Translate this page into:
Enhancing plant growth under municipal wastewater irrigation by plant growth promoting rhizospheric Bacillus spp
⁎Corresponding author. siriruk@g.swu.ac.th (Siriruk Sarawaneeyaruk)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Wastewater treatment is often addressed with phytoremediation. However, excessive amount of salts in wastewater can cause toxicity in plants. To mitigate the problem, plant growth-promoting rhizobacteria (PGPR) can be used to promote plant growth under this stressful condition. This research aimed to screen PGPR for their application in enhancing plant growth under municipal wastewater irrigation and to identify their characteristics. We isolated PGPR from soil contaminated with municipal wastewater and from weeds grown around solid waste dump. The isolates were tested in vitro for their plant growth promotion characteristics such as phosphate solubilization, auxin phytohormone and siderophore production. The isolates showing high ability of siderophore production were chosen and tested for plant growth promotion in vivo. Inoculation of certain isolates, including Bacillus amyloliquefaciens isolate WE15, to Chinese kale increased its root and shoot weight when it was irrigated with sterile tap water or municipal wastewater containing high concentration of ammonium, magnesium, and iron ions. Additionally, Bacillus firmus isolate WD19 reduces iron ions in culture medium. Thus, B. amyloliquefaciens isolate WE15 and B. firmus isolate WD19 are potential candidates for phytoremediation enhancement of iron ions contaminated soil.
Keywords
Bacillus
Rhizobacteria
Siderophore
Wastewater
Phytoremediation
1 Introduction
Inhabiting around plant roots, plant growth-promoting rhizobacteria (PGPR) are known to promote plant growth because of their inherent characteristics, such as siderophore and phytohormone production, nitrogen fixation, or phosphate solubilization. Certain PGPR inadvertently reduce soil toxicity around plant roots by producing siderophore as a high affinity Fe3+ chelating compound. Moreover, because of the ability of siderophores to bind Mg2+, Mn2+, Cd2+, Cu2+, As3+, Pb2+, and Zn2+, these PGPR also protect plants from being affected by toxic heavy metals (Gamalero and Glick, 2011). In addition to protection from heavy metals, phytohormone such as indole acetic acid (IAA) produced by PGPR stimulates root growth. Some PGPR supply the plant with nutrients, for example, nitrogen-fixing bacteria that convert N2 into NH3, which can be used by plants as a nitrogen source. Some PGPR solubilize phosphate from either organic or inorganic bound phosphates (Lugtenberg and Kamilova, 2009). Furthermore, PGPR can be used for phytoremediation enhancement. Phytoremediation is a process of reducing pollution in soil and water by using plants. Usually, contaminated soil reduces plant growth due to abiotic stress. However, some PGPR can produce 1-aminocyclopropane-1-carboxylate (ACC) deaminase to lower plant ethylene levels under stress. Bacillus sp., which is tolerant to Pb2+ and As3+, produces ACC deaminase that induces germination, increases plant growth, and reduces toxicity in rice (Pandey et al., 2013).
Municipal wastewater is a global issue for big cities. Feces, urine, fat, soap, synthetic detergents, as well as potential toxic elements such as Zn2+, Cu2+, Pb2+, Cd2+, and As3+, are usually found in wastewater (Pescod, 1992). Some of these elements are required for plant growth, but high concentration of the elements in municipal wastewater becomes a hindrance (Pescod, 1992). Phytoremediation can be applied for municipal wastewater treatment (Ijas et al., 2015, 2016). Brassica species are shown to accumulate metals well and often used in the context of phytoremediation (Mourato et al., 2015). Sarawaneeyaruk et al. (2014) found that the use of municipal wastewater for irrigation reduced growth of Brassica alboglabra (Chinese kale) and the number of its rhizobacteria. To remedy the situation, exploiting PGPR is a potential solution to improve plant growth by inoculating into plant roots. Complementary to this line of research, a review by Khan et al. (2013) shed light on how plant-bacteria partnerships can be applied for mitigating environmental pollution. Another supporting evidence is found in Khan et al. (2015). Specifically, Cr resistant endophytic and rhizosphereric bacteria from Prosopis juliflora improved plant growth and the remediation of heavy metal contaminated soil. In this study, we screen PGPR that are capable of enhancing plant growth under municipal wastewater irrigation, and identify their characteristics for plant growth enhancement. The municipal wastewater was sampled from Saen Saeb Canal at the middle area of Bangkok, Thailand. The area we took water sample is an upstream of surrounding areas where water is heavily used for agriculture and fishery (Charuvan et al., 2013, Suwandee et al., 2013). The source area is currently unsuitable for any domestic use. Therefore, addressing this issue at the source area is imperative before it becomes a widespread problem elsewhere.
2 Materials and methods
2.1 Water quality test
Wastewater was sampled from the Saen Saeb Canal, Prasan Mit pier, Bangkok, on non-rainy days. Water was analyzed by standard methods (APHA, 2012, 2005) for pH, electrical conductivity, total dissolved solids, total phosphorous (Ptot), NO3−, NH4+, Mg2+, Mn2+, SO42−, Cl−, total iron ions (Fetot), As3+, Cd2+, Cu2+, Pb2+, Ni2+, and Zn2+.
2.2 Bacterial isolation
The bacteria were isolated from rhizosphere soil taken from three sources: (1) Chinese kale grown on wastewater treated soil, (2) tomato grown on wastewater treated soil, and (3) weeds growing around municipal solid waste dumps near the Saen Saeb Canal, Wattana District, Bangkok, Thailand. Wastewater treated soil was prepared from sterile soil, which was soaked with wastewater from Saen Saeb canal, for one month. Weeds growing around the waste dump and the one-month-old Chinese kale and tomato were uprooted and the rhizosphere soil was collected. The rhizosphere soil was ten-fold diluted and followed by the pour plate method with nutrient agar (NA). After incubation at room temperature for two days, colonies were isolated. Next, the isolates were cultured on NA that was prepared from Saen Saeb wastewater (NA-SW) instead of distilled water. The isolates that grew on NA-SW were selected to test for plant growth-promoting characteristics.
2.3 In vitro screening of PGPR
Four tests were conducted for detecting characteristics of PGPR. First, the isolates were tested for growth capacity by inoculating on nitrogen-free (N-free) Jensen’s medium. The nitrogen-fixing bacteria are expected to grow well on N-free medium. Second, the isolates were inoculated to Pikovskaya’s (PVK) agar. The isolates that show clear zone on PVK agar are phosphate-solubilizing bacteria. Third, the isolates were inoculated on chrome azurol s (CAS) agar (Louden et al., 2011). An orange halo zone around an isolate on the CAS agar indicates the presence of siderophore producing bacteria. Last, the isolates were cultured in nutrient broth (NB) supplemented with tryptophan (100 µg/mL), for two days at room temperature. Then the cultures were centrifuged at 5000 rpm for 10 min. The supernatant was mixed with equal volume of Salkowski reagent (2% 0.5 M FeCl3 in 35% HClO4). The development of red-pink color indicates IAA production.
2.4 In vivo test of plant growth promotion
There are only two conditions in our experiment: sterile and wastewater irrigation. Two groups of ornamental soil, which is commercially available in the market, were prepared. The ingredients contain an equal ratio of loam, rice husk, and coconut flake. The first group was sterile and the second group was treated with wastewater after sterile. Chinese kale (B. alboglabra) seeds were soaked with 108 cell/mL of isolate for 2 h. Then, the seeds were sowed on sterile soil (for sterile condition) and wastewater treated soil (for wastewater irrigation condition) in pots (10 seeds per pot). One week after sowed, ungerminated seeds were removed and abnormal seedlings (i.e., too high or too short) were uprooted, which finally resulted in one seedling per pot. One-month-old and two-month-old plants were inoculated again with 3 mL of 108 cell/mL of isolate by dropping the isolate suspension in adjacent to the root zone. Plants which were not inoculated were used as a control. The initial sample size was 25 seedlings in each treatment. Plants were grown in a greenhouse under two conditions of irrigating with sterile water and wastewater. After ten weeks the plants were uprooted and the shoot and root weights were measured.
The data were analyzed by ANOVA, and means were compared using a Fishers Least Significant Difference test (LSD).
2.5 Iron ions reduction characteristic of PGPR
One milliliter of the isolates whose cell density was 105 cell/mL was inoculated in 50 mL NB containing 35 mg/L Fe2(SO4)3. The isolates were incubated at room temperature with shaking at 120 rpm for two days. Then, the culture was centrifuged at 10,000 g for 10 min. The cell free supernatant was subjected to colorimetric o-phenanthroline method. Briefly, one milliliter of the cell free supernatant were mixed with 0.05 mL of 0.1 g/mL hydroxylamine, 0.4 mL of 1 g/L 1,10-phenanthroline (C12H8N2, ortho-phenanthroline or o-phenanthroline), and 0.25 mL of buffer pH 5.0. Then, distilled water was added to make a final volume of 2.5 mL. From reaction with hydroxylamine, ferric was reduced to ferrous and the o-phenanthroline reacted with ferrous to form a red complex of [(C12H8N2)3Fe]2+. This complex was measured with spectrophotometry at 490 nm.
2.6 Bacterial identification
To identify the isolates, morphological, physiological, and biochemical characteristics of the isolates were tested according to Bergey’s manual of systematic bacteriology 2nd volume 3: The Firmicute (Logan and de-Vos, 2009). The isolates were identified via 16S rRNA gene sequencing. Briefly, Genomic DNA of an isolate was extracted by a kit from Machery-Nagel. Then, the 16S rRNA gene was amplified by PCR with universal Primer (27F and 1492R). The PCR product was sent to Macrogen (in Seoul, South Korea) for sequencing. The sequences were compared pairwise using a BLASTN search and were aligned with sequences of related species retrieved from GenBank using multiple alignment program CLUSTAL_X version 1.8 (Thompson et al., 1997). The phylogenetic trees were constructed by the neighbor joining (Saitou and Nei, 1987), tree making algorithms by using the software packages MEGA version 5.0 (Tamura et al., 2011). Topology of phylogenetics tree was tested by performing 1000 replicates bootstrap resampling.
3 Results
3.1 Municipal wastewater characteristics
From the Saen Saeb Canal, we investigated the wastewater characteristics by analyzing the quantity of elements and metals in the water. The results were shown in Table 1. We found that the amount of NH4+, Mg2+, and iron ions exceeds the limit of water quality for irrigation specified by Ayers and Westcot (1985). These results indicated that municipal wastewater from Saen Saeb Canal is not suitable for agricultural use. The results are consistent with those of our previous study that showed plant growth reduction from utilizing wastewater from the Saen Saeb Canal. The wastewater with high concentration of NH4+ but low concentration of NO3− might hinder plant growth. Britto et al. (2001) reported that plants showed toxicity symptoms when NH4+ was the sole source of nitrogen. High amount of NH4+ around plant cell roots leads to the excessive accumulation of NH4+ in cytosol. Although it is essential for photosynthesis and respiration in plants, excessive iron ions in the wastewater might reduce plant growth due to the increase of hydroxyl radical, which can damage plant cells (Connolly and Guerinot, 2002). The n.d. means not detected.
Parameter
Value
Usual range in irrigation water (*) and recommended maximum concentration of trace element in irrigation water (**)
pH
8.03
6.00–8.50*
Electrical conductivity (dS/m)
0.704
0.00–3.00*
Total dissolved solids (mg/L)
580
450–2000*
Ptot (mg/L)
0.86
0.00–2.00*
NO3¯ (mg/L)
0.08
0.00–10.00*
NH4+ (mg/L)
11.6
0.00–5.00*
SO42- (mg/L)
37.4
0.00–960*
Cl¯ (mg/L)
70
0.00–1065*
Mg2+ (mg/L)
84
0.00–61.00*
Mn2+ (mg/L)
0.21
0.20**
Fetot (mg/L)
33.64
5.00**
As3+ (mg/L)
n.d.
0.10**
Cd2+ (mg/L)
n.d.
0.01**
Cu2+ (mg/L)
<0.01
0.20**
Pb2+ (mg/L)
0.034
5.00**
Ni2+ (mg/L)
0.0112
0.20**
Zn2+ (mg/L)
0.27
2.00**
3.2 Bacterial isolation
A total of 187 isolates were isolated from the rhizospheric soil of Chinese kale roots (36 isolates), tomato roots (36 isolates), and weed roots (115 isolates). We found that about half of the isolates, especially those isolated from weeds (Table 2), did not grow well in NA-SW compared to their growth in NA. We hypothesize that the isolates that did grow in NA-SW were tolerant to some toxic effects from the wastewater. Thus, all of the isolates that grew in NA-SW were selected to test for plant growth-promoting characteristics.
Sources
Number of Isolates in NA
Number of Isolates in NA-SW
Chinese Kale
36
26
Tomato
36
36
Weeds
115
53
Total
187
115
3.3 In vitro screening of PGPR
The isolates were tested for plant growth-promoting characteristics. Out of 115 isolates, 102 grew on N-free medium, 88 solubilized phosphate in PVK agar, 93 showed siderophore production ability, and 69 showed IAA production ability. To further test plant growth-promoting characteristics in vivo, we selected the isolates that produced a wide halo zone on CAS agar. The wider the zone, the higher the ability to produce siderophore, which has the potential to eliminate ferric in wastewater. The selected isolates were CK1, CK2, CK3, CK6, CK12, and CK16 (obtained from Chinese kale); T3, T6, and T24 (obtained from tomato); and WA15, WB6, WC2, WD19, and WE15 (obtained from weeds). The plant growth promoting characteristics of the isolates were shown in Table 3.
Isolates
Growth on N-free medium
IAA Production
Clear zone size on PVK agar (cm)
Halo zone size on CAS agar (cm)
CK1
+
+
0.2
0.2
CK2
+
−
0.6
0.3
CK3
+
+
0.6
0.2
CK6
+
−
0.7
0.4
CK12
+
−
0.6
0.3
CK16
+
−
0.6
0.3
T3
+
+
0.3
0.5
T6
+
+
0.5
0.4
T24
+
+
0
0.3
WA15
+
+
0.9
0.2
WB6
+
+
1.2
0.2
WC2
+
+
0.7
0.5
WD19
+
+
0.3
0.4
WE15
+
+
0.3
0.4
3.4 In vivo test of plant growth promotion
The isolates were inoculated to Chinese kale to test whether they could enhance Chinese kale growth. In sterile condition, T24, WC2, WD19, and WE15 significantly enhanced the root growth (Fig. 1B, D, and F). The shoot weight of inoculated plants was relatively comparable to that in the control in all cases (Fig. 1A, C, and E). However, root and shoot dry weight for all plants under sterile condition was not statistically significant from that of control (data not shown). The T24, WC2, WD19, and WE15 isolates were selected to test for plant growth promotion in the wastewater irrigation condition. We found that isolates WC2, WD19, and WE15 significantly enhanced the root growth (Fig. 2). Only isolate WE15 significantly enhanced shoot weight (Fig. 2). These results indicated that isolates WC2, WD19, and especially WE15, enhanced plant growth under a stressful condition, while isolate T24 could not.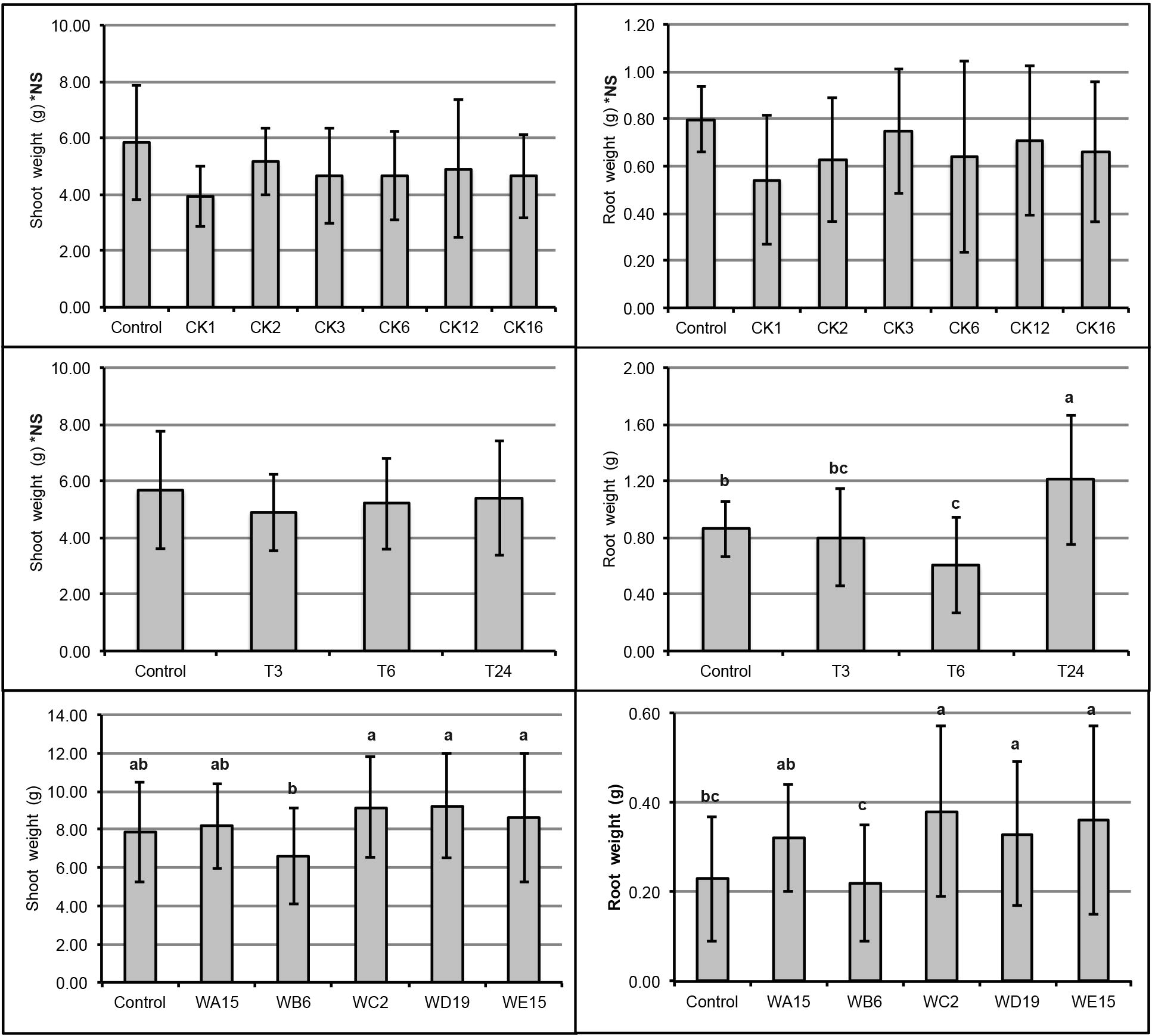
In vivo test of isolates for plant growth promotion in sterile soil. The isolates were taken from rhizosphere of Chinese kale (A and B), tomato (C and D), and weeds (E and F). Data shown are mean ± standard deviation of shoot weight (A, C, and E) and root weight (B, D, and F). *NS indicates not statistically significant. Lowercase letters indicate significant difference (P < 0.05). The P-values of the data in (A–F) are 0.05, 0.31, 0.51, <0.01, 0.01, and <0.01, respectively.
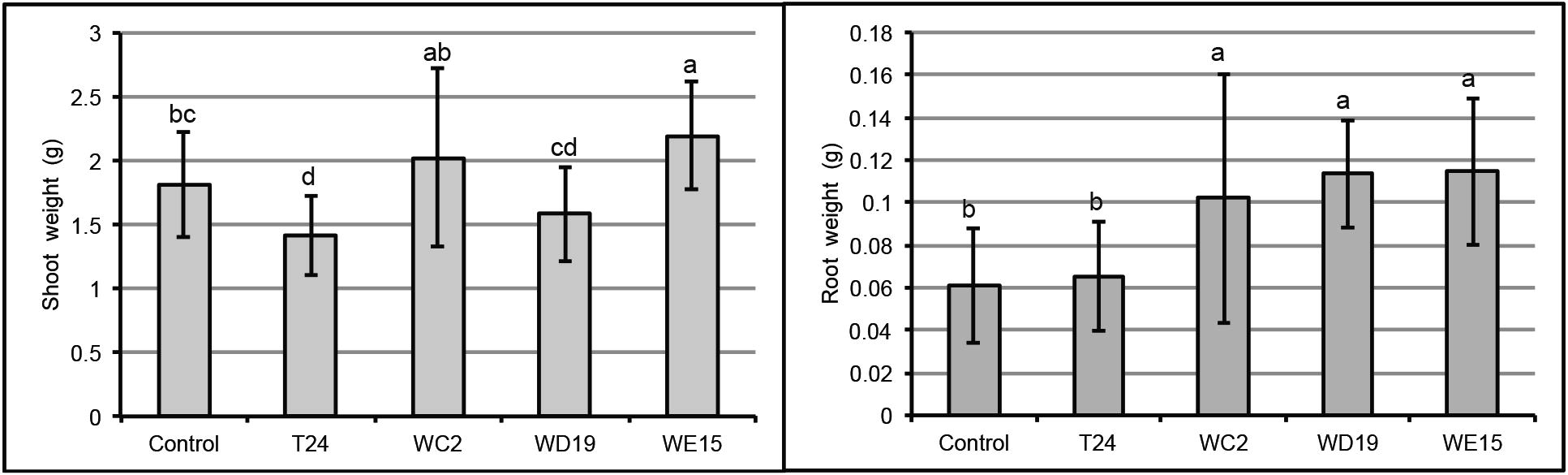
In vivo test of isolates for plant growth promotion in wastewater irrigated soil. Data shown are mean ± standard deviation of shoot weight (A) and root weight (B). Lowercase letters indicate significant difference (P < 0.01).
3.5 Bacterial identification
The isolates T24, WC2, WD19, and WE15 were identified bacterial species. The morphology, physiology, and biochemistry of isolates are shown in Table 4. The above four isolates belong to the Bacillus genus according to the methods outlined in Bergey’s manual of systematic bacteriology 2nd volume 3: The Firmicute (Logan and de-Vos, 2009). From the 16S rDNA sequence analysis with BLASTn pairwise alignment, we found that isolates T24 had a similarity score of 100% to B. aryabhattai B8W22T and isolates WD19 had a similarity score of 99% to B. firmus NCIMB 9366T. Isolates WC2 and WE15 had a similarity score of 99% to B. amyloliquefaciens subsp. plantarum FZB42T. Moreover, the isolates WC2 and WE15 are identical according to resulting phylogenetic tree. Phylogenetic tree based on 16S rRNA gene sequences was shown in Fig. 3. Bacterial isolates 1, 2, 3, 4, 5, 6, and 7 are T24, B. aryabhattai B8W22T, WC2, WE15, B. amyloliquefaciens subsp. Plantarum FZB42T, WD19, B. firmus NCIMB 9366T, respectively. n means no data. Superscripts a, b, and c indicate data from Shivaji et al. (2009), Borriss et al. (2011), Logan and de-Vos (2009), respectively.
Character
Bacterial isolates
1
2a
3
4
5b
6
7c
Cell shape
Rod
Rod
Rod
Rod
Rod
Rod
Rod
Gram’s staining
+
+
+
+
+
+
+
Endospore formation
+
+
+
+
+
+
+
Motility
−
+
+
+
+
+
+
Growth at 6.5% NaCl
+
+
+
+
+
+
+
Catalase
+
+
+
+
+
+
+
Citrate
−
−
−
−
+
−
−
Starch hydrolysis
+
+
+
+
+
+
+
Acid production from:
- Glucose
+
+
+
+
+
+
+
- Lactose
+
+
+
+
+
−
n
- Mannitol
+
+
+
+
+
+
+
- Arabinose
+
+
+
+
n
+
+
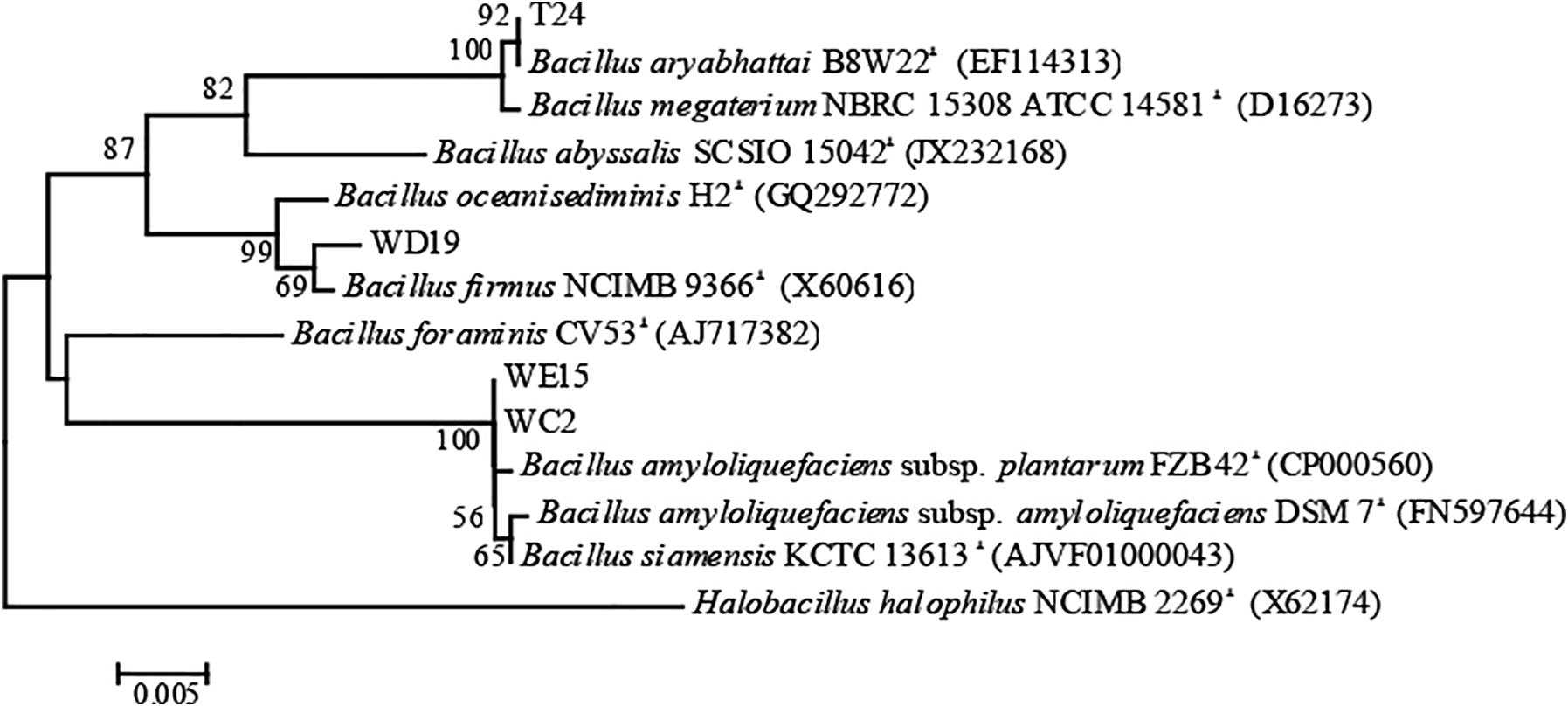
Phylogenetic tree based on 16S rRNA gene sequences showed T24, WD19, WE15, and WC2, all of which were related to the Bacillus species. The numbers represent the percentage from 1000 replicates bootstrap resampling (frequencies less than 50% are not shown).
3.6 Iron ions reduction characteristic of the Bacillus spp
The B. aryabhattai isolate T24, B. firmus isolate WD19, and B. amyloliquefaciens isolates WE15 were tested for iron ions reduction in culture medium. The control experiment was sterile NB containing 35 mg/L Fe2(SO4)3. As shown in Fig. 4, we found that cell-free culture obtained from B. aryabhattai isolate T24 and B. firmus isolate WD19 showed significantly lower amount of ferrous-phenanthroline complex than that from the control (p = 0.01 and p < 0.01, respectively). However, as for B. amyloliquefaciens isolates WE15, the amount of ferrous-phenanthroline complex was comparable with that of control.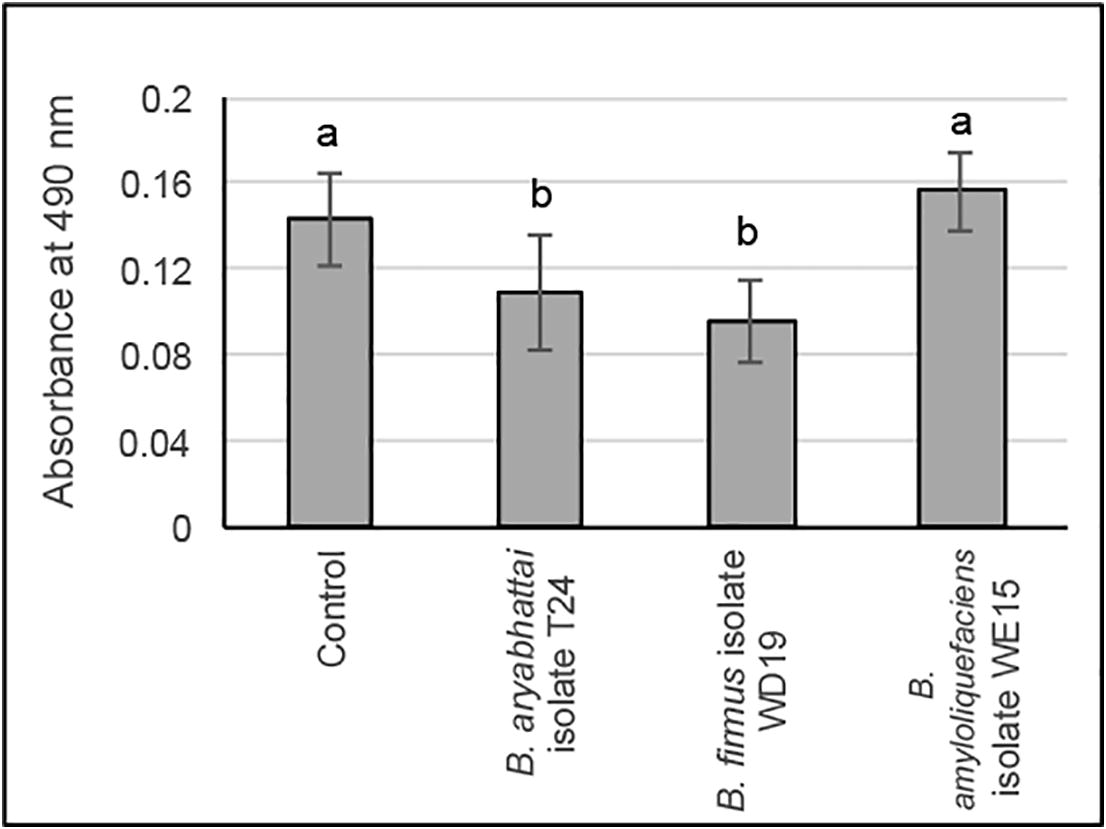
Iron ions reduction characteristic of the Bacillus spp. Data shown are mean ± standard deviation of absorbance of cell-free culture of the Bacillus spp. Lowercase letters indicate significant difference (P < 0.05).
4 Discussion
Compared to other PGPR, Bacillus spp. are commonly commercialized for the use of plant growth promotion and biocontrol because of their long-term viability and various secondary metabolite production. ACC deaminase produced by bacteria can enhance plant growth because the enzyme degrades ethylene precursor, which is a phytohormone known to reduce plant growth under stress (Yang et al., 2009). We found that B. aryabhattai isolate T24 slightly grew on Dworkin and Foster (DF) salt medium that contains ACC as a sole nitrogen in the medium. However, B. amyloliquefaciens isolate WC2, isolate WE15, B. firmus isolate WD19 could not grow on the medium (data not shown). Thus, the ACC deaminase did not play a role in plant growth promotion in the wastewater irrigation condition. As shown in Fig. 2, Chinese kale root growth was stimulated by B. amyloliquefaciens isolate WC2, isolate WE15, and B. firmus isolate WD19. The growth was possibly resulted from auxin phytohormone (IAA) produced by the Bacillus spp. Sadeghi et al. (2012) reported that siderophore and IAA producing Streptomyces isolate C enhanced germination rate and dry weight of wheat in normal and saline condition. Sharma et al. (2013) found that using PGPR in rice plants increases ferric uptake by the plants and increases ferric translocation into the grains. Additionally, the Bacillus spp. might enhance plant growth by reducing toxic effect from highly concentrated iron ions in soil. B. firmus isolate DS1 is often exploited for bioremediation of heavy metal from industrial wastewater (Bachate et al., 2014). The genome sequence of B. firmus isolate DS1 contains toxic compound resistance genes (Geng et al., 2014). In this study, B. firmus isolate WD19 shows PGPR traits in addition to heavy metal removal ability.
Siderophore producing bacteria could reduce heavy metal in polluted environment (Gaonkar and Bhosle, 2013). A varying degree of siderophore production depends on type of bacteria and iron ions concentration in environment. Pseudomonas aeruginosa strain PSS was inhibited when ferric level exceeds 10 µM (Villegas et al., 2002), while only 1.23 µM of ferric inhibited the siderophore production of Pseudomonas fluorescens strain P5-18 (Rachid and Ahmed, 2005). From result in the Fig. 4, B. amyloliquefaciens isolate WE15 could not reduce iron ions in culture medium while B. aryabhattai isolate T24 and B. firmus isolate WD19 could. This counter-intuitive result is probably caused by incapability of producing enough siderophore of B. amyloliquefaciens isolate WE15 when it was cultured in NB containing 35 mg/L (87.53 µM) Fe2(SO4)3. It should be noted that the concentration of iron ions in the wastewater used in this study was 33.64 mg/L. Gaonkar and Bhosle (2013) claimed in an independent study that ferric level exceeding 30 µM is required to suppress siderophore production in B. amyloliquefaciens strain NAR 38.1.
B. amyloliquefaciens isolates WE15 was closely similar to B. amyloliquefaciens subsp. plantarum FZB42T, which was PGPR with IAA producing, and plant-colonizing ability (Borriss et al., 2011). Chen et al. (2007) found that over 8.5% of the genome of B. amyloliquefaciens strain FZB42 was responsible for synthesizing antibiotics and siderophores. The genome also contained a set of genes implicated in biofilm formation, essential for PGPR to colonize plant root surfaces. The ability of PGPR to colonize plant roots and enhance microbial diversity is a plant growth-promoting characteristic often found in the Bacillus genus. B. amyloliquefaciens strain NBRISN13 promoted rice growth under salt stress and changed the microbial diversity in the rice rhizosphere (Nautiyal et al., 2013). Stamenov et al. (2012) reported that English ryegrass inoculated with B. subtilis produced higher yields of fresh and dry mass than that without inoculation, and increased the number of Azotobacter in the rhizospheric soil.
5 Conclusion
B. firmus isolate WD19, B. amyloliquefaciens isolate WC2 or WE15 showed plant growth-promoting characteristics and enhanced plant growth in vivo under high concentration of NH4+ and iron ions. Therefore, under irrigating condition with municipal wastewater, these bacteria could be exploited to enhance crop yield. Moreover, the B. amyloliquefaciens isolate WE15 and B. firmus isolate WD19 or consortia of these Bacillus strains, are promising plant-associated bacteria for heavy metal phytoremediation enhancement.
Acknowledgments
This study was financially supported by the Srinakharinwirot University (Grant number 014/2558). We thank Supattra Wichalek, Thondanee Mookhhilanphan, and Hasawanus Pengsantia for technical support.
References
- Standard Methods for the Examination of Water and Wastewater (21st ed.). Washington DC: American Public Health Association; 2005.
- Standard Methods for the Examination of Water and Wastewater (22nd ed.). Washington DC: American Public Health Association; 2012.
- Ayers, R.S., Westcot, D.W., 1985. Water quality for agriculture. Irrigation and drainage Paper 29, FAO, Rome.
- Simultaneous reduction of Cr(VI) and oxidation of As(III) by Bacillus firmus TE7 isolated from tannery effluent. Chemosphere. 2014;90:2273-2278.
- [Google Scholar]
- Relationship of Bacillus amyloliquefaciens clades associated with strains DSM 7T and FZB42T: a proposal for Bacillus amyloliquefaciens subsp. amyloliquefaciens subsp. nov. and Bacillus amyloliquefaciens subsp. plantarum subsp. nov. based on complete genome sequence comparisons. Int. J. Syst. Evol. Microbiol.. 2011;61:1786-1801.
- [Google Scholar]
- Futile transmembrane NH4+ cycling: a cellular hypothesis to explain ammonium toxicity in plants. Proc. Natl. Acad. Sci. U.S.A.. 2001;98:4255-4258.
- [Google Scholar]
- Water quality index of Saen Saeb canal. In: Proceeding of Science and Engineering. I-SEEC 2012; 2013. p. :404-408.
- [Google Scholar]
- Comparative analysis of the complete genome sequence of plant growth-promoting bacterium Bacillus amyloliquefaciens FZB42. Nat. Biotechnol.. 2007;25:1007-1014.
- [Google Scholar]
- Mechanisms used by plant growth-promoting bacteria. In: Maheshwari D.K., ed. Bacteria in Agrobiology: Plant Nutrient Management. Berlin: Springer; 2011. p. :17-46.
- [Google Scholar]
- Effect of metals on a siderophore producing bacterial isolate and its implications on microbial assisted bioremediation of metal contaminated soils. Chemosphere. 2013;93:1835-1843.
- [Google Scholar]
- Remediation of sewage and industrial effluent using bacterially-assisted floating treatment wetlands vegetated with Typha domingensis. Water Sci. Technol.. 2016;74:2192-2201.
- [Google Scholar]
- Enhanced remediation of sewage effluent by endophyte-assisted floating treatment wetlands. Ecol. Eng.. 2015;84:58-66.
- [Google Scholar]
- Cr-resistant rhizo-and endophytic bacteria associated with Prosopis juliflora and their potential as phytoremediation enhancing agents in metal-degraded soils. Front. Plant Sci.. 2015;5:1-10.
- [Google Scholar]
- Plant-bacteria partnerships for the remediation of hydrocarbon contaminated soils. Chemosphere. 2013;90:1317-1332.
- [Google Scholar]
- Family I. Bacillaceae. In: de-Vos P., Garrity G.M., Jones D., Krieg N.R., Ludwig W., Rainey F.A., eds. Bergey’s manual of systematic bacteriology. New York: The Firmicutes. Springer; 2009. p. :20-126.
- [Google Scholar]
- Use of blue agar CAS assay for siderophore detection. J. Microbiol. Biol. Educ.. 2011;12:51-53.
- [Google Scholar]
- Effect of heavy metals in plants of the genus Brassica. Int. J. Mol. Sci.. 2015;16:17975-17998.
- [Google Scholar]
- Plant growth promoting bacteria Bacillus amyloliquefaciens NBRISN13 modulates gene expression profile of leaf and rhizosphere community in rice during salt stress. Plant Physiol. Bioch.. 2013;66:1-9.
- [Google Scholar]
- Role of heavy metal resistant Ochrobactrum sp. and Bacillus spp. strains in bioremediation of rice cultivar and their PGPR Like activities. J. Microbiol.. 2013;51:11-17.
- [Google Scholar]
- Pescod, M.B., 1992. Wastewater treatment and use in agriculture. Irrigation and drainage paper 47, FAO, Rome.
- Effect of iron and growth inhibitors on siderophores production by Pseudomonas fluorescens. Afr. J. Biotechnol.. 2005;4:697-702.
- [Google Scholar]
- Plant growth promoting activity of an auxin and siderophore producing isolate of Streptomyces under saline soil conditions. World J. Microbiol. Biotechnol.. 2012;28:1503-1509.
- [Google Scholar]
- The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol.. 1987;4:406-425.
- [Google Scholar]
- The effect of domestic wastewater from Thailand’s Saen Saeb canal on plant growth, rhizosphere microorganisms. Songklanakarin J. Sci. Technol.. 2014;36:627-632.
- [Google Scholar]
- Enhancing grain iron content of rice by the application of plant growth promoting rhizobacteria. Plant Soil Environ.. 2013;59:89-94.
- [Google Scholar]
- Janibacter hoylei sp. nov., Bacillus isronensis sp. nov. and Bacillus aryabhattai sp. nov., isolated from cryotubes used for collecting air from the upper atmosphere. Int. J. Syst. Evol. Microbiol.. 2009;59:2977-2986.
- [Google Scholar]
- Plant growth promoting rhizobacteria in the production of English ryegrass. Plant Soil Environ.. 2012;10:477-480.
- [Google Scholar]
- Quality of life and environment of communities along Saen Saeb canal: a survey foundation of the physical and the current situation (Phase I) Procedia Soc. Behav. Sci.. 2013;88:205-211.
- [Google Scholar]
- MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol.. 2011;28:2731-2739.
- [Google Scholar]
- The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res.. 1997;25:4876-4882.
- [Google Scholar]
- Evaluation of the siderophores production by Pseudomonas aeruginosa PSS. Rev. Latinoam. Microbiol.. 2002;44:112-117.
- [Google Scholar]
- Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci.. 2009;14:1-4.
- [Google Scholar]







