Translate this page into:
Enhanced production antibiotics using green gram husk medium by Streptomyces sp. SD1 using response surface methodology
⁎Corresponding author. venzymes@gmail.com (Ponnuswamy Vijayaraghavan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Discovery of various antibiotics from the genus Streptomyces attained good attention from Industrialists and Pharmacologists. Streptomyces sp. produce various antibiotics to treat various diseases. The ability of Streptomyces sp. SD1 to synthesize antibiotics using various agro industrial wastes as solid substrate was evaluated. The agro wastes used were pineapple peel, wheat bran, apple pomace, rice bran, tapioca powder, orange peel and green gram husk for the production of antibiotics. The culture medium was optimized by using various carbon, nitrogen, ions, and minerals. To enhance maximum metabolite production, fermentation of Streptomyces sp. SD1 was optimized through response surface methodology. RSM is also used to analyze the interactions among the factors and screening the culture medium components. Streptomyces sp. SD1 had the ability to produce antibiotics at highest level with green gram husk as the substrate. Among the carbon sources, starch, and maltose enhanced the production of antibiotics. However, the selected sources such as, sucrose and glucose negatively influenced on antibiotics production. Beef extract, peptone and yeast extract positively influenced on antibiotics production. However, addition of casein and oat meal inhibited the production of antibiotics. Among the divalent ions, calcium chloride, magnesium sulphate and manganese chloride enhanced the production of antibiotics, however supplementation of cobalt chloride and mercuric chloride affected antibiotics production. All selected mineral sources enhanced the production of antibiotics and the yield was higher than control. 3D response surface graphs showed maximum yield of antibiotics at higher concentration of selected nutrient sources. Antibiotics production enhanced 2.9 fold by Streptomyces sp. SD1 in optimized medium than unoptimized medium. Among the isolates, Streptomyces sp. SD1 showed high antimicrobial activity. The selected actinomycetes utilized green gram husk for antibiotics production. Also addition of nutrient sources stimulated the production of antibiotics. Green gram husk is highly useful for the production of antibiotics in large scale.
Keywords
Streptomyces sp.
Antibiotics
Agro-residues
Solid state fermentation
Response surface methodology
1 Introduction
Solid-state fermentation (SSF) describes to the fermentation solid substrates using microorganisms with less water or no free water (Al-Dhabi et al., 2016). In SSF, two kinds of solid substrates used in SSF, these are inert solid substrates supplemented with various nutrient sources and naturally available solid substrates such as, agro-industrial wastes. These include, crop residues such as, wheat and rice, fruit processing wastes, including, pineapple, grapes, carrot etc. (Al-Dhabi et al., 2019a). These substrates act as the important nutrient source and the microorganism secretes extracellular enzymes for modifying and breaking down these solid substrates. In the case of synthetic inert solid materials required nutrient sources were substituted for the growth and product formation of the microorganism (Al-Dhabi et al., 2019b). In SSF, the agro-industrial wastes not only provide required nutrients to the organism, but also anchoring the growth and production of biomolecules (Vijayaraghavan and Vincent, 2012; Al-Dhabi et al., 2020). Generally, antibiotics have been frequently produced by liquid culture and in recent years, SSF is used for antibiotics production. SSF has many advantages over submerged fermentation. SSF is very simple to perform, has high productivity, requires very minimum capital investment, required very low energy, simple culture medium, and application of less water and produces less wastewater and simple downstream processing (Vijayaraghavan and Vincent, 2014; Al-Dhabi et al., 2019c). In bioprocess, product formation is influenced by culture medium and culture conditions applied. The good relationship between metabolite secretion and the composition of the culture medium has been investigated earlier. In Streptomyces sp. metabolite secretion is mainly based on both media components and culture conditions. The concentrations and components of the nutrients in culture medium influenced on metabolite production (Elibol, 2004; Al-Dhabi et al., 2018a). In SSF, requirements of nutrients vary from organism to organism, hence, the important fermentation variables, the suitable culture medium and its components and various other process parameters are required to identify and optimize (Singh et al., 2017; Al-Dhabi et al., 2018b). In Streptomyces sp. various secondary metabolites can be synthesized by the same organism at various pH environments and optimization of factors is very important to achieve a desired antibiotics. Streptomyces are the versatile organisms, produced various degree of secondary metabolite production. In these organisms, intracellular mechanism effectively controls metabolic accumulation, which mainly depends on culture conditions and concentrations of nutrients (Kirk et al., 2000). Hence, the selection and study of suitable culture medium composition is very useful to ensure low costs and high productivity of the secondary metabolite production process.
Streptomyces utilize both complex and simple molecules as energy and nutrient sources (Al-Dhabi et al., 2019d) The genus Streptomyces produces various bioactive secondary metabolites (Arasu et al., 2013a; Al-Dhabi et al., 2014; Arasu et al., 2017). These organisms have lot of biosynthetic potential that remains unchallenged without a valid competitor among other group of organisms. Many Streptomyces species were screened for the production of secondary metabolites from various sources (Liu et al., 2015; Manivasagan et al., 2013; Arasu et al., 2019; Arasu et al., 2015). A total of 500 Streptomyces strains contribute about 70–80% of total antibiotic production (Arasu et al., 2013b). The other genera such as Actinoplanes, Micromonospora, Amycolatopsis and Saccharopolyspora contribute very little (Azman et al., 2015; Arokiyaraj et al., 2015; Balachandran et al., 2015). The important reasons for the discovery of novel antibiotics are to solve the problem of disease resistant, which are no longer effective against some pathogenic organisms. Discovery of various antibiotics from this genus attained good attention from Industrialists and Pharmacologists. SSF was also useful for the production of antimicrobial agents (Rajkumaria et al., 2019; Valsalam et al., 2019a). S. clavuligerus has been cultured under SSF culture condition in optimized medium to enhance the production of cephalosporin C. In another study, S. rimosus was employed for the production of oxytetracycline under SSF (Yang and Swei, 1996). The antimicrobial agent, polyetherin A, also called Nigericin, is a polycyclic polyether secreted by various strains from the genus Streptomyces. It showed very strong activity as antifungal, antibacterial and antimalarial activities (Taechowisan et al., 2013; Gumila et al., 1997; Valsalam et al., 2019b). Industries include vegetables, fruits, meat, dairy, poultry, brewery, marine and grain processing which produced a large amount of waste residues. These industries discard these naturally available nutrient sources as waste material. The Food and Agriculture Organization estimated that, in every year more than 1.3 billion tons of food materials wasted. This waste includes, 35% sea food, 20% dairy products, 30% cereals and 20% meat. Agro residues are widely used as the substrate for the production various novel antibiotics
Rice bran contains rich of protein, fiber and edible oil and has been used frequently for the production of various biomolecules (Zhang et al., 2012). In SSF, the medium components are initially screened for the production of products and further optimized to enhance the product yield by statistical approach. In antibiotics production, nutrient components and physical conditions are the limiting factors. Designing of experiments by statistical method is an efficient tool to design the experiment by statistical mean with relatively small number of experimental run and minimum effort. The aim of the present study is to optimize the bioprocess variables by response surface methodology.
2 Materials and methods
2.1 Isolation of actinomycetes species
Sampling and isolation of actinomycetes
A total of 10 mangrove samples were collected from Manakudy mangrove at three different stations using pre-labelled plastic containers using a spatula and tightly closed. The collected samples were stored in an ice box and transferred to the laboratory. In this study, the physico-chemical properties of soil samples of three sampling sites were analyzed to study the nature of soil. The properties such as, texture, colour, salinity, nitrogen, phosphorus content, pH, magnesium, chloride and calcium content were analyzed. Actinomycetes were initially isolated from the sample using spread plate method using actinomycetes isolation agar (g/L) (sodium caseinate-2, L-Asparagine-0.1, sodium propionate-4, dipotassium phosphate-0.5, magnesium sulphate-0.001 and agar-15) and starch casein agar (g/L) soluble starch-10, casein-0.3, potassium nitrate-2, magnesium sulphate-0.05, di-potassium hydrogenphosphate-2, sodium chloride-2, ferrous sulphate-0.01 and agar-18), and incubated for 14 to 21 days at 28 °C. The culture media were supplemented with nystatin (50 µg/ml) and gentamycin sulphate (25 µg/ml) to reduce fungal and bacterial contamination, respectively. After 28 days of incubation, the culture was purified by standard method (Hayakawa and Nonomura, 1987; Crawford et al., 1993; Yu et al., 2013).
2.2 Primary screening of actinomycetes for antibiotics production
The antibacterial activity was tested against Staphylococcus aureus MTCC96, Bacillus subtilis MTCC 441 and Escherichia coli MTCC 64. The isolated Actinomycetes were screened for antagonistic activity by inoculating a single line streak of the Actinomycetes in the centre of the petri plate. Then the plates were incubated for 6 days at 28 °C and seeded with various bacterial strains by a single streak and the plates were further incubated at 28 °C for 1–2 days. The antagonistic activity was then determined as zone of inhibition (mm) (Wu, 1984). Streptomyces strain showing good activity against test bacteria was further tested by agar well method.
2.3 Secondary screening
The isolated actinomycetes which showed potent activity against B. subtilis MTCC 441 were inoculated into the Actinomycetes culture medium and incubated for 2 weeks at 175 rpm (Madigan et al., 1997). After 2 weeks, the secondary metabolites were extracted using solvent such as, methanol, ethanol, ethyl acetate, hexane and acetone. The solvent was evaporated and antibacterial assay was performed by Kirby-Bauer method. The ethyl acetate fraction was loaded on a sterile 6 mm disc, dried and placed on B. subtilis MTCC 441 culture plates. To the control disc, ethyl acetate was loaded and dried. The zone of diameter was obtained and the size of zone of inhibition was considered as antibiotic titre as described by Maxwell et al. (1994).
2.4 Morphological characterization and 16S rDNA sequencing
The maximum antibiotics producing Streptomyces strain was grown on ISP medium and the culture was identified based on morphological characters, including pigment production, morphology of spores and aerial hyphae. The isolated potent actinomycete was grown actinomycetes isolation agar medium. 16S rDNA gene sequencing was performed by standard method. Genomic DNA of the potent Streptomyces sp. was performed using DNA purification kit (Qiagen, USA). The 16S rRNA gene was amplified by using the forward (5′-GTTGGATCCAGAGTTTGATCMTGGCT-3′) and reverse (5′- GTTGGATCCACGGYTACCTTGTTA CG-3′), primer (Sambrook et al., 1989).
2.5 Agro-industrial wastes for the production of antibiotics
Pineapple peel, wheat bran, apple pomace, rice bran, tapioca powder, green gram husk and orange peel were dried at 60 °C for overnight. All agro residues were ground well and passed through using a standard mesh (30 mesh sieve). All substrates were directly used without any pretreatment.
2.6 Antibiotics production by solid-state fermentation (SSF)
About 5 g of substrate (pineapple peel, wheat bran, apple pomace, rice bran, tapioca powder, green gram husk and orange peel) was taken and the moisture content was adjusted to 65%. The flask was sterilized and 0.5 ml inoculum was introduced. The culture was mixed frequently and incubated for 8 days at 30 ± 2 °C. After 8 days, the crude secondary metabolites were extracted, filtered, centrifuged and used as the crude antibiotics.
2.7 Screening of variables by traditional method
Antibiotics production was performed using green gram husk as the solid substrate until otherwise stated. The process parameters such as, carbon source, nitrogen sources, divalent ions and minerals were evaluated. The selected medium components were supplemented into the Erlenmeyer flask containing green gram husk as the major nutrient medium. After sterilization, cooled the culture medium, 0.5 ml inoculum (10%, v/w) was inoculated in the Erlenmeyer flasks. The antibiotics production of Streptomyces sp. was measured after 8 days of incubation at 28 ± 2 °C.
2.8 Optimization of medium components
CCD and Response Surface Methodology (RSM) were used to screen maltose, CaCl2 and NaH2PO4 for enhanced production of antibiotics based on the activities obtained from traditional method of optimization. The selected medium components were studied to determine the optimum value. The experiment was performed in 20 different experiment trials with triplicate experiment analysis. For the calculation of statistical model, the matrix was designed based on the following equation −1.where xi – denotes selected independent variables for coded value, Xi – original value of the selected variable, X0 – centre point value of the actual value of the independent variable, and –Xi step change value.
Central composite design and response surface methodology was employed to optimize the process variables. The quadratic model equation is useful to analyze the behaviour of quadratic equation.where Y = predicted response, β0 = intercept term, βi = linear coefficient, βij = quadratic coefficient, β = interaction coefficient, XiXi = independent variables.
Analysis of variance (ANOVA) was used to verify the designed model. The significance of the designed model was determined using F-test and the p-value < 0.05 was considered as the designed CCD model was significant. In this experiment, the response which was predicted was validated by triplicate experiment.
3 Result and discussion
3.1 Antibiotics producing actinomycete
Totally, 36 mangrove actinomycetes were screened from three selected sites in Manakudy mangrove forest, Southern India (Fig. 1). The physico-chemical parameters of these three sites were varied widely and the results were tabulated in Table 1. The number of isolated actinomycetes varied among stations. Among all the selected sites, site 3 showed 71% of actinomycetes. Ethyl acetate extract of these isolates effectively inhibited the growth of bacterial pathogens. Actinomycetes isolated from the mangrove soil sediments showed diverse colony shape, colour and texture. Initially, the antimicrobial activity screening was evaluated from the selected Actinomycetes against Staphylococcus aureus MTCC96, Bacillus subtilis MTCC 441 and Escherichia coli MTCC 64. About 59% of the isolates showed antimicrobial properties and most of the samples were found to be effective against Bacillus subtilis MTCC 441. Among the actinomycetes, Streptomyces sp. SD1 showed hyper production; hence this was retained for enhanced production of antimicrobial agents. The morphological characters revealed that it comes under Streptomyces sp. The selected isolate was identified by molecular approach. The growth characteristics of the selected Streptomyces sp. was carried out at various pH and temperature levels. The present finding revealed that this isolate was able to grow well between 22 and 37 °C and at pH ranges from 6.5 to 8.0. The maximum growth was obtained at 28 °C and pH 7.2. This isolate was positive for chitinase, catalase and oxidase test, starch hydrolysis, and showed negative result for indole test and positive for the production of melanin. Actinomyces have been used to produce various antimicrobial lead molecules. The threat of various multiple drug resistant organisms is a serious concern in various countries (Hamers et al., 2012). The present study highlighted on the isolation and screening of Streptomyces from mangrove originating from three locations was screened for potent antimicrobials. The isolated actinomycetes mainly from the genus Streptomyces sp. showed various biological properties. Previously, many Streptomyces sp. were screened for antimicrobials isolated from aquatic environment (Bruntner et al., 2005; Rao et al., 2012). Based on the present finding, Streptomyces sp. SD1 was selected for optimized production of antibiotics in SSF. SSF is effective to synthesize broad spectrum of antimicrobial agents with novel properties, because the process conditions are very closely resemble with natural habitats of Streptomyces sp. than submerged fermentation.
Soil sampling station of Manakudy mangrove forest. Samples were collected from these three stations at 10 places. The colour of the soil was black and was collected using a sterile spatula.
Soil properties
Sampling sites
Station 1
Station 2
Station 3
Texture
Sandy clay
Sandy loamy clay
Sandy clay
Colour
Black
Black
Black
Salinity (ppt)
17.2
10.3
28.3
Nitrogen (g/kg)
57
87
65
Phosphorus (g/kg)
65
53
49
pH
7.6
7.2
7.3
Magnesium (g/kg)
0.41
0.26
0.18
Chloride (mg/kg)
1.3
0.93
1.42
Calcium (mg/kg)
1.6
0.91
2.2
3.2 Agro-industrial residues and antibiotics
The important factors for bacterial growth and production of various biomolecules are particle size, substrate type, and moisture holding capacity of the medium and nutrient value of the raw material in SSF. The small substrate material provides very large surface area for anchoring of microbial cells. At the same time, very small powdery substrates may heavily interfere with respiration of microbial cells, and thus leads to poor growth (Vijayaraghavan and Vincent, 2012; Pandey, 1991). The antibacterial activity of crude extract from Streptomyces sp. SD1 cultured in various substrates was tested against B. subtilis MTCC 441 and the zone of inhibition was determined (Fig. 2a). In the present study, green gram husk stimulated the production of antibiotics than other selected substrates (212 ± 8 U/g substrate) (Fig. 2b). In Streptomyces sp. strain MAR01, wheat bran supported Meroparamycin production than the substrate such as, quaker, bread and ground corn (El-Naggar et al., 2009). Earlier, many Streptomyces sp. have been used for the production of antimicrobial agents using peanut shell, corncob, cassava peels and corn pomace. Among the selected substrates, peanut enhanced tetracycline production (Asagbra et al., 2005).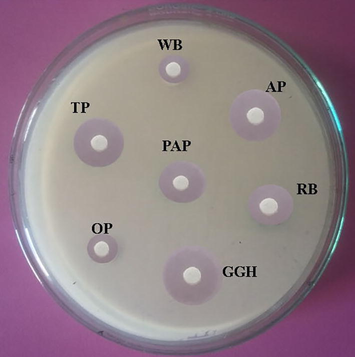
Antibacterial activity of Streptomyces sp. SD1 against B. subtilis MTCC 441. The strain was cultured in various agro-industrial wastes in solid state fermentation and antibacterial activity was assayed (WB – wheat bran; AP – Apple peel; RB – Rice bran; GGH – Green gram husk; OP – Orange peel; TP – Topioca powder and PAP – Pine apple peel). About 20 µl sample was loaded and incubated for 24 h.
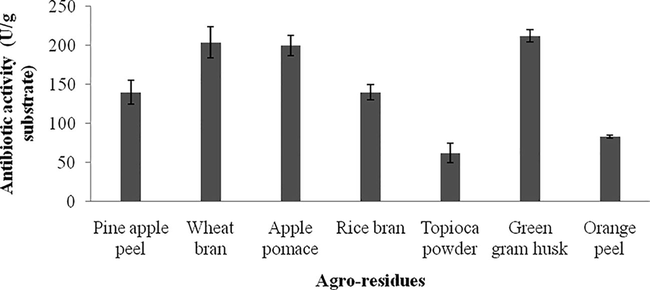
Effect of agro-industrial residues for the production of antibiotics. Streptomyces sp. SD1 was grown on these wastes and antibiotic activity was assayed.
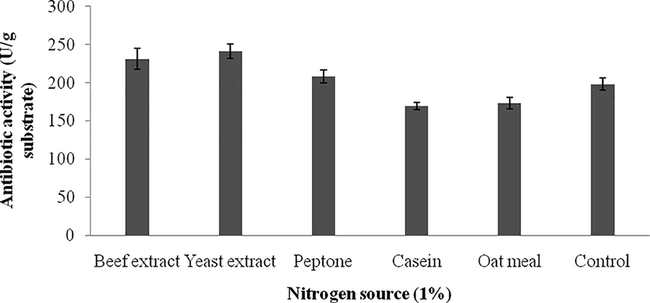
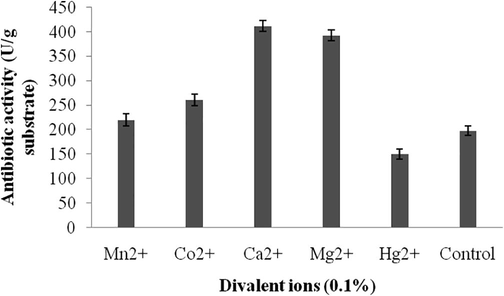
3.3 Screening of nutrient factors by one-variable at a time approach
Carbon sources influenced on antibiotics production and the results were analyzed. Starch, and maltose maximized antibiotics production, however, sucrose and glucose negatively influenced on the production of antibiotics. The zone of inhibition against the tested bacterial strain was described in Fig. 3a and the total enzyme yield was described in Fig. 3b. The stimulatory effect of various nitrogen sources at 1% (w/w) level on antibiotics synthesis was analyzed. Among the nitrogen sources, beef extract, peptone and yeast extract positively influenced on antibiotics production. The zone of inhibition of secondary metabolites against indicator bacterium was described in Fig. 4a. The antibiotics yield vary widely based on the nitrogen sources used in SSF (Fig. 4b). The green gram husk medium was supplemented with divalent ions and minerals, individually to evaluate the stimulatory effect on antibiotics production. The effect of supplementation of various divalent ions at 0.1% (w/w) concentration was tested and the zone of inhibition was described in Fig. 5a. Ions, such as, calcium chloride, magnesium sulphate and manganese chloride enhanced the production of antibiotics, however supplementation of cobalt chloride and mercuric chloride affected antibiotics production (Fig. 5b). Antibiotics production was critically enhanced by the supplementation of various carbon sources as nutrient supplements in fermentation medium (Mahalaxmi et al., 2010). All selected mineral sources, enhanced the production of antibiotics and the yield was higher than control and the zone of inhibition was calculated (Fig. 5c). The production of secondary metabolite was maximum in the culture medium containing sodium dihydrogen phosphate, followed by potassium dihydrogen phosphate (Fig. 5d). In Streptomyces fradiae NCIM 2418, neomycin production was enhanced by copper sulphate and zinc sulphate with the coconut oil cake (Vastrad and Neelagund, 2014). Also, sodium nitrate and ammonium chloride positively influenced on neomycin production, however, the carbon sources, glucose, sucrose, maltose, starch, ferrous sulphate showed less impact on neomycin production. In S. aureofaciens, production of tetracycline was critically influenced when 10% soluble starch was enriched with sweet potato residues (Yang and Ling, 1989). In Streptomyces rimosus, supplementation of 1% (w/w) CaCO3, enhance the production of tracycline in SSF when corncob was used as the solid medium (Yang and Swei, 1996).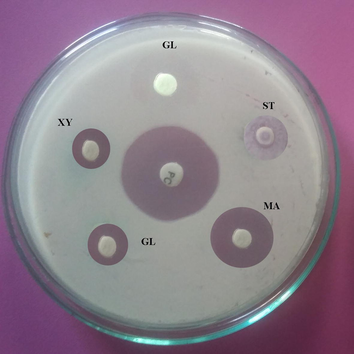
Antibacterial activity of Streptomyces sp. SD1 against B. subtilis MTCC 441. The strain was cultured in green gram husk medium containing various carbon sources in SSF and antibacterial activity was assayed (ST – Starch; MA – Maltose; GL – Glucose; XY – Xylose and SU – Sucrose). About 20 µl sample was loaded and zone of inhibition was measured.
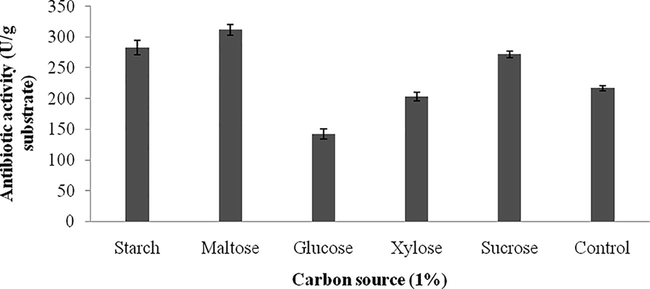
Effect of carbon source (1%) for the production of antibiotics from green gram husk by using Streptomyces sp. SD1 in SSF.
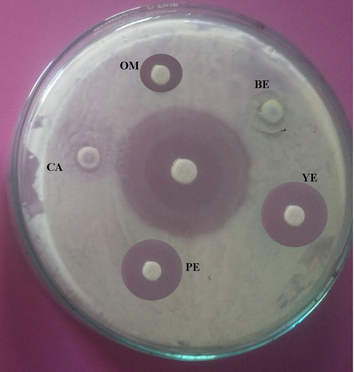
Antibacterial activity of Streptomyces sp. SD1 against B. subtilis MTCC 441. The strain was cultured in green gram husk medium containing various nitrogen sources in SSF and antibacterial activity was assayed (BE – Beef extract; YE – Yeast extract; PE – Peptone; CA – Casein and OM – Oat meal). About 20 µl sample was loaded and zone of inhibition was measured.
Effect of nitrogen source (1%) for the production of antibiotics from green gram husk by using Streptomyces sp. SD1 in SSF.

Antibacterial activity of Streptomyces sp. SD1 against B. subtilis MTCC 441. The strain was cultured in green gram husk medium containing various ionic sources in SSF and antibacterial activity was assayed. The divalent ions, Co2+, Ca2+, Mg2+, Hg2+ and Mn2+ were incorporated with the culture medium. About 20 µl sample was loaded and zone of inhibition was measured.
Effect of ionic source (1%) for the production of antibiotics from green gram husk by using Streptomyces sp. SD1 in SSF.
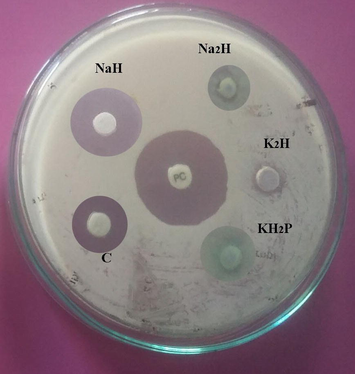
Antibacterial activity of Streptomyces sp. SD1 against B. subtilis MTCC 441. The strain was cultured in green gram husk medium containing various mineral sources (Na2H – disodium hydrogen phosphate; K2H – dipotassium hydrogen phosphate; KH2P – potassium dihydrogen phosphate; NaH – sodium dihydrogen phosphate; C – control). About 20 µl sample was loaded and zone of inhibition was measured.
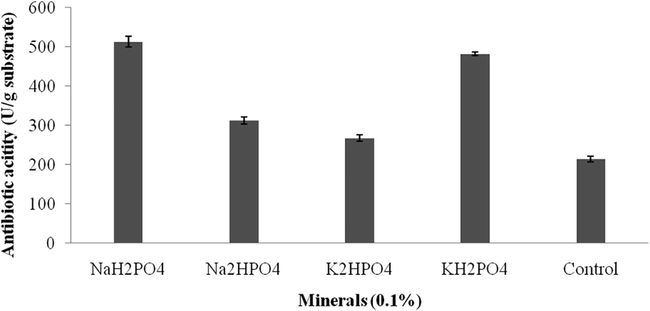
Effect of minerals (1%) for the production of antibiotics using green gram husk by Streptomyces sp. SD1 in SSF.
3.4 Central composite design and response surface methodology
The selected nutrient factors enhanced the production of antibiotics and the yield varies from 246 U/g to 1459 U/g. The designed matrix and the response of CCD were tabulated (Table 2a). The F-value of the fitted quadratic design was 167.79 implies the designed quadratic model is statistically significant. In this designed model all factors and interactive effects were statistically significant. The predicted R2 value was 0.9663 and the adjusted R2 value was 0.987. The lack of fit value was 0.3654 and was statistically insignificant (Table 2b). As described in the results, RSM are key tool for identifying and determining the concentration of nutrients for antibiotics production. The 3D graph described the interaction between Maltose and CaCl2, Maltose and NaH2PO4, Maltose and NaH2PO4. All three 3D response surface graphs showed maximum yield of antibiotics at higher concentration of nutrient sources (Fig. 6a–c). The predicted response was 1468 U/g and this was almost similar with experimental value (1473 U/g) validate the designed model. In this study, antibiotics production enhanced 2.9 fold by Streptomyces sp. SD1 in optimized medium than unoptimized medium. In recent years, RSM has been extensively used for the production of various antibiotics. RSM was used for the synthesis of neomycin (Vastrad and Neelagund, 2014), fibrinolytic enzymes (Vijayaraghavan et al., 2016) and streptomycin (Saval et al., 1993).
Std
A:Maltose
B:CaCl2
C:NaH2PO4
Antibiotic activity (U/g)
1
0.10
1.50
1.00
479
2
0.30
0.90
0.55
990
3
0.30
0.90
0.55
976
4
0.50
1.50
0.10
872
5
0.30
0.90
0.55
908
6
0.30
−0.11
0.55
375
7
0.50
0.30
1.00
900
8
0.30
0.90
1.31
919
9
0.30
0.90
0.55
976
10
0.10
0.30
1.00
513
11
0.30
0.90
−0.21
1459
12
0.50
0.30
0.10
1047
13
0.64
0.90
0.55
745
14
−0.04
0.90
0.55
475
15
0.30
1.91
0.55
246
16
0.50
1.50
1.00
430
17
0.10
0.30
0.10
600
18
0.30
0.90
0.55
987
19
0.10
1.50
0.10
945
20
0.30
0.90
0.55
976
Source
Sum of Squares
df
Mean Square
F-value
p-value
Model
1.667E + 06
9
1.852E + 05
167.79
<0.0001
significant
A-Maltose
99565.54
1
99565.54
90.19
<0.0001
B-CaCl2
22226.74
1
22226.74
20.13
0.0012
C-NaH2PO4
3.078E + 05
1
3.078E + 05
278.79
<0.0001
AB
1.142E + 05
1
1.142E + 05
103.48
<0.0001
AC
162.00
1
162.00
0.1467
0.7097
BC
56784.50
1
56784.50
51.44
<0.0001
A2
2.049E + 05
1
2.049E + 05
185.57
<0.0001
B2
7.303E + 05
1
7.303E + 05
661.56
<0.0001
C2
1.053E + 05
1
1.053E + 05
95.38
<0.0001
Residual
11039.54
10
1103.95
Lack of Fit
6406.71
5
1281.34
1.38
0.3654
not significant
Pure Error
4632.83
5
926.57
Cor Total
1.678E + 06
19
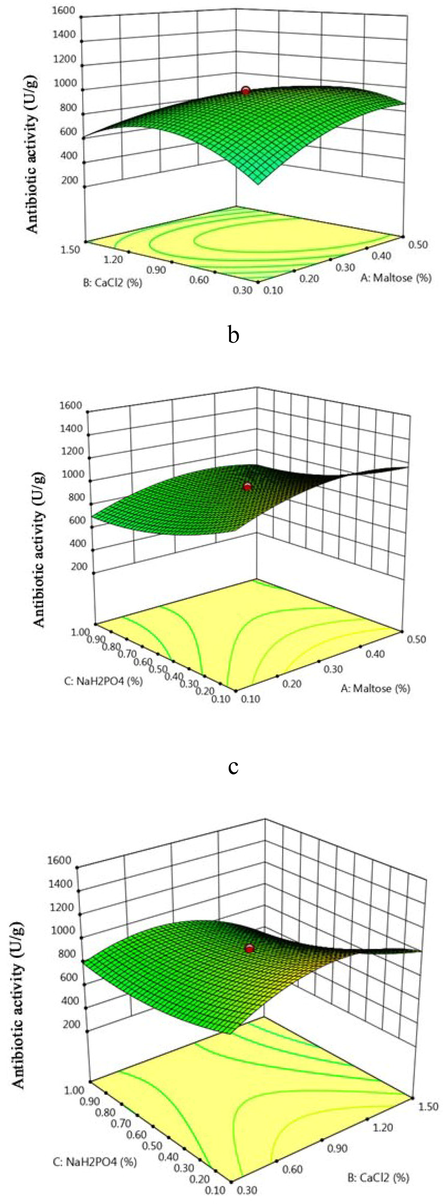
a–c. Response surface plots explaining the individual and interactive effects of factors on the antimicrobial activity of Streptomyces sp. SD1.
4 Conclusion
Thirty-six morphologically different actinomycete strains were isolated from mangrove soil sediments. Among the isolates, Streptomyces sp. SD1 showed high antimicrobial activity against Gram positive bacterial pathogen. The selected Streptomyces sp. SD1 utilized green gram husk as the cheap substrate for antibiotics production. Also addition of carbon, nitrogen and ionic sources stimulated the production of antibiotics. RSM optimized medium enhanced 2.9-fold antibiotics production.
Acknowledgement
The authors extend their appreciation to The Researchers Supporting Project Number (RSP-2019/116) King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Antifungal metabolites from sponge associated marine Streptomyces sp. strain (ERIMA-01) J. Pure Appl. Microbiol.. 2014;8(2):115-128.
- [Google Scholar]
- Isolation, identification and screening of antimicrobial thermophilic Streptomyces sp. Al-Dhabi-1 isolated from Tharban hot spring, Saudi Arabia. Extremophiles. 2016;20:79-90.
- [Google Scholar]
- Characterization and fermentation optimization of novel thermo stable alkaline protease from Streptomyces sp. Al-Dhabi-82 from the Saudi Arabian environment for eco-friendly and industrial applications. J. King Saud Univ. – Sci.. 2020;32(1):1258-1264.
- [Google Scholar]
- Al-Dhabi, N.A., Ghilan, A.K.M., Esmail, G.A., Arasu, M.V., Duraipandiyan, V., Ponmurugan, K., 2019. Bioactivity assessment of the Saudi Arabian Marine Streptomyces sp. Al-Dhabi-90, metabolic profiling and its in vitro inhibitory property against multidrug resistant and extended-spectrum beta-lactamase clinical bacterial pathogens, J. Infection Public Health 549–556.
- Characterization of silver nanomaterials derived from marine Streptomyces sp. Al-Dhabi-87 and its in vitro application against multidrug resistant and extended-spectrum beta-lactamase. Clin. Pathogens. Nanomater.. 2018;8(5)
- [Google Scholar]
- Green biosynthesis of silver nanoparticles produced from marine Streptomyces sp. Al-Dhabi-89 and their potential applications against wound infection and drug resistant clinical pathogens. J. Photochem. Photobiol., B. 2018;189:176-184.
- [Google Scholar]
- Chemical profiling of Streptomyces sp. Al-Dhabi-2 recovered from an extreme environment in Saudi Arabia as a novel drug source for medical and industrial applications. Saudi. J. Biol. Sci.. 2019;26:758-766.
- [Google Scholar]
- Isolation and screening of Streptomyces sp. Al-Dhabi-49 from the environment of Saudi Arabia with concomitant production of lipase and protease in submerged fermentation. Saudi. J. Biol. Sci. 2019
- [CrossRef] [Google Scholar]
- Environmental friendly synthesis of silver nanomaterials from the promising Streptomyces parvus strain Al-Dhabi-91 recovered from the Saudi Arabian marine regions for antimicrobial and antioxidant properties. J. Photochem. Photobiol., B: Biol.. 2019;197:111529
- [Google Scholar]
- Antifeedant, larvicidal and growth inhibitory bioactivities of novel polyketide metabolite isolated from Streptomyces sp. AP-123 against Helicoverpa armigera and Spodoptera litura. BMC Microbiol.. 2013;13(1):105.
- [Google Scholar]
- Antibacterial and antifungal activities of polyketide metabolite from marine Streptomyces sp. AP-123 and its cytotoxic effect. Chemosphere. 2013;90(2):479-487.
- [Google Scholar]
- Identification of novel quinine metabolite from marine actinomycetes with antifungal and anticancer bio-prospective. Fresenius Environ. Bull.. 2015;24(10a):3281-3287.
- [Google Scholar]
- Green chemical approach towards the synthesis of CeO2 doped with seashell and its bacterial applications intermediated with fruit extracts. J. Photochem. Photobiol., B. 2017;172:50-60.
- [Google Scholar]
- One step green synthesis of larvicidal, and azo dye degrading antibacterial nanoparticles by response surface methodology. J. Photochem. Photobiol., B. 2019;190:154-162.
- [Google Scholar]
- Green synthesis of Silver nanoparticles using aqueous extract of Taraxacum officinale and its antimicrobial activity. South Indian J. Bio. Sci.. 2015;2:115-118.
- [Google Scholar]
- Solid-state fermentation production of tetracycline by Streptomyces strains using some agricultural wastes as substrate. World J. Microbiol. Biotechnol.. 2005;21:107-114.
- [Google Scholar]
- Mangrove rare actinobacteria: taxonomy, natural compound, and discovery of bioactivity. Front. Microbiol.. 2015;6:856.
- [Google Scholar]
- Antimicrobial and cytotoxic properties of Streptomyces sp. (ERINLG-51) isolated from Southern Western Ghats. South Indian J. Biol. Sci.. 2015;1:7-14.
- [Google Scholar]
- Frigocyclinone, a novel angucyclinone antibiotic produced by a Streptomyces griseus strain from Antarctica. J. Antibiot.. 2005;58:346-349.
- [Google Scholar]
- Isolation and characterization of actinomycete antagonists of a fungal root pathogen. Appl. Environ. Microbiol.. 1993;59(11):3899-3905.
- [Google Scholar]
- Optimization of medium composition for actinorhodin production by Streptomyces coelicolor A3(2) with response surface methodology. Process Biochem.. 2004;39:1057-1062.
- [Google Scholar]
- El-Naggar, Moustafa, Y., El-Assar, S.A., Abdul-Gawad, S.A., 2009. Solid-state fermentation for the production of meroparamycin by Streptomyces sp. strain MAR01. J. Microbiol. Biotechnol. 19, 468–473.
- Characterization of the potent in vitro and in vivo antimalarial activities of ionophore compounds. Antimicrob. Agents Chemother.. 1997;41:523-529.
- [Google Scholar]
- Global threat from drug resistant HIV in sub Saharan Africa. British Med. J.. 2012;344:e4159
- [Google Scholar]
- Humic acid-vitamin agar, a new medium for the selective isolation of soil actinomycetes. J. Ferment. Technol.. 1987;65(5):501-509.
- [Google Scholar]
- Growth limiting substrate affects antibiotic production and associated metabolic flues in Streptomyces clavuligerus. Biotechnol. Lett.. 2000;22:1803-1809.
- [Google Scholar]
- Microbacterins A and B, new peptaibols from the deep sea actinomycete Microbacterium sediminis sp. nov. YLB-01(T) Org. Lett.. 2015;17:1220-1223.
- [Google Scholar]
- Madigan, M.T., Martiko, J.M., Parker, J., 1997. Antibiotics: isolation and characterization, in Brock Biology of Microorganisms, 8th Edn., ed M. T. Madigan (New Jersey: Prentice-Hall International Inc., 440–442.
- Corn husk as a novel substrate for the production of rifamycin B by isolated Amycolatopsis sp. RSP 3 under SSF. Process Biochem.. 2010;45:47-53.
- [Google Scholar]
- Marine actinobacterial metabolites: current status and future perspectives. Microbiol. Res.. 2013;168:311-332.
- [Google Scholar]
- Stability and activities of antibiotics produced during infection of the insect Galleria mellonella by two isolates of Xenorhabdus nematophilus. Appl. Environ. Microbiol.. 1994;60:715-721.
- [Google Scholar]
- Effect of particle size of substrate on enzyme production in solid-state fermentation. Bioresour. Technol.. 1991;37:169-172.
- [Google Scholar]
- Rajkumaria, J., Magdalane, M., Siddhardh, B., Madhavan, J., Ramalingam, G., Al-Dhabi, N.A., Arasu, M.V., Ghilan, A.K.M., Duraipandiayan, V., Kaviyarasu, K., 2019. Synthesis of titanium oxide nanoparticles using Aloe barbadensis mill and evaluation of its antibiofilm potential against Pseudomonas aeruginosa PAO1. J. Photochem. Photobiol. B: Biol. 111667.
- Isolation and characterization of antagonistic actinobacteria from mangrove soil. J. Biochem. Technol.. 2012;3:361-365.
- [Google Scholar]
- Molecular Cloning: A Laboratory Manual. Woodbury, NY: Cold Spring Harbor Laboratory Press; 1989.
- Optimization of a culture medium for streptomycin production using response-surface methodology. Bioresour. Technol.. 1993;43:19-25.
- [Google Scholar]
- Strategies forfermentation medium optimization: an in-depth review. Front. Microbiol.. 2017;7:2087.
- [Google Scholar]
- Antibacterial activity of 1-methyl ester-nigericin from Streptomycxes hygroscopicus BR10: An endophyte in Alpinia galanga. J. Appl. Pharm. Sci.. 2013;3:104-109.
- [Google Scholar]
- Biosynthesis of silver and gold nanoparticles using Musa acuminata colla flower and its pharmaceutical activity against bacteria and anticancer efficacy. J. Photochem. Photobiol., B 2019111670
- [Google Scholar]
- Rapid biosynthesis and characterization of silver nanoparticles from the leaf extract of Tropaeolum majus L. and its enhanced in-vitro antibacterial, antifungal, antioxidant and anticancer properties. J. Photochem. Photobiol., B. 2019;191:65-74.
- [Google Scholar]
- Optimization of medium composition for the production of neomycin by Streptomyces fradiae NCIM 2418 in solid state fermentation. Biotechnol. Res. Int. 2014:11. Article ID 674286
- [Google Scholar]
- Vijayaraghavan, P., Prakash Vincent, S.G., 2014. Statistical optimization of fibrinolytic enzyme production by Pseudoalteromonas sp. IND11 using cow dung substrate by response surface methodology. SpringerPlus, 2014, 3, 60.
- Cow dung as a novel, inexpensive substrate for the production of a halo-tolerant alkaline protease by Halomonas sp. PV1 for eco-friendly applications. Biochem. Eng. J.. 2012;69:57-60.
- [Google Scholar]
- Cow dung is a novel feedstock for fibrinolytic enzyme production from newly isolated Bacillus sp. IND7 and its application in In Vitro clot lysis. Front. Microbiol.. 2016;7:361.
- [Google Scholar]
- Studies on the Streptomyces SC4. II Taxonomic and biological characteristics of Streptomyces strain SC4. Bot. Bull. Acad. Sin.. 1984;25:111-123.
- [Google Scholar]
- Tetracycline production with sweet potato residue by solid-state fermentation. J. Biotechnol. Bioeng.. 1989;33:1021-1028.
- [Google Scholar]
- Cultural condition and oxytetracycline production by Streptomyces rimosus in solid state fermentation of corncob. World J. Microbiol. Biotechnol.. 1996;12:43-46.
- [Google Scholar]
- Diversity and abundance of phosphonate biosynthetic genes in nature. Proc. Nat. Acad. Sci. USA. 2013;110(51):20759-20764.
- [Google Scholar]
- Preparation and functional properties of rice bran proteins from heat-stabilized defatted rice bran. Food Res. Int.. 2012;47:359-363.
- [Google Scholar]







