]
- Selective toxicity of zinc oxide nanoparticles to prokaryotic and eukaryotic systems. Appl. Phys. Lett.. 2007;90:2139021-2139023.
- [CrossRef] [Google Scholar]
- Characterization of bioactive compounds from Acacia concinna and Citrus limon, silver nanoparticles’ production by A. concinna extract, and their biological properties. Molecules. 2022;27:2715.
- [CrossRef] [Google Scholar]
- DNA damaging potential of zinc oxide nanoparticles in human epidermal cells. Toxicol. Lett.. 2009;185:211-218.
- [CrossRef] [Google Scholar]
- Difference in surface properties between Escherichia coli and Staphylococcus aureus as revealed by electrophoretic mobility measurements. Biophys. Chem.. 1995;55:273-277.
- [CrossRef] [Google Scholar]
- Photosynthesis of silver nanoparticles using medicinal and dye yielding plant of Bixa orellana L. leaf extract. J. Pharm. Sci. Innovation. 2013;2:9-13.
- [Google Scholar]
- Evaluation of green synthesized gold nanoparticles from abrus precatorius seeds for their antibacterial, anti-inflammatory, anti-proliferative and antidiabetic properties. Lat. Am. J. Pharm.. 2022;41:420-427.
- [Google Scholar]
- Green synthesis and characterization of zinc oxide nanoparticle using insulin plant (Costus Pictus D. Don) and investigation of its antimicrobial as well as anticancer activities. Adv. Nat. Nanosci. Nanotechnol. Sci. 2018
- [Google Scholar]
- Green synthesis of Zinc Oxide Nanoparticles by Calotropis gigantea. IJCET 2013:118-120.
- [Google Scholar]
- The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int. J. Nanomed.. 2017;12:1227-1249.
- [CrossRef] [Google Scholar]







