Translate this page into:
Enhancement of cancer-type specific cytotoxicity of natural killer cells via pre-conditioning with cancer cell culture medium
⁎Corresponding author. sam1017@ish.ac.kr (Seahyoung Lee)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University. Production and hosting by Elsevier.
Abstract
Recently, we have established a method for culturing NK cell-enriched lymphocytes (NKELs) with high anti-cancer activity. These NKELs are found to be highly cytotoxic against various types of cancer cells, and even the extracellular vesicles derived from those NKELs were also demonstrated high anti-cancer activity. In the present study, to find a way to enhance cancer type specificity of these NKELs, the impact of tumor cell-priming of these NKELs on their cytotoxic effect against particular type of cancer cells was investigated. According to our data, when these NKELs were primed with conditioned media (CM) from three different lines of liver cancer cell lines, their cytotoxicity against liver cancer cell lines was significantly increased compared to that of the NKELs without priming. Based on cytokine arrays of the tumor CM and subsequent network analysis, 6 proteins were selected as a potential candidate factors that facilitated the liver cancer cell-specific priming of those NKELs. When these 6 candidate proteins (human recombinant proteins) were separately applied to prime NKELs, 2 out of 6 proteins (DKK1 and THBS1) showed enhanced liver cancer-specific cytotoxicity. With further optimization and elucidation of the underlying mechanisms, it may be possible to prime NKELs for learned cancer type specificity.
Keywords
Natural killer cell-enriched lymphocytes (NKELs)
conditioned medium (CM)
Tumor priming
Liver cancer
Dickkopf WNT signaling pathway inhibitor 1 (DKK1)
Thrombospondin 1 (THBS1)
1 Introduction
Adoptive cell transfer (ACT) using immune cells such as T cells, dendritic cells (DCs), and natural killer (NK) cells in combination with other therapy or alone is a treatment emerging as potential therapeutics for cancers (Kroemer et al., 2015; Vahedi et al., Ashkar, 2017). Especially, ACT using NK cells has been tried in various cases due to its safety and potent anti-cancer effect (Cichocki et al., 2016; Lanuza et al., 2019; Vahedi et al., 2017), and the efforts to improve its clinical effects, including optimization of conditions for NK expansion, activation, and post-transplantation survival, are being made.
For example, various NK cell culture conditions have been developed to overcome the difficulties of ex vivo-expansion and to secure enough number of NK cells for clinical application (Shimasaki et al., 2016; Yang et al., 2015). Recently, we also have developed a method for expansion and activation of the NK cell-enriched lymphocytes (NKELs) in a short time period (Choi et al., 2019). Furthermore, more importantly, various approaches to enhance the anti-cancer effect of NK cells are being actively tested.
To enhance the NK cell cytotoxicity against cancers, interleukin-12 (IL-12)/IL-18 and ADAM metallopeptidase domain 17 (ADAM-17) inhibitor had been tried. The IL-12 and IL-18 induced priming of a memory-like NK cells with improved anti-cancer effects (Cooper et al., 2009; Leong et al., 2014; Uppendahl et al., 2019) and ADAM-17 inhibitor maintained the CD16 expression which related to NK cell activation (Grzywacz et al., 2007). On the other hand, blocking inhibitory mediators such as killer-cell immunoglobulin-like receptor (KIR), NK group 2 member A (NKG2A/CD94), or programmed cell death protein-1 (PD-1)/PD-ligand (L) 1 that compromise the cytotoxicity and post-transplantation survival of NK cells also enhances the anti-cancer function of NK cells (Benson et al., 2010; Korde et al., 2014; Nguyen et al., 2005; Nguyen et al., 2008). All of these approaches to enhance the functional properties of the NK cells can be referred to as “priming”.
In addition to the above mentioned approaches, cancer cells themselves have been used for priming of NK cells. North J et al. have reported that leukemic cell line, CTV-1 primed NK cells for improved effector functions (North et al., 2007). The K562 and Daudi cells induced NKp30 expression to render more potent response of NK cells in target cell recognition (Fauriat et al., 2010; Sabry et al., 2019). Reported mechanisms of cancer cell-based priming of NK cells includes, but are not limited to, increased expressions of activation markers (CD25 and CD69), pro-inflammatory cytokines such as macrophage inflammatory proteins (MIP-1) α/β and IL-1β/6/8, and genes related to NK cell cytotoxicity and immunomodulation namely, FAS, tumor necrosis factor (TNF), interferon γ (IFNG), and mitogen activated protein kinase 11 (MAPK11) (Sabry et al., 2019).
As such, it seems that exposing NK cells to the cancer microenvironment can enhance the anti-cancer effect of NK cells. Nevertheless, the underlying mechanisms of cancer cell-mediated priming of NK cells have not been completely elucidated, and considering cancer cell secretome includes key regulators of the tumorigenic process (da Cunha et al., 2019), the possibility that the cancer type-specific secretome may render cancer type-specific activation of NK cells cannot be simply dismissed.
Therefore, the purpose of this study was to examine whether a certain type of cancer secretome enhances the NK cell cytotoxicity against that particular type of cancers. As a proof of concept study, we investigated the effect of liver and colon cancer-derived conditioned media (CM) on the cytotoxic effect of NKEL, especially against corresponding types of cancer cells from which the CM was derived, and the causative factors that may have facilitated the cancer type-specific cytotoxicity of NKEL are suggested.
2 Materials and methods
2.1 Donors and preparation of NKELs
Blood samples were obtained from 4 healthy donors who were recruited at the International St. Mary’s Hospital of Catholic Kwandong University. All donors have written consent to participation and the study protocol was approved by the Institutional Review Board of the International St. Mary’s Hospital, Catholic Kwandong University. Preparation of natural killer cell-enriched lymphocytes (NKELs) was performed as descried previously (Choi et al., 2019). Briefly, human peripheral blood mononuclear cells (PBMCs) isolated from buffy coats by density gravity centrifugation (Ficoll-Paque, GE Healthcare, Piscataway, NJ, USA) was supplemented with the autologous human plasma, 3 different agonistic antibodies (anti-human CD56, anti-human CD16, and anti-human CD355 (NKp46); BD Biosciences, San Jose, CA, USA), and 3 different cytokines (IL-2: Novartis, Whippany, NJ, USA, IL-12: PeproTech, Rocky Hill, NJ, USA, and IL-18 (R&D systems, Minneapolis, MN, USA) and cultured for 2 weeks.
2.2 Culture of cancer cell lines
Three liver cancer cell lines (HepG2 (hepatoblastoma), Hep3B (hepatocellular carcinoma), and SK-Hep1 (adenocarcinoma) cells) and one colon cancer cell line (COLO320-DM (adenocarcinoma) cells) were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). The HepG2 cells were cultured in Minimum Essential Medium, the Hep3B and SK-Hep1 cells were cultured in Dulbecco Modified Eagle Medium (DMEM), and the COLO320-DM cells were in RPMI 1640 Medium containing 2 mM L-glutamine, which were supplemented with 10 % heat-inactivated FBS, 1 mM sodium pyruvate, 25 mM HEPES, and 1 % penicillin/streptomycin in a humidified atmosphere of 5 % CO2 at 37 °C. All media and supplements were purchased from Gibco by Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
2.3 Preparation and treatment of CM and cytotoxicity assay
Cancer cells were seeded at 5 × 106 cells in 7 mL medium per 100 mm culture dish and the CM was collected after 3 days of incubation from cells grown. The cell debris was then removed via centrifugation for 30 min at 1000 × g. The CM was treated by mixing the cell culture medium with different concentrations (0, 10, 25, or 50 %) with NKELs (5 × 106 cells/100 mm dish) and incubated for 24 hrs. The harvested CM-treated NKELs (1 × 104 cells/well) were co-cultured with pre-seeded cancer cells (1 × 104 cells/well) in 96-well plate. After 24 hrs, cytotoxicity assay was performed. Cytotoxicity of CM-treated NKELs against each cancer cell lines was determined using a LDH Cytotoxicity Detection Kit (Takara, Nojihigashi, Kusatsu, Shiga, Japan), following manufacturer’s instructions.
2.4 Cytokine array
Culture supernatants of three liver cancer cells and one colon cancer cell were analyzed with a Proteome Profiler human XL cytokine array (ARY022B, R&D Systems) by the manufacturer’s protocol. Cancer cells were seeded at 5 × 106 cells in 7 mL medium per 100 mm culture dish and the CM was collected after 3 days and centrifuged at 1000 xg for 30 min. Membranes were incubated with 400 μL of the supernatant overnight at 4 °C and chemiluminescence was detected by using the Davinch-K imaging system (Seoul, Republic of Korea). The DMEM, RPMI, and MEM medium were examined as negative controls.
2.5 Priming of NK cells with candidate cytokines
To prime NKELs with the candidate cytokines, NKELs (5 × 106 cells/100 mm dish) were separately treated with each purified cytokine (0.5 μg/mL) and incubated for 24 hrs. The cytokine-primed NKELs (1 × 104 cells/well) were harvested and co-cultured with pre-seeded cancer cells (1 × 104 cells/well) in 96-well plate. After 24 hrs, cytotoxicity assay was performed. Cytotoxicity of cytokine-treated NKELs against each cancer cell lines was determined using a LDH Cytotoxicity Detection Kit (Takara, Nojihigashi, Kusatsu, Shiga, Japan), following manufacturer’s instructions.
2.6 Statistical analysis
One-way analysis of variance (ANOVA) by the Statistical Package for the Social Sciences (SPSS, version 17) program was used for all comparison of experimental results. The data were means ± SEM and they were considered to be significantly different at p < 0.05, as determined by the protected least-significant difference (LSD) test.
3 Results
3.1 Cytotoxicity of cancer cell-derived CM-treated NKELs on cancer cells
Whether a certain type of cancer cell-derived CM increases the cytotoxicity of NKEL against the particular type of cancer cells from which the CM was derived was examined using two different cancer cell lines; HepG2 (liver cancer) and COLO320-DM (colon cancer). According to the data, control NKELs without priming showed approximately 70 % of cytotoxicity against HepG2 (Fig. 1A and 1B). However, when NKELs were primed with HepG2 CM, their cytotoxicity against HepG2 significantly and proportionally increased as the volume of HepG2 CM used for the priming increased (Fig. 1A). On the other hand, NKELs primed with COLO320-DM CM did not show such priming effect against HepG2 cells (Fig. 1B). Vice versa, the cytotoxicity of NKELs against COLO320-DM was enhanced only with the priming using COLO320-DM CM (Fig. 1C), while priming with HepG2 CM failed to enhance the cytotoxicity of NKELs against COLO320-DM (Fig. 1D).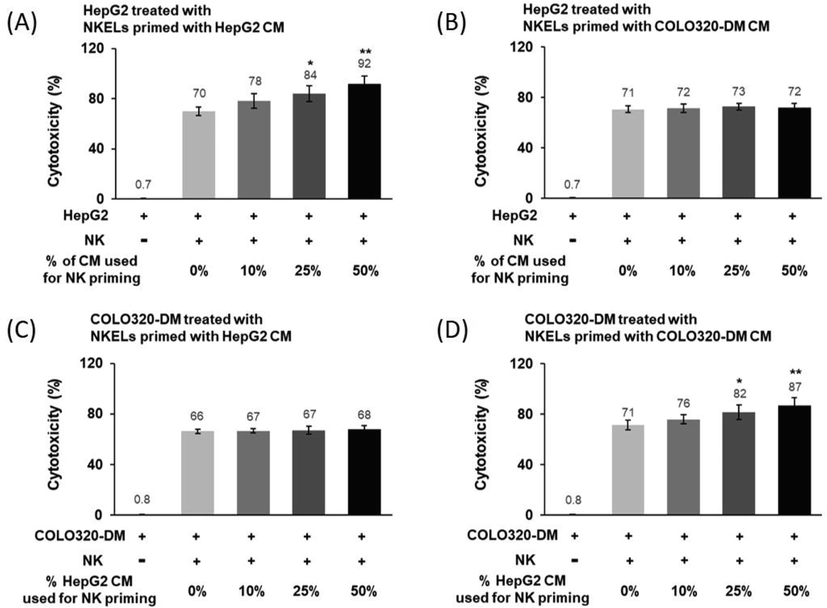
Cytotoxicity of HepG2 or COLO320-DM-derived CM-treated NKELs against HepG2 or COLO320-DM cells. (A) Cytotoxicity of NKELs primed with increasing % volume of HepG2 CM on HepG2 cells. (B) Cytotoxicity of NKELs primed with COLO320-DM CM on HepG2 cells. (C) Cytotoxicity of NKELs primed with HepG2 CM on COLO320-DM cells. (D) Cytotoxicity of NKELs primed with COLO320-DM CM on COLO320-DM cells. The data are cytotoxic mean values of NKELs from four individuals on cancer cells. Significant differences between untreated control and CM-treated groups were determined via ANOVA, with p values indicated as *p < 0.05 and **p < 0.01.
3.2 Cytotoxicity of liver cancer cell-derived CM-treated NKELs on liver cancer cells
To further examine whether the observed cancer type-specific priming of NKELs was truly dependent on the type of tissue from which the cancer cells were derived, liver cancer cell CMs derived from 3 different liver cancer cell lines (HepG2, Hpe3B, and SK-Hep1) were used to prime NKELs. The results indicated that all 3 different liver cancer cell CMs increased the cytotoxicity of NKELs as the volume of CMs used for priming increased (Fig. 2). These results suggested that there may be liver cancer-specific soluble factors that facilitate the increased cytotoxicity of NKELs against liver cancer cells.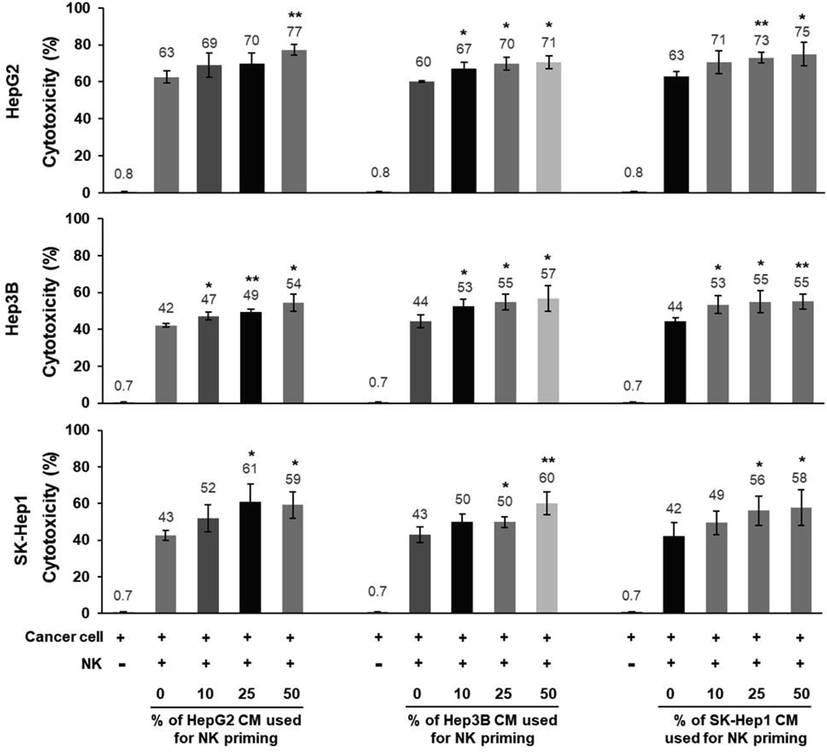
Cytotoxicity of 3 different liver cancer cell-derived CM-treated NKELs against 3 different liver cancer cells. NKELs were primed with increasing % volume of CMs derived from 3 different liver cancer cells (HepG2, Hep3B, and SK-Hep1), and their cytotoxicity on each liver cancer cell line was examined in combinations. Significant differences between untreated control and CM-treated groups were determined via ANOVA, with p values indicated as *p < 0.05 and **p < 0.01.
3.3 Differential cytokine expression between liver and colon cancer cell-derived CMs
To identify liver cancer-specific soluble factors that may facilitate the liver cancer-specific priming of NKELs, cytokines differentially expressed in the liver and colon cancer-derived CMs were determined by using a cytokine array that can detect 105 different human cytokines. In Hep3B CM, 19 cytokines showed more than 5-fold increase compared to COLO320-DM CM which served as control. For HepG2 CM and SK Hep1 CM, 14 and 13 cytokines, respectively, showed more than 5-fold increase compared to the control (Fig. 3A). Among these cytokines increased, 6 of them were commonly increased in all 3 liver cancer-derived CMs, and they were namely, Dickkopf-related protein 1 (DKK1), intercellular adhesion molecule 1 (ICAM1), interleukin 8 (IL-8), growth/differentiation factor 15 (GDF15), Serpin Family E Member 1 (Serpin E1/PAI-1), and thrombospondin 1 (THBS1) (Fig. 3B).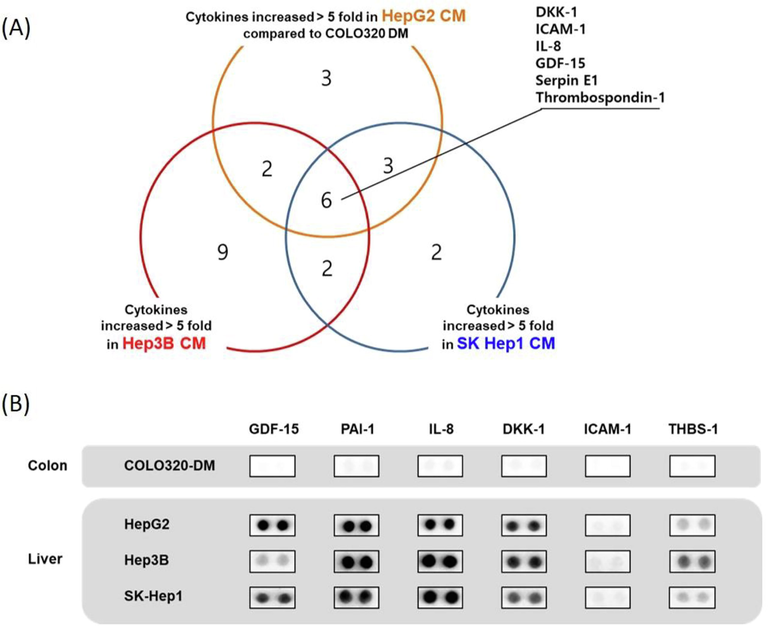
Identification of liver cancer cell derived CM enriched cytokines. (A) Using a cytokine array that can detect 105 different cytokines, liver cancer cell derived CM enriched cytokines whose expressions are greater than 5 folds compared to that of colon cancer cell derived CM were screened. (B) Expressions of 6 common liver cancer cell derived CM enriched cytokines in comparison to those of colon cancer cell derived CM.
3.4 Cytotoxicity of cytokine-primed NKELs on liver cancer cells
To further examine whether the 6 cytokines increased in the CM of liver cancer cells were responsible for the observed enhanced liver cancer-specific cytotoxicity of NKELs, NKELs were primed with each individual cytokine, and the cytotoxicity of the cytokine-primed NKELs against 3 different liver cancer cell lines and 1 colon cancer cell line was examined. When the 6 candidate cytokines separately primed NKELs, both DKK1 and THBS1 significantly increased the cytotoxicity of NKELs at a concentration of 0.5 μg/mL against all 3 liver cancer cell lines tested. For HepG2, the cytotoxicity induced by the DKK1 and THBS1-primed NKELs was 73.35 ± 5.97 and 72.35 ± 6.56, respectively. Considering the cytotoxicity induced by control NKELs was 61.87 ± 3.62, both DKK1 and THBS1 increased the cytotoxicity of NKELs by approximately 10 % (Fig. 4A). The cytotoxicity of NKELs against Hep3B cells was also increased by DKK1 and THBS1 priming (49.02 ± 3.18 and 49.73 ± 4.16 of the DKK1 and THBS1-primed NKELs, respectively vs. 43.53 ± 2.64 of control NKELs) (Fig. 4B). Such enhanced cytotoxicity of NKELs by DKK1 and THBS1 priming was also observed in SK-Hep1 cells as well (54.54 ± 3.23 and 53.84 ± 3.97 of the DKK1 and THBS1-primed NKELs, respectively vs. 43.74 ± 2.98 of control NKELs) (Fig. 4C). On the other hand, priming of NKELs with DKK1 and THBS1 failed to enhance the cytotoxicity of NKELs against COLO-DM cells (38.25 ± 5.64 and 35.45 ± 4.39 of the DKK1 and THBS1-primed NKELs, respectively vs. 42.12 ± 4.98 of control NKELs) (Fig. 4D), suggesting the priming of NKELs with DKK1 and THBS1 preferentially enhanced the cytotoxicity of NKELs against liver cancer cells.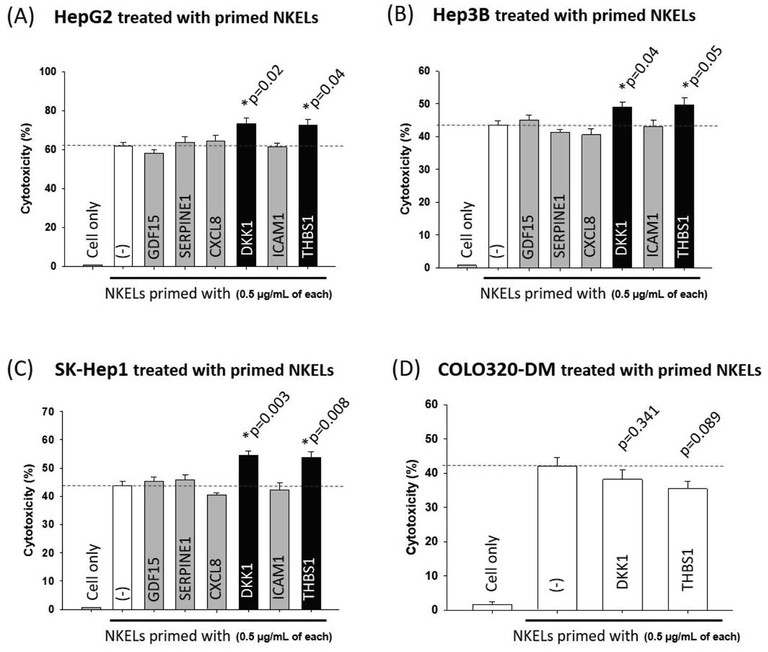
Cytotoxicity of purified liver cancer cell derived CM enriched cytokine-treated NKELs. (A) NKELs were primed with 6 different liver cancer cell derived CM enriched cytokines (0.5 μg/mL, each), and their cytotoxicity on HepG2 cells was examined. (B) Cytotoxicity of NKELs primed with 6 different cytokines on Hep3B cells. (C) Cytotoxicity of NKELs primed with 6 different cytokines on SK-Hep1 cells. (D) NKELs were primed with DKK1 or THBS1 (0.5 μg/mL, each), and their cytotoxicity on COLO320-DM cells was examined. Significant differences between untreated control and cytokine primed groups were determined via ANOVA.
4 Discussion
Using activated NK cells is important for the ACT to treat cancer, because activated NK cells show potent cytotoxicity against various cancer cells and produce a multitude of immunoregulatory cytokines and inflammatory reaction-related chemokines (Bakker et al., 2000; Sabry et al., 2019). Therefore, various methods for NK cell activation have been studied, and priming NK cells with tumor cells is one of them. Previous studies examined the effect of tumor cell mediated priming of NK cells demonstrated that priming of NK cells with tumor cells increased some of the NK cell activation markers, secretion of pro-inflammatory cytokines, and expression of genes associated with enhanced NK cell cytotoxicity and immunomodulatory functions (Sabry et al., 2019), indicating improved effector functions and target cell recognition of the primed NK cells (Fauriat et al., 2010; North et al., 2007).
Although these studies well demonstrated the feasibility and importance of NK cell priming, the priming strategy involving direct contact of tumor cells with NK cells (i.e., co-incubation) may not very useful if it were used to develop a primed NK cell-based anti-tumor therapeutics. It is because if tumor cells are in direct contact with NK cells during priming process, isolation of NK cells for clinical use would be virtually impossible. Furthermore, even if it can be done, the risk of possible tumor cell contamination is too high to be simply dismissed. Therefore, in the present study, we used cancer cell-derived CM to prime NKELs in order to avoid such issue of possible tumor cell contamination. This is not the first study to use CM for NK cell priming. In fact, macrophage-derived CM (Mattiola et al., 2015) and mesenchymal stem cell-derived CM (Cui et al., 2016) have been used to prime NK cells for improved NK cell function. Nevertheless, to our best knowledge, cancer cell-derived CM has never been used to prime NK cells, especially to enhance the cancer type-specific cytotoxicity. Here, we report that the priming of NK cells with cancer cell-derived CM increases the cytotoxicity of the primed NK cells especially against the cancer cells from which the CM was derived.
In the present study, we have empirically demonstrated that the priming of NKELs with liver cancer cell-derived CM significantly increased the cytotoxicity of the primed NKELs against liver cancer cells, while the priming using colon cancer cell-derived CM specifically increased the cytotoxicity of the primed NKELs against colon cancer cells (Fig. 1). We also have demonstrated that the CM was responsible for the increased cytotoxicity of NKELs by demonstrating the cytotoxicity of the primed NKELs was proportional to the amount of CM used to prime NKELs (Fig. 2). These data strongly suggested that something, most likely soluble factor(s), contained in the CM derived from cancer cells stimulated NKELs so that they can be ready to search and destroy the specific type of cancer cells from which the CM was derived.
In effort to identify such soluble factors, cytokine array that can detect 105 different cytokines simultaneously was performed on the CMs derived from liver cancer cells and colon cancer cells. Overall, a lot more cytokines were detected in the CM derived from liver cancer cells compared to that from colon cancer cells, and therefore, the CM derived from colon cancer cell was used as a control since it was logical to start the screening with something detected or increased. In fact, since this was a proof of concept study, which CM (or cancer type) serve as a control really was not the issue, as long as key factors facilitate the cancer type specific priming of NKELs were to be found.
Our data indicated that a higher amount of 6 cytokines were commonly detected in CMs derived from 3 different liver cancer cell lines compared to the control CM derived from colon cancer cells (Fig. 3), and 2 of them (DKK1 and THBS1) actually enhanced the liver cancer specific cytotoxicity of the NKELs primed with (Fig. 4). These data indicated that, although there still is a possibility that another soluble factors not included in the cytokine array panel, yet also contributed to the observed cancer cell type specific priming of NKELs, at least DKK1 and THBS1 have altered the biology of the NKELs primed with so that they have enhanced cytotoxicity against liver cancer cells. To understand the underlying mechanisms of how those 2 cytokines enhance the liver cancer specific cytotoxicity, first, the biological effect of those cytokines on NK cells was searched, but no known mechanism even remotely explains the observed effect was found.
For example, DKK1 is an endogenous inhibitor of Wnt signaling pathway (Zorn, 2001), and it has been known to induce excessive tumor growth and suppression of anti-cancer immune responses by down-regulating the expression of activating ligands on NK cells (Kagey and He, 2017). However, such mechanisms cannot explain the observed effect, and are even contradictory to what is observed in the present study. Nevertheless, this does not necessarily mean that the observed liver cancer specific priming of NK cells by DKK1 is impossible since the effect of DKK1 is highly context-dependent so that it can act as a cancer promoter as well as a suppressor (Kagey and He, 2017; Mazon et al., 2016). Therefore, a different approach was taken to understand the mechanisms.
For the other approach, a few premises had to be laid out. First of all, since the washing process following the priming with cytokines was sufficient and identical for both the NKELs applied to liver cancer cells and the NKELs applied to colon cancer cells, the observed priming effect was less likely facilitated by any leftover cytokines that might have been remained following washes. In other words, the observed effect was probably not mediated by a direct contact of cytokines with cancer cells. Consequently, the mechanisms have to be based on certain biological changes of NKELs primed with those cytokines. Furthermore, the priming was only effective against liver cancer cells, and therefore, the mechanisms of the observed cytotoxicity have to be something only applicable to liver cancer cells.
That being said, theoretically speaking, mainly 2 scenarios seem to be plausible for the observed priming effect. The first possibility is a NKEL production of soluble factors inducing cytotoxicity only to liver cancer cells. However, to date, no such naturally occurring factors selectively destroy a certain type of cancer cell ever have been found, not just for liver cancers but for all type of cancers. Therefore, unless proven otherwise, the NKEL production of liver cancer specific cytotoxic factor is less likely responsible for the observed priming effect.
Another, yet a bit more plausible, scenario is that the priming induces or enhances the expression of certain NKEL surface molecules that can physically interact with the surface molecules only specific to liver cancer cells. For example, according to the human protein atlas (proteinatlas.org), 37 genes are listed as “tissue enriched” genes of the liver, and complement C8a (C8A) and complement C9 (C9) are also included in the list. This is interesting because both complements are known to bind to CD59 (Bryceson et al., 2006), one of the complementary receptors of NK cells (Min et al., 2014). Furthermore, in cooperation with NKp46 and NKp30 activating receptors (Pegram et al., 2011), CD59 triggers signals to induce the NK cell mediated cytotoxicity (Marcenaro et al., 2003). Therefore, it may be possible that the priming of NKELs with cytokines induce or enhance the expression of CD59 so that the primed NKELs are more prone to physically contact C8a and C9 expressing liver cancer cells over colon cancer cells. Furthermore, the transcription of CD59 is known to be regulated by the transcription factor sox2 (Chen et al., 2017). Since sox2 competes with β-catenin, a key regulator of activated Wnt signaling (MacDonald et al., 2009), for binding to TCF (T cell factor) to be transcriptionally active (Kormish et al., 2010), Wnt signaling inhibitor DKK1 may down-regulate the β-catenin available for TCF binding so that sox2 can be more active and increase CD59 expression. However, although we are currently examining such possibility, we are not able to provide any empirical data at this point so that such scenario remains as a speculation and how those 2 cytokines teach NKELs to preferentially kill liver cancer cells is yet to be empirically proved.
5 Conclusion
The significance of this study is that this study experimentally demonstrated a possibility of priming NK cells for cancer type specific cytotoxicity, and with further optimization and elucidation of the underlying mechanisms, the findings of this study can lead to the development of effective NK cell-based cancer type specific therapeutics.
Acknowledgement
This work was supported by Basic Science Research Program through the National Research Foundation of Korea NRF funded by the Ministry of Education (2020R1I1A2064710) and the NRF grant funded by the Korea government (MSIT) (NRF-2021R1A2C1005280).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- NK cell activation: distinct stimulatory pathways counterbalancing inhibitory signals. Hum. Immunol.. 2000;61:18-27.
- [Google Scholar]
- The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood. 2010;116:2286-2294.
- [Google Scholar]
- Activation, coactivation, and costimulation of resting human natural killer cells. Immunol. Rev.. 2006;214:73-91.
- [Google Scholar]
- CD59 Regulation by SOX2 Is Required for Epithelial Cancer Stem Cells to Evade Complement Surveillance. Stem Cell Rep.. 2017;8:140-151.
- [Google Scholar]
- Cytotoxic effects of ex vivo-expanded natural killer cell-enriched lymphocytes (MYJ1633) against liver cancer. BMC Cancer. 2019;19:817.
- [Google Scholar]
- The Past, Present, and Future of NK Cells in Hematopoietic Cell Transplantation and Adoptive Transfer. Curr. Top. Microbiol. Immunol.. 2016;395:225-243.
- [Google Scholar]
- Human mesenchymal stromal/stem cells acquire immunostimulatory capacity upon cross-talk with natural killer cells and might improve the NK cell function of immunocompromised patients. Stem Cell Res Ther. 2016;7:88.
- [Google Scholar]
- Cellular Interactions in the Tumor Microenvironment: The Role of Secretome. J. Cancer. 2019;10:4574-4587.
- [Google Scholar]
- Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood. 2010;115:2167-2176.
- [Google Scholar]
- Grzywacz, B., Kataria, N., Verneris, M. R. 2007. CD56(dim)CD16(+) NK cells downregulate CD16 following target cell induced activation of matrix metalloproteinases. Leukemia 21,356-9; author reply 359.
- Rationale for targeting the Wnt signalling modulator Dickkopf-1 for oncology. Br. J. Pharmacol.. 2017;174:4637-4650.
- [Google Scholar]
- A phase II trial of pan-KIR2D blockade with IPH2101 in smoldering multiple myeloma. Haematologica. 2014;99:e81-e83.
- [Google Scholar]
- Interactions between SOX factors and Wnt/beta-catenin signaling in development and disease. Dev. Dyn.. 2010;239:56-68.
- [Google Scholar]
- Natural and therapy-induced immunosurveillance in breast cancer. Nat. Med.. 2015;21:1128-1138.
- [Google Scholar]
- Recalling the Biological Significance of Immune Checkpoints on NK Cells: A Chance to Overcome LAG3, PD1, and CTLA4 Inhibitory Pathways by Adoptive NK Cell Transfer? Front. Immunol.. 2019;10:3010.
- [Google Scholar]
- Preactivation with IL-12, IL-15, and IL-18 induces CD25 and a functional high-affinity IL-2 receptor on human cytokine-induced memory-like natural killer cells. Biol. Blood Marrow Transplant.. 2014;20:463-473.
- [Google Scholar]
- Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev. Cell. 2009;17:9-26.
- [Google Scholar]
- CD59 is physically and functionally associated with natural cytotoxicity receptors and activates human NK cell-mediated cytotoxicity. Eur. J. Immunol.. 2003;33:3367-3376.
- [Google Scholar]
- Priming of Human Resting NK Cells by Autologous M1 Macrophages via the Engagement of IL-1beta, IFN-beta, and IL-15 Pathways. J. Immunol.. 2015;195:2818-2828.
- [Google Scholar]
- Modulating Dickkopf-1: A Strategy to Monitor or Treat Cancer? Cancers (Basel). 2016;8
- [Google Scholar]
- Expression and regulation of complement receptors by human natural killer cells. Immunobiology. 2014;219:671-679.
- [Google Scholar]
- NK-cell reconstitution after haploidentical hematopoietic stem-cell transplantations: immaturity of NK cells and inhibitory effect of NKG2A override GvL effect. Blood. 2005;105:4135-4142.
- [Google Scholar]
- Involvement of mature donor T cells in the NK cell reconstitution after haploidentical hematopoietic stem-cell transplantation. Leukemia. 2008;22:344-352.
- [Google Scholar]
- Tumor-primed human natural killer cells lyse NK-resistant tumor targets: evidence of a two-stage process in resting NK cell activation. J. Immunol.. 2007;178:85-94.
- [Google Scholar]
- Activating and inhibitory receptors of natural killer cells. Immunol. Cell Biol.. 2011;89:216-224.
- [Google Scholar]
- Tumor- and cytokine-primed human natural killer cells exhibit distinct phenotypic and transcriptional signatures. PLoS One. 2019;14:e0218674.
- [Google Scholar]
- Expanded and armed natural killer cells for cancer treatment. Cytotherapy. 2016;18:1422-1434.
- [Google Scholar]
- Cytokine-induced memory-like natural killer cells have enhanced function, proliferation, and in vivo expansion against ovarian cancer cells. Gynecol. Oncol.. 2019;153:149-157.
- [Google Scholar]
- Ex Vivo Expanded Human NK Cells Survive and Proliferate in Humanized Mice with Autologous Human Immune Cells. Sci. Rep.. 2017;7:12083.
- [Google Scholar]
- A New Ex Vivo Method for Effective Expansion and Activation of Human Natural Killer Cells for Anti-Tumor Immunotherapy. Cell Biochem. Biophys.. 2015;73:723-729.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102717.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







