Translate this page into:
Environmentally friendly low-cost graphene oxide-cellulose nanocomposite filter for dye removal from water
⁎Corresponding author at: Office of the Dean (Research) & Division of Chemistry, Department of Science, Alliance College of Engineering and Design, Faculty of Science &Technology, Alliance University (Central Campus), Chikkahagade Cross, Chandapura-Anekal Main Road, Bengaluru, Karnataka 562 106, India. siva.chavali@alliance.edu.in (Murthy Chavali),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
This study primarily aims to develop a simple, cost-effective, eco-friendly nanocomposite filter for dye removal by adsorption. In this work, graphite oxide and graphene oxide were synthesized using a modified Hummers’ process and used to fabricate nanocomposite filters by co-precipitation reactions. Scanning electron microscopy and X-ray diffraction were performed on nanofillers revealing crystallographic and morphological properties of the nanofillers.
Methods
Furthermore, bio-based cellulose composite filters were fabricated using graphene oxide, graphite oxide, and graphene on the leaf of Ficus religiosa (L.) (i.e., Peepal leaf) in various concentrations and were studied for the adsorption of organic dye (i.e., methylene blue). Scanning electron microscopy was also performed on nano filters where a good dispersion of nanofillers in the composite was observed.
Results and Conclusions
The efficiency and capacity of these materials as filters were compared via column studies. These findings offer a direction for the development of an eco-friendly, efficient nano filter for the removal of organic dye from water. The composite containing graphene oxide and cellulose in the weight ratio of 4:1 (wt:wt) was significantly useful in combination with Peepal leaf as a filter to adsorb organic dyes from water.
Keywords
Graphene Oxide
Nanocomposites
Dye removal
Peepal Leaf
Bio-filter
1 Introduction
In the past few decades, rapid and aggressive industrialization and urbanization have led to the production of various toxic pollutants, both inorganic (e.g. Mercury, Lead), and organic (e.g. dyestuff wastewater (Dotto and Pinto, 2011), oil spills from sea transportation (Yang et al., 2014; Zhu et al., 2011), which have disturbed the balance of the aquatic ecosystem, seriously damaging environmental and human health. Industrial wastewater contains harmful contaminants or pollutants and needs further treatment required to remove them before discharge. For example, wastewater discharged from the textile industry contains harmful dyes such as Crystal Violet, Congo Red, and Methylene Blue. (Robinson et al., 2001; Mahmoodi and Arami, 2009) Dyes are coloured carcinogenic organic compounds or mixtures that may be used to give colour to a substrate and are often found in the releases from textile, rubber, plastic, printing, leather, and cosmetic industries, which are among the most exponentially growing industries. (Malik and Grohmann, 2012; Foroughi-Dahr et al., 2015) Among these methods, adsorption is a promising technique that is highly effective and widely applied due to its high efficiency and simplicity, specifically, many effective carbon-based nanocomposites have also been prepared for the adsorption of dyes in wastewater. (Rashed, 2013; Sadegh et al., 2017)
Various cellulose-based bio-filtration methods are applied to further enhance the adsorption capacity of adsorbents including large specific surface area fillers like graphite and graphene materials into biofilter networks could effectively increase the capacity of decontamination for dyes. (Feng et al., 2020; Balathanigaimani et al., 2009) Graphene nanoplatelets (GNP) contain sp (Yang et al., 2014)-hybridized carbon atoms that form a two-dimensional network structure that retains a C⚌C conjugated ring structure. Graphene oxide (GO) is one of the derivatives of GNP which possesses many functional groups of oxygen such as carboxylic acid, phenol, hydroxyl, and epoxide groups on the surface. (Park and Ruoff, 2009; Fan et al., 2008; Li and Kaner, 2008; Lerf et al., 1998; Chen et al., 2009; Dreyer et al., 2009) In addition to this, graphite oxide (GrO) is a relatively unexplored yet readily available and inexpensive material that can be modified into a novel material for low-cost water purification (Lerf et al., 1998; Gao et al., 2011) including radioactive waste (Romanchuk et al., 2013) and dyes (Rajabi et al., 2017; Cheng et al., 2013; Stoller et al., 2008) Furthermore, Graphene, GrO, and GO nanofiller are liable to develop the 3D network structure in polymer composites. Several researchers have reported that a 3D network structure in the composite can demonstrate excellent adsorption capacity towards cationic dyes by controlling the porous structures and stability properties of the nanocomposite. (Feng et al., 2020) Since dye adsorption activity exists predominantly occurs on the inter-molecular level of the adsorbent and dye which means the molecular interaction between them may greatly influence the adsorption property of the composite. Over the past years, GO as an adsorbent material to remove several types of pollutants from wastewater has attracted excessive attention due to its combined advantages and other promising properties such as high surface area, oxygenated functional groups, strong affinity with dye molecules (Rajabi et al., 2017), and excellent thermal stability. (Ersan et al., 2017; Zhang et al., 2011; Yan et al., 2015) Previously, several researchers have reported the adsorption mechanism that the decontamination of dyes from aqueous solution could be driven by hydrogen bonding, electrostatic force, or others in the adsorption process. (Wei et al., 2015; Ahmad et al., 2019; Yu et al., 2017; Self-Assembly, 2021; Travlou et al., 2013) However, the adsorption mechanism of graphene, GrO and GO nanofiller on different organic dyes still lacks systematic study due to complexities such as the type of reagents used in the process, its microstructure, and so on of both dye and adsorbent. Furthermore, cellulose is the most abundant biopolymer available in nature. (Holtzapple, 2003; Khandelwal and Windle, 2013) Generally, cellulose has been used widely as a raw material for the production of biocompatible and biodegradable materials. Cellulose sources include paper, cotton, wood, agricultural residues, and other plant or plant-derived substances that are abundantly available. (Lavanya et al., 2011) This provides an answer to the rising need for biodegradable and environment-friendly materials and also a solution for waste management.
Ficus religiosa (L.) (sacred fig, see Fig. 1) is a large perennial tree found throughout the plains of India up to 170 m altitudes in the Himalayas. It belongs to the Moraceae family or Mulberry family (Ficus religiosa - Peepal https://web.archive.org/web/, 2021), and its medicinal leaves contain phytochemicals like flavonoids, terpenoids, and tannins. (Bhalerao, 2014) Though literature suggest on heavy metal removal using peepal leaves there is no literature on dye removal. (Zaheer Aslam et al., 2010)
Leaf of Ficus religiosa (L.), its taxonomy and selective vernacular names.
Literature studies reveal the usefulness of graphene-based composites of Graphene with metal oxides (MOx), carbon derivatives, metal hybrids, and polymers for the removal of organic dyes from contaminated water. (Rouhi et al., 2019; Apul et al., 2013) Graphene oxide and cellulose are generally used for applications like flexible conductive films, but there is immense potential in the application in the field of wastewater purification as they are inexpensive, effective, and in an eco-friendly especially for toxic dyes. (Tian et al., 2019; Lv et al., 2018)
In this manuscript, the authors focus on creating enhanced graphene oxide-cellulose (GO-C) composite materials based on peepal leaf for application as a simple and low-cost filter for the removal of methylene blue dye (the most popular dye used in cotton, wool, and silk dying). Besides, the concept of introducing a leaf skeleton to a filter has been presented, which may serve as a model for the preparation of biodegradable filter materials in the future. The microstructure of the prepared composites with varying weight ratios of GO and C (4:1, 2:1, 1:1, 1:2, 1:4) were characterized using XRD and SEM and their efficiency and capacity as filter materials have also been compared via column studies.
2 Experimental
2.1 Materials
Graphite (99%), 10% v/v Sulphuric acid (H2SO4), Nitric acid (HNO3), Potassium permanganate (KMnO4), Copper Sulfate (CuSO4), Sodium Hydroxide (NaOH), Ammonium Hydroxide (NH4OH, 30%), Sodium Carbonate (Na2CO3), Methylene Blue (C16H18ClN3S) were obtained from Sigma Aldrich (India). All reagents were of AR grade ≥ 98.5% and used as received without further purification. PTFE filter paper was used. Standard cotton was used as a source of cellulose and a Peepal leaf was procured from a healthy tree. Double distilled water was used throughout the work.
2.2 Methods
2.2.1 Synthesis of graphene oxide (GO) sheets
A novel modified Hummers’ process was used to synthesize graphene oxide. 130 mL H2SO4 and 6 mL HNO3 were added to 6 g of graphite in a round bottom flask and stirred continuously using a magnetic stirrer. The entire system was kept in an ice bath until the temperature reached about 5–10 °C. Then 18 g KMnO4 was gradually added such that the temperature remained constant. The mixture was stirred continuously at room temperature for 24 h. Then 140 mL double distilled water (DDW) was added while maintaining the temperature below 80 °C. After 15 min, 450 mL DDW was added and filtered. To this 200 mL of 20% HNO3 was added, and the mixture was heated at a constant temperature of 80 °C for 30 min. The suspension then turned brilliant yellow and was immediately washed and centrifuged at 7000 rpm. The settled semi-solid was sequentially washed with 30 vol% HCl, DI water, and ethanol until the washing solution became neutral. The settled semi-solid was transferred to a container and oven-dried for about 18 h at 60 °C. The obtained product was graphite oxide which was then thoroughly scraped out, and roughly ground in a mortar pestle. Then it was exfoliated by heating in an RB flask with a gas outlet pipe at 300 °C, 1 atm pressure) to get graphene oxide (nanosheets).
2.2.2 Preparation of graphene oxide-cellulose nanocomposites
The samples prepared in various compositions as given in Table 1 were used for the removal of methylene blue dye. Graphene oxide Graphite oxide Graphene oxide and Cellulose Graphene oxide and Cellulose Graphene oxide and Cellulose Graphene oxide and Cellulose Graphene oxide and Cellulose Bare Peepal Leaf
S. No. Material
Composition
Ratio (w/w)
–
Pure
–
Pure
0.05 g + 0.05 g
1:1
0.1 g + 0.05 g
2:1
0.2 g + 0.05 g
4:1
0.05 g + 0.1 g
1:2
0.05 g + 0.2 g
1:4
–
–
2.2.3 Graphene oxide-cellulose nanocomposite (GO-C NC) preparation
Solutions of 2.5 g CuSO4 in 250 mL H2O and 0.8 g NaOH in 7.5 mL H2O were prepared. Then these solutions were mixed after complete individual dissolution, and the mixture was allowed to stand to precipitate Cu(OH)2. Then this precipitate was washed, and about 48–50 mL 30% NH4OH was added while stirring to dissolve it completely. Now (depending upon the composite to make) 0.05 g cotton (as a source of cellulose) was added to the mix in small portions periodically to ensure complete dissolution. Then the required amount of graphene oxide (here 0.05 g, for 1:1 composition) was added to the mixture to get the graphene oxide-cellulose nanocomposites. Next, this solution was allowed to form fibrous aggregates with 200 mL of H2SO4 solution, it was filtered and removed as the desired product.
2.2.4 Preparation of the peepal leaf filters
Fresh Peepal leaves were collected and cleaned with water to remove any dirt. A 10 wt% solution of washing soda (Na2CO3) and double distilled water was prepared and the leaves were boiled in it for 6 h. They were then taken out and washed with double distilled water, after which a soft brush was used to gently rub and remove the chlorophyll to leave only the leaf skeleton, which was later left to dry (see Fig. 2(a)).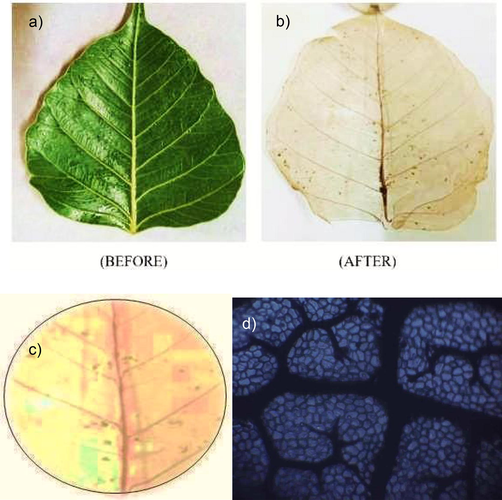
(a) Peepal Leaf surface preparation (b) Dried peepal leaf (c) Dried peepal leaf cut to fit the size of silica crucible (c) Optical microscopy of porous prepared peepal leaf.
2.2.5 Peepal leaf surface preparation
Optical microscopy was performed on the leaf as shown in Fig. 2(c), to view its microstructure, and confirm the sub-hypothesis that the peepal leaf provides a porous base for the substrate to be loaded. A slice of the dried peepal leaf of the size of silica crucible was cut out as shown in Fig. 2(c), to be used as the base. A probe-sonicated solution of 0.05 g of each sample in 5 mL of distilled water was made. The leaf was put in the crucible, and the solution was poured on the assembly, and it was left to dry.
2.2.6 Characterization
The crystalline properties of Graphite oxide, Graphene oxide, bare peepal leaf, synthesized GO-C NC Peepal leaf were recorded using a Rigaku Miniflex X-ray diffractometer. XRD analysis was carried out using CuKᾳ radiation (λ = 1.5406 nm) at 40 kV voltage and 30 mA current over a 2θ angle range of 5° to 55° at a scan rate of 2°/min. The microstructures of nanoparticles were recorded using a scanning electron microscope (FEI-APREO, Thermo Fisher). 58 The chemical composition of Graphite oxide, Graphene oxide and C-GO NC were determined from the energy dispersive spectrum (EDS) recorded. A Perkin Elmer LAMBDA 650 Series spectrophotometer was used for analyzing the filtrate collected via UV–vis spectroscopy in the range of 190 nm to 900 nm. Filter capacity and capability testing was done via the column method, and the column design is constructed as shown in Fig. 3. The filter material of 0.1 g being investigated was packed into a column with an inner diameter of 15 mm and an overall height of 75 cm. The filter material was packed below and above with cotton (0.05 g) to prevent loss of material during the water flow.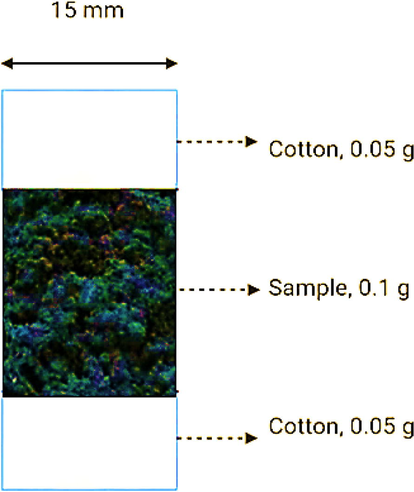
Basic column design.
3 Results and discussion
3.1 Xrd
To investigate the crystalline structures, X-ray diffraction (XRD) was performed on the graphite oxide, graphene oxide, bare peepal leaf, synthesized graphene oxide cellulose nanocomposite, in the range of 2θ from 5° to 55° and results were shown in Fig. 4.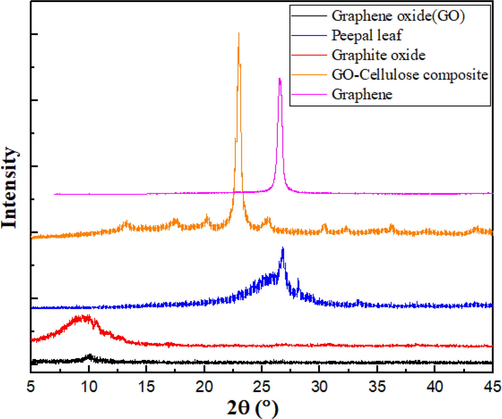
XRD patterns for Graphene, Graphite oxide, Graphene oxide, Bare peepal leaf, and Graphene oxide cellulose composite samples.
The XRD data for Graphene shows a single significant diffraction peak at 2θ = 26.54° (d = 0.336 nm), which corresponds to the (002) plane. It can also be observed that the intensity of the prominent XRD peak at 10.66° sharply decreased in the case of graphite oxide (GrO). This is because after the oxidation of Graphite to Graphite oxide, the diffraction peak shifts from 2θ = 26° (d = 0.34 nm) 60 to 2θ = 10.66° (d = 0.83 nm), reflecting a significant increase in the inter-planar distance due to the addition of oxygenated functional groups. The absence of excessive peak broadening of the characteristic peak of Graphite shows that the stacking was well ordered. Similar observations were reported in the literature.
After oxidation/exfoliation of graphite oxide to graphene oxide, these peaks are shifted to smaller angles; here, to a 2θ value of 10.2°, which corresponds to d = 0.87 nm using Bragg’s Law. This increased interplanar distance is due to a further introduction of additional oxygen moieties and oxygenated groups on the GO surface. Similar observations were reported in the literature. In addition to this, the disappearance of the sharp peak in graphene oxide can be attributed to the exfoliation of layered structures of graphene oxide. The broader peak may stem from the partial restacking of exfoliated graphene oxide layers, which effectively prevented the restacking of the graphene layers. The XRD patterns of peepal leaf were shown in Fig. 4. It was demonstrated that the prominent peak was observed at a 2θ angle of 26.8° and another peak at a 2θ angle of 28.1°. The peepal leaf was observed to be semi-crystalline in structure. It can be suggested that the lower crystal size structure tends to absorb more water than the higher crystal size structures. In all cases, more intense peaks indicate a more crystalline structure for the sample in question, implying that the GO-C composite was the most crystalline, followed by Graphene, Peepal leaf, Graphite oxide, and Graphene oxide.
3.2 Sem
The surface morphologies of graphite oxide and graphene oxide samples were characterized using a scanning electron microscope (SEM) and the figures were shown in Fig. 5(a and b) respectively.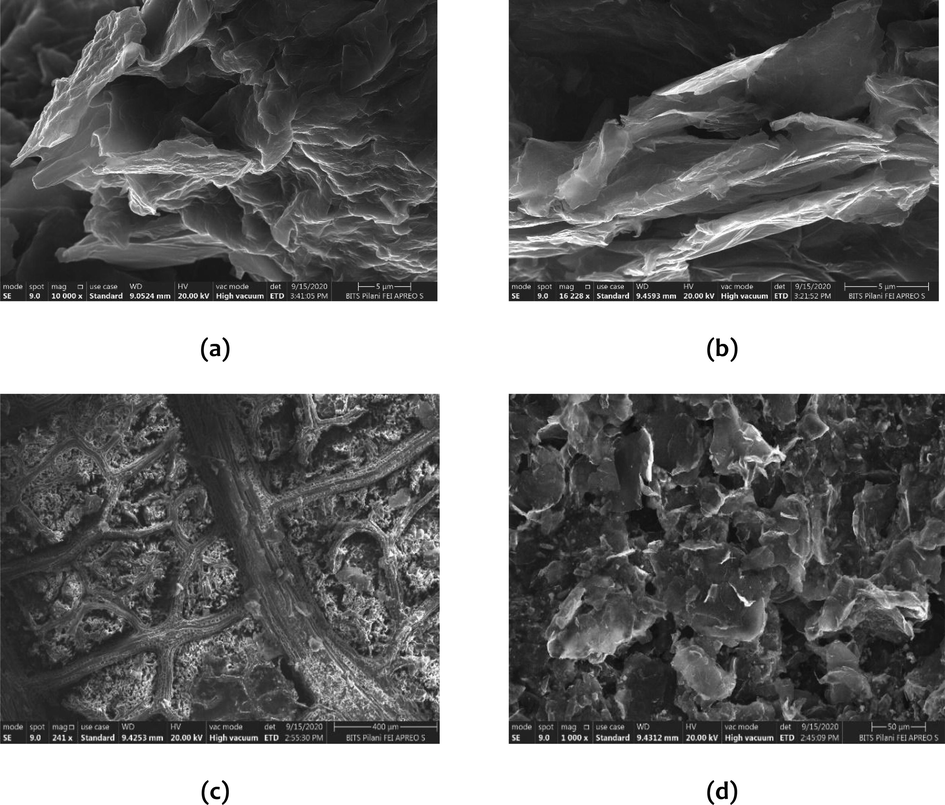
SEM images of (a) Graphite oxide, (b) Graphene oxide, (c) Graphene oxide cellulose composite on the leaf at the center, (d) Graphene oxide cellulose composite on the leaf at the edge.
In Fig. 5(a), the SEM image of GrO exhibits its standard layered structure, identical to graphite, which is due to the addition of a huge number of oxygen-containing groups, resulting in an increase in the interlayer spacing and additionally making the atomic-thick layers hydrophilic. In Fig. 5(b), the SEM image of GO, the creased overlapping exfoliated sheets containing only one or a few layers of carbon atoms like graphene can be observed. The particulate structure and an irregular, wrinkled surface of GO are observed as well. This may indicate a large number of “defective” adsorption sites favorable for capturing dye molecules. Similar observations were reported in the literature. (Feng et al., 2020) Furthermore, to analyze the surface morphology in the graphene oxide cellulose composite, SEM was performed. The GO nanofillers were uniformly distributed in the cotton fibers and confirmed the completion of preparation. The optical microscope image of the bare leaf shown in Fig. 5(c) was favorable results in that the surface was almost free of any leaf blade remnants and the porous leaf support structure was intact, and not degraded by the use of chemicals. This further established the usage of peepal leaf as appropriate support for the composite. The microstructure of the GO-cellulose composite deposited on the leaf at the center and the edge were characterized using SEM was shown in Fig. 5(c and d) respectively. In Fig. 5(c), the midrib and the porous structure of the treated leaf can be seen, and the composite is loaded on it uniformly. In Fig. 5(b), the layered nature of the leaf blade at the edge is seen, and the expectancy of uniform and proper embedding of the composite is achieved and further confirmed.
Fig. 5(c and d) reveals the interaction between graphene oxide and cellulose, and the network structure of GO cellulose composite loaded on the peepal leaf. This microstructure of GO cellulose composite loaded on the peepal leaf can help to exhibit better adsorption efficiency than its parental ingredients due to this system’s observed rough surface, increased porosity and enhanced surface area. The SEM images of GO cellulose composite-leaf samples were shown that the incorporation of GO did not create significant morphological changes in the surface roughness; composite film surfaces were similarly homogeneous and smooth. Nonetheless, smoothness observed in the SEM images indicates the GO sheets were uniformly dispersed, demonstrating films with high homogeneity without aggregation. Therefore, the GO oxygenated groups might be interacting with fibres of cellulose in the filter can provide good compatibility between the GO filler and the composite.
3.3 Column study and UV spectroscopy
For analysing the capabilities of water filtration of the various materials, dye removal tests were performed at set wavelengths. The column studies performed as batch experiments. On analyzing a pure MB sample to ascertain the reference value of absorbance to be subtracted from the data points observed for filtrates from different samples, the characteristic peak wavelengths values in order of decreasing absorbance were observed at 663.71 nm (1.3 A), 291.51 nm (0.8 A), and 245.45 nm (0.5 A). The λmax, or the most significant peak, for MB, was observed at 663.71 nm, and dye removal tests were performed at this set wavelength to check the water filtration and dye removal capabilities of the various materials. The molecular structure and UV-Vis spectrum of MB are shown in Fig. 6(b).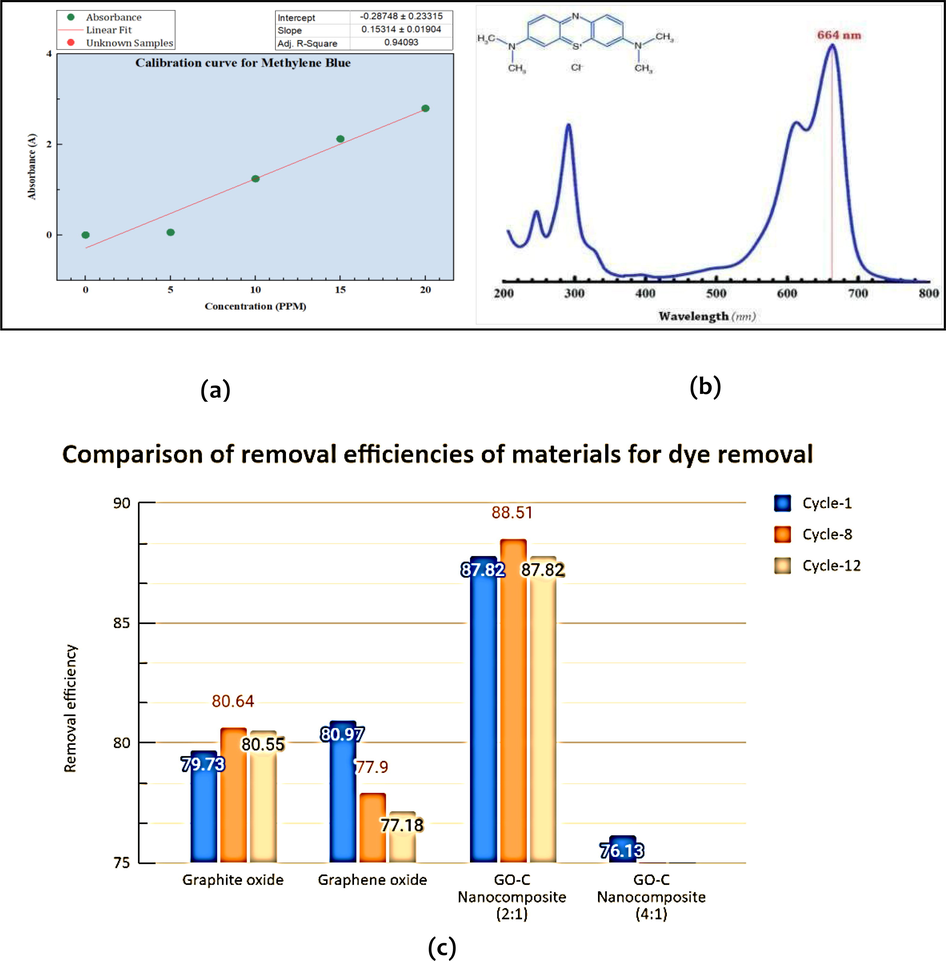
(a) Calibration curve of Methylene Blue (MB) dye, (b) Molecular formula and UV–vis spectrum of methylene blue with λmax at 663.71 nm, (c) Comparison of removal efficiencies of all the materials used for dye removal via column studies.
In the pure MB sample, the characteristic peak wavelength values in order of decreasing absorbance were observed at 663.71 nm (1.3 A), 291.51 nm (0.8 A), and 245.45 nm (0.5 A). The λmax, or the most significant peak, for MB, was observed at 663.71 nm. The molecular formula and UV–vis spectrum of methylene blue are shown in Fig. 6(b). Initially, the absorbance of methylene blue with concentrations 10 ppm and 20 ppm was investigated by passing 15 mL of solution (per cycle) through the column and subsequent UV–vis spectrophotometer measurement at λmax = 663.71 nm for graphene oxide, graphite oxide, and graphene oxide-cellulose composites (1:1, 2:1, 4:1, 1:2, 1:4 wt%. graphene oxide-cellulose ratios); this was termed ‘Cycle-1′. This procedure was repeated till 12 cycles, or the system got choked, i.e. there was no filtrate- whichever occurred last.
Furthermore, MB solutions of different concentrations were treated through the columns in batches of 15 mL, the time taken for the solutions to filter out completely was noted. The resulting filtrates were analysed using UV-vis spectroscopy to obtain the absorbance, and consequently, the concentration, at 663.71 nm. Double distilled water was used as blank and the absorbance value of double distilled water was subtracted from all the recorded values for the samples. A calibration plot was constructed for MB to convert the absorbance into the concentration values for easier and more practical analysis using the Beer-Lambert Law as shown in Eq. (1) below: where α is the absorbance, k is the proportionality constant, c is the concentration of the sample being analysed, and l (cm) is the length of the path (width of the cuvette between the two translucent sides).
The formula used to convert absorbance to concentration in this paper is:
Since slope = k * l and R = y-intercept of the calibration curve’s trendline with an R2 (regression coefficient) value of (Fig. 6(a)).
The regression coefficient R2 was 0.9403 as shown in Fig. 6(a). To observe dye removal using bare peepal leaf, graphite oxide loaded peepal leaf, graphene oxide loaded peepal leaf, and graphite oxide cellulose nanocomposite loaded on peepal leaf filters, a silica crucible was placed on a conical flask, and the filter material was loaded. Subsequently, 15 mL of MB was transferred in regular intervals of 5 mL each to avoid overflow owing to the small size of the crucible. From Fig. 6(c), it can be understood that the filter choked out after 12 cycles (filtrations) (13th in series). During the last cycle (12th) for 2:1 graphene oxide-cellulose nanocomposite material, it was found that 10 ppm of MB was reduced by 80.76% based on filtrate absorption studies. Overall, the composite materials of graphene oxide, graphite oxide, graphene oxide and cellulose (1:1, 2:1, 4:1, 1:2, 1:4 wt ratios), bare peepal leaf), the absorbance decreased slowly. Graphite oxide was very effective with lower absorbance than graphene oxide till the eighth cycle. Since GrO and GO are negatively charged adsorbents (Yu et al., 2017), it exhibits an especially high affinity for cationic dyes like MB due to the strong electrostatic attraction observed between them. (Self-Assembly, 2021) Because of these advantages, GO (and GrO) has proven to be a promising adsorbent for the removal of cationic dyes like MB from water. Furthermore, there is no leaching of MB despite the number of cycles of filtration; only the choking of the bed was a hindrance which is to be expected while dealing with nanomaterials due to their small size, the tendency of aggregation, and the resulting lower porosity. This non-leaching of dye could prove to be a benefit of using bio-nano composites for water filtration.
During the last cycle (12th) for the synthesized 2:1 GO-C (Graphene oxide-cellulose) nanocomposite material, it was found that 10 ppm of MB was reduced by 87.82% based on the observed filtrate absorbance. For the 1:4 GO-C NC Peepal leaf composite, the removal efficiency was 80.17%, and comparable to standard adsorption materials like Graphene oxide and Graphite oxide. For all the materials analyzed (graphene oxide, graphite oxide, graphene oxide, and cellulose (1:1, 2:1, 4:1, 1:2, 1:4 wt ratios), bare peepal leaf), the adsorption removal efficiency decreased very gradually with subsequent cycles. This is because the number of adsorption sites left decreases as the number of MB molecules adsorbed on the material keeps increasing. According to the average values throughout the 12 cycles, the decreasing order of average efficiency across cycles and purification performance was:
GO-Peepal (80.93%) > Graphite oxide (80.82%) > 2:1 GO-C NC (80.76%) > 1:4 GO-C + Peepal (80.17%) > Graphene oxide (79.61%) > 4:1 GO-C + Peepal (73.85%) > 1:2 GO-C (68.48%) > Bare Peepal leaf (41.44%).
Fig. 6(c) shows that the filter choked out after 12 cycles (filtrations) (13th in series). Additionally, no leaching of MB was observed despite the number of cycles of filtration; only the choking of the bed was a hindrance- which is to be expected while dealing with nanomaterials due to their small size, the tendency of aggregation, and the resulting lower porosity. This non-leaching of dye could prove to be a benefit of using (bio-) nanocomposites for water filtration. Finally, noticed that the GO-C composites have comparable removal efficiencies to traditionally used and well-developed and commercialized materials like GrO and GO.
4 Conclusions
In this manuscript, the authors prepared novel filtration materials: enhanced graphene oxide-cellulose and graphite oxide-cellulose (GO-C) composites combined with a peepal leaf filter to be used as simple and low-cost resources for the removal of methylene blue dye from water, and potential for wider water purification. The novel concept of introducing a porous Peepal leaf as a filter ‘substrate’ for loading the prepared GO-C composite was presented, which may serve as a model for the preparation of biodegradable filter materials in the future. Additionally, cotton (cellulose) was used here as an effective loading substrate for graphene oxide (GO) to make a graphene oxide-cellulose nanocomposite (GO-C NC) to avoid leakage of both the GO solution and dye solution. These included XRD, SEM, EDS, Optical Microscopy, and UV-Vis spectroscopy, and were completed for all five samples: Graphite oxide, Graphene oxide, bare peepal leaf, synthesized C-GO NC, and the GO-C NC Peepal leaf. The efficiency and capacity of the composite versus the parent materials (graphite oxide and graphene oxide) as filters were evaluated via column studies. According to these, GO-C NC membranes were found to be efficient at removing dye from water to an exceptional level in the laboratory, showing a reduction efficiency of 99.41% even after 12 cycles. In comparison with different materials, graphene oxide-cellulose nanocomposite (GO-C NC) loaded Peepal Leaf was found to be the best low-cost filter in terms of efficiency, effectiveness, and reusability (indicated by the fact that repeated cycles still produced clear filtrate). The order of effectiveness among all filtration materials was observed to be: GO-Peepal (80.93%) > Graphite oxide (80.82%) > 2:1 GO-C NC (80.76%) > 1:4 GO-C + Peepal (80.17%) > Graphene oxide (79.61%) > 4:1 GO-C + Peepal (73.85%) > 1:2 GO-C (68.48%) > Bare Peepal leaf (41.44%). Graphite oxide-loaded Peepal leaf showed exceptional water cleansing potential, with graphene oxide showing the approximately same value of reduction efficiency. Further, a more detailed analysis is required for a concrete establishment of the feasibility of peepal leaf and graphene oxide-based materials. Results are attributed to since GO is a negatively charged adsorbent (Yu et al., 2017), it exhibits an especially high affinity for cationic dyes like Methylene Blue (MB) (Self-Assembly, 2021) due to the strong electrostatic attraction observed between them. Because of these advantages, GO (and GrO) has proven to be a promising adsorbent for the removal of cationic dyes like MB from water.
Acknowledgements
The authors would like to thank the staff of the Centre for Fire, Explosive and Environment Safety (CFEES), Defence Research and Development Organisation (DRDO), Ministry of Defence, Timarpur, Delhi, and Dr. Bharti Moni, Scientist ‘D’ for their help in preliminary experimental work. The authors also acknowledge the support of Taif University Researchers Supporting Project number (TURSP-2020/123), Taif University, Taif, Saudi Arabia.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Murthy CHAVALI reports financial support was provided by Alliance University. Murthy Chavali reports a relationship with Taif University that includes: funding grants.
References
- Adsorption of Food Dyes Acid Blue 9 and Food Yellow 3 onto Chitosan: Stirring Rate Effect in Kinetics and Mechanism. J. Hazard. Mater.. 2011;187(1):164-170.
- [CrossRef] [Google Scholar]
- Magnetic Graphene Foam for Efficient Adsorption of Oil and Organic Solvents. J. Colloid Interface Sci.. 2014;430:337-344.
- [CrossRef] [Google Scholar]
- Zhu, S.; An, W.; Li, G.; Zhao, Y.; Niu, Z.; You, X. R&D on Oil Spill Emergency Decision Support System. In Computer Science for Environmental Engineering and EcoInformatics; Yu, Y., Yu, Z., Zhao, J., Eds.; Communications in Computer and Information Science; Springer: Berlin, Heidelberg, 2011; pp 180–186. https://doi.org/10.1007/978-3-642-22694-6_25.
- Remediation of Dyes in Textile Effluent: A Critical Review on Current Treatment Technologies with a Proposed Alternative. Bioresour. Technol.. 2001;77(3):247-255.
- [Google Scholar]
- Degradation and Toxicity Reduction of Textile Wastewater Using Immobilized Titania Nanophotocatalysis. J. Photochem. Photobiol. B. 2009;94(1):20-24.
- [CrossRef] [Google Scholar]
- Malik A., Grohmann E., eds. Environmental Protection Strategies for Sustainable Development. Dordrecht: Springer Netherlands; 2012.
- Adsorption Characteristics of Congo Red from Aqueous Solution onto Tea Waste. Chemical Engineering Communications. 2015;202(2):181-193.
- [Google Scholar]
- Adsorption Technique for the Removal of Organic Pollutants from Water and Wastewater. Org. Pollut. – Monit. Risk Treat. 2013
- [CrossRef] [Google Scholar]
- The Role of Nanomaterials as Effective Adsorbents and Their Applications in Wastewater Treatment. J. Nanostructure Chem.. 2017;7(1):1-14.
- [CrossRef] [Google Scholar]
- Graphene/Waste-Newspaper Cellulose Composite Aerogels with Selective Adsorption of Organic Dyes: Preparation, Characterization, and Adsorption Mechanism. New J. Chem.. 2020;44(6):2256-2267.
- [CrossRef] [Google Scholar]
- Adsorption of Methane on Novel Corn Grain-Based Carbon Monoliths. Microporous Mesoporous Mater.. 2009;119(1):47-52.
- [CrossRef] [Google Scholar]
- Chemical Methods for the Production of Graphenes. Nat. Nanotechnol.. 2009;4(4):217-224.
- [CrossRef] [Google Scholar]
- Deoxygenation of Exfoliated Graphite Oxide under Alkaline Conditions: A Green Route to Graphene Preparation. Adv. Mater.. 2008;20(23):4490-4493.
- [CrossRef] [Google Scholar]
- Materials Science: Graphene-Based Materials. Science. 2008;320(5880):1170-1171.
- [CrossRef] [Google Scholar]
- Self-Assembled Free-Standing Graphite Oxide Membrane. Adv. Mater.. 2009;21(29):3007-3011.
- [CrossRef] [Google Scholar]
- Engineered Graphite Oxide Materials for Application in Water Purification. ACS Appl. Mater. Interfaces. 2011;3(6):1821-1826.
- [Google Scholar]
- Graphene Oxide for Effective Radionuclide Removal. Phys. Chem. Chem. Phys.. 2013;15(7):2321-2327.
- [CrossRef] [Google Scholar]
- Removal of Dye Molecules from Aqueous Solution by Carbon Nanotubes and Carbon Nanotube Functional Groups: Critical Review. RSC Adv.. 2017;7(74):47083-47090.
- [CrossRef] [Google Scholar]
- Toward 3D Graphene Oxide Gels Based Adsorbents for High-Efficient Water Treatment via the Promotion of Biopolymers. J. Hazard. Mater.. 2013;263:467-478.
- [Google Scholar]
- Adsorption of Organic Contaminants by Graphene Nanosheets: A Review. Water Res.. 2017;126:385-398.
- [CrossRef] [Google Scholar]
- Fast and Considerable Adsorption of Methylene Blue Dye onto Graphene Oxide. Bull. Environ. Contam. Toxicol.. 2011;87(1):86.
- [CrossRef] [Google Scholar]
- Influence of the Surface Structure of Graphene Oxide on the Adsorption of Aromatic Organic Compounds from Water. ACS Appl. Mater. Interfaces. 2015;7(12):6690-6697.
- [CrossRef] [Google Scholar]
- Selective Adsorption and Separation of Dyes from an Aqueous Solution on Organic-Inorganic Hybrid Cyclomatrix Polyphosphazene Sub micro-Spheres. J. Mater. Chem. A. 2015;3(8):4314-4322.
- [CrossRef] [Google Scholar]
- Neodymium Embedded Ordered Mesoporous Carbon (OMC) for Enhanced Adsorption of Sunset Yellow: Characterizations, Adsorption Study and Adsorption Mechanism. Chem. Eng. J.. 2019;359:814-826.
- [CrossRef] [Google Scholar]
- KOH-Activated Carbon Aerogels Derived from Sodium Carboxymethyl Cellulose for High-Performance Supercapacitors and Dye Adsorption. Chem. Eng. J.. 2017;310:300-306.
- [CrossRef] [Google Scholar]
- Three-Dimensional Self-Assembly of Graphene Oxide and DNA into Multifunctional Hydrogels | ACS Nano https://pubs.acs.org/doi/10.1021/nn1027104 (accessed Feb 2, 2021).
- Graphite Oxide/Chitosan Composite for Reactive Dye Removal. Chem. Eng. J.. 2013;217:256-265.
- [CrossRef] [Google Scholar]
- Holtzapple, M. T. Cellulose. In Encyclopedia of Food Sciences and Nutrition (Second Edition); Caballero, B., Ed.; Academic Press: Oxford, 2003. 998–1007. https://doi.org/10.1016/B0-12-227055-X/00185-1.
- Hierarchical Organisation in the Most Abundant Biopolymer-Cellulose. MRS Online Proc. Libr. OPL 2013:1504.
- [CrossRef] [Google Scholar]
- Sources of Cellulose and Their Applications- A Review. Int. J. Drug Formul. Res.. 2011;2:19-38.
- [Google Scholar]
- Ficus religiosa – Peepal https://web.archive.org/web/20120214150730/http://www.flowersofindia.in/catalog/slides/Peepal.html (accessed Jan 28, 2021).
- Bhalerao, S. A.; Sharma, A. S. Ethnomedicinal. Phytochemical and Pharmacological Profile of Ficus Religiosa Roxb. 2014. 11.
- Ni(II) removal by biosorption using Ficus religiosa (Peepal) leaves. J. Chil. Chem. Soc.. 2010;55(1):81-84.
- [CrossRef] [Google Scholar]
- Novel Carbon Based Bioactive Nanocomposites of Aniline/Indole Copolymer for Removal of Cationic Dyes from Aqueous Solution: Kinetics and Isotherms. New J. Chem.. 2019;43(5):2400-2410.
- [CrossRef] [Google Scholar]
- Adsorption of Aromatic Organic Contaminants by Graphene Nanosheets: Comparison with Carbon Nanotubes and Activated Carbon. Water Res.. 2013;47(4):1648-1654.
- [CrossRef] [Google Scholar]
- Preparation of Cellulose/Graphene Oxide Composite Membranes and Their Application in Removing Organic Contaminants in Wastewater. J. Nanosci. Nanotechnol.. 2019;19(4):2147-2153.
- [CrossRef] [Google Scholar]
- Graphene Oxide-Cellulose Nanocrystal (GO-CNC) Composite Functionalized PVDF Membrane with Improved Antifouling Performance in MBR: Behavior and Mechanism. Chem. Eng. J.. 2018;352:765-773.
- [CrossRef] [Google Scholar]







