Translate this page into:
Enhanced cellulase enzyme production by Aspergillus niger using cellulase/iron oxide magnetic nano-composites
⁎Corresponding author. thirubiotech@gmail.com (Poovanalingam Thirumalai Vasan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
The cellulase enzyme is used for various industrial applications such as textile, paper, food and biofuel industries. Industrially, the fungal strain, Aspergillus niger, is widely used to produce cellulase enzymes. Emerging evidence has indicated the possible role of magnetic nanocomposites in enhancing cellulase production by A. niger. The cellulase/iron oxide nanocomposites are already reported to be an eco-friendly method for modulating microbial biochemical characteristics.
Methods
The present study was used to assess the efficacy of cellulase/iron oxide magnetic composites (CMNPS) for the enhanced production of cellulase enzyme. The fungal strain Aspergillus niger was isolated from the soil samples by using standard techniques. The fungi were then transfected with CMNPS and further evaluated for the production of cellulase enzyme.
Results
Synthesis of the cellulase/iron oxide magnetic composites was characterized by UV, FTIR, and XRD. The produced CMNPS was used as substrate and enzyme production. The cellulase enzyme production by Aspergillus niger was analyzed by CMC (0.82 IU/ml enzyme activity) and FPA assay (0.039 IU/ml enzyme activity). CMNPS was also analyzed by CMC (0.74 IU/ml enzyme activity) and FPA assay (0.039 IU/ml enzyme activity). Reuse of CMNPS is the ultimate application and ecofriendly approach for the synthesis of cellulase enzymes.
Conclusion
The study concludes that the cellulase/ iron oxide nanocomposites may be a useful tool for the enhanced production of cellulase enzyme from the soil fungus A. niger.
Keywords
Cellulase
Cellulase/iron oxide magnetic composites
Carboxymethyl cellulose assay
Filter paper assay
Aspergillus niger
1 Introduction
Cellulases are a kind of enzyme that breaks down cellulose, the predominant component of the cell walls of the plant biomass (Garg et al., 2016). Cellulases are important industrial enzymes synthesized by microorganisms such as fungi and bacteria using cellulosic materials (EFSA Panel on Food Contact Materials et al., 2019). The action of the Cellulase enzyme mainly comes from three major enzymes that confirm the efficiency of this process. They are endoglucanases, exoglucanases, and β-glucosidase. Β-1,4 linkages present in cellulose chains can be easily broken up by the cellulase enzymes. Enzymes are one of the key materials that are widely recognized for their diverse applications at the industrial level (Kuhad et al., 2011a). Cellulases enzymes are utilized for textile, biofuel, paper, food, and detergent industries (Li et al., 2018).
Cellulose is one of the richest biomass found on Earth. It is the prime product of photosynthesis in terrestrial environments and the richest renewable bioresource formed in the biosphere (Yamazawa et al., 2013). The cellulosic waste materials are classified as industrial, agricultural and municipal wastes (Omojasola and Jilani, 2008). The cellulosic waste materials are pretreated after usage. The common methods available for pretreatment are physical pretreatment, chemical pretreatment, and biological pretreatment methods. In the industrial sectors, widely used pretreatment methods are acid or alkaline treatment, the explosion of steam, wet oxidation, hot water and organic solvent pretreatment. Among them, acid pretreatment is the method of option in several industries processes (Kumar and Sharma, 2017).
Cellulase enzyme can be produced by various industrially important microorganisms, which includes fungi (Aspergillus fumigatus, Fusarium solani, Trichoderma reesei and Sclerotium rolfsii) as well as bacteria (Clostridium thermocellum, Ruminococcus albus and Streptomyces species) using different substrates (Cheese whey, Baggase and Rice straw) through submerged and solid-state fermentation (Mrudula and Murugammal, 2011). Filamentous fungi are the best choice for commercial enzyme production because the level of the enzymes produced by filamentous fungi is higher than those collected from yeast and bacteria. Almost all species of the genus Aspergillus is synthesizing cellulase (Mrudula and Murugammal, 2011). A large volume of cellulase enzyme is required for the industrial processes to break down cellulose. To minimize this demand, cellulase enzymes must be reused or recycled effectively.
In order to reuse the cellulase enzyme, the current study is planned to synthesize CMNPS, which may enable the reuse of cellulase. In recent years, “nanotechnology” has spread to almost all aspects of science and technology with several beneficial applications (Singh, 2017). In specific, magnetic nanoparticles based on magnetite (Fe3O4) for enzymes immobilization have gained high attention in different fields, which includes biomedical and environmental applications, due to their tiny size, large specific surface area, very little toxicity and ecofriendly nature (Joseph et al., 2020). The main advantages of using these magnetic composites are their easy and immediate separation from the mixture by creating an external magnetic field that may be appropriate for the reuse of cellulase enzyme (Sánchez-Ramírez et al., 2017).
Overall, the present study aims to isolate the soil Aspergillus niger strain and the enhancement of cellulase production by using metal nanocomposites, especially derived from iron oxide nanoparticles.
2 Materials and methods
2.1 Isolation of microbes from a soil sample
The soil sample was collected from the garden area located at Thiruvanaikovil, Tiruchirappalli, Tamil Nadu. The collected sample was kept in a sterile container and transferred to the laboratory. In the laboratory it was serially diluted, plated and fungus was isolated as per standard protocols described by Jin et al. (2019).
2.1.1 Identification and characterization of the isolated fungus
The isolated fungus was identified and characterized through their morphological and microscopic observation. Smears of the isolated fungi were prepared in Lactophenol cotton blue and examined with a microscope (McClenny, 2005).
2.2 Screening of Aspergillus niger for cellulase activity
Primary qualitative analysis was carried out using Congo red dye for the confirmation of cellulolytic microorganisms. The isolated fungus was inoculated in the CMC agar plate and incubated for 24 hrs at 25 °C. After successful incubation, the culture plate was flooded with Congo red (1%) and allowed to stand for 15 min at room temperature. After 15 min, the material was counterstained with NaCl (1 M) solution and left undisturbed for another 15 min. Finally, the tested plates which showed a clear zone around the line of growth confirm cellulose hydrolysis (Ahmad et al., 2020).
2.3 Synthesis of CMNPS
The CMNPS was prepared using the co-precipitation method. Ferric chloride (Three equivalent), Ferrous sulphate (Two equivalent), and 5μM of commercial A. niger cellulase were added and stirred for 15 min. Once the mixing is complete, about 50 ml of NaCl (0.2 M) was added to it and stirring was continued for another 1 h. The ammonium hydroxide 200 ml (w/v) was added at a slow rate with continuous stirring for 15 min. The black colour precipitate formed was washed with 5% ammonium hydroxide solution and the precipitate was then dried in the presence of nitrogen flow (Hassan et al., 2019).
2.4 Characterization of synthesized CMNPS
The structure and composition of the CMNPS were confirmed by UV, FTIR and XRD analysis. UV–visible spectroscopic analysis was performed on Hitachi double beam equipment (Model Lambda 35), in the 200–1100 nm range. In the FTIR spectrum (Spectrum RX 1-One), all spectral transmittance was acquired over the mid-infrared region (4000–400 cm−1) using 32 scanning at a resolution of 8 cm−1. For quantitative analysis of functional groups, single-beam attenuated total reflectance (ATR) spectra were collected from each sample and recorded. As a background, an air spectrum was used. Spectra were recorded repeatedly and average calculated before being used for model optimization (Rohman et al., 2014).
In XRD the crystalline nature and average size of the particles of the synthesized CMNPS were analyzed by X-ray diffraction (XRD) at 25 °C with a D8 Advanced X-ray diffractometer (Gonio model) using CuKα (λ = 1.54060 Å) radiations as an X-ray source and nickel (Ni) as a filter (Yu et al., 2013).
2.5 Production of cellulase enzyme
The Czapek dox media was used for the production of the cellulase enzyme. The media was dispensed into a 250 ml conical flask using a measuring cylinder and 1.0 g of each of the CMNPS were added into the Erlenmeyer flask and labelled properly. CMC was used as a control and sterilized in Autoclave at 121 °C for 15 min (Ghose, 1987).
2.6 Cellulase enzyme assay
The sample was collected and centrifuged at 2800 g for 10 min. The supernatant collected from centrifugation contains the enzyme. The supernatant was analyzed for the Endo-β-1,4- Glucanase assay and filter paper assay (FPA) (Ferrari et al., 2014).
2.6.1 CMC (Carboxymethylcellulose) assay
The Citrate buffer (0.1 M) was taken and about 1 ml of cell-free supernatant was added to the tube; it was then incubated at 50 °C for 30 min followed by the addition of 3 ml of 3, 5-dinitro salicylic acid (DNS). The tubes were incubated at 100 °C in a boiling water bath for 15 min and the optical density of the solution was determined spectrophotometrically at 540 nm. The glucose was used as a standard for final calculations (Yan and Chai, 2021).
2.6.2 FPA assay (filter paper assay)
Sodium citrate, filter paper strip, and 1 ml of culture supernatant were added to a test tube, and the tube was incubated at 50 °C at 60 min. After the incubation period, about 3 ml of DNS was added to a test tube and further maintained at 100 °C in a boiling water bath (15 min). The optical density of the solution was read at 540 nm using a spectrophotometer (Yu et al., 2016).
3 Results
3.1 Screening of Aspergillus niger for cellulase activity
The preliminary qualitative analysis was conducted by using Congo red dye for cellulolytic microorganisms. Fig. 1 shows isolated fungus produced the cellulase enzyme. 2 cm of clear zone appeared. The plates that showed a zone of clearance around the line of growth indicated cellulose hydrolysis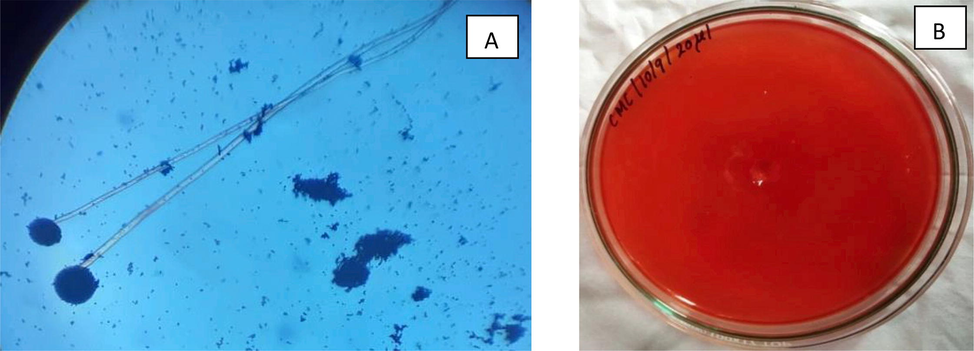
Identification of Aspergillus niger from the soil sample, cultured using standard protocols (A) and Screening of Aspergillus niger colony using the cellulose hydrolysis assay (B).
3.2 Characterization of synthesized CMNPS
The CMNPS is separated using a magnet (Fig. 2) from the medium and the CMNPS is reused for the next industrial process. The Czapek dox media was used for the production of the cellulase enzyme. The cellulase enzyme assay and FPA were used to analyze enzyme production.
Demonstration of the attraction potential of Cellulase/Iron oxide magnetic composites (CMNPS) towards a magnet kept aside.
3.2.1 UV–VIS spectroscopy
The UV/visible absorption spectra for CMNPS are shown in Fig. 3. The magnetite composites show a surface Plasmon resonance (SPR) band at 400 nm. The SPR band of the magnetite composites shows a redshift and broadening of the peak in the spectrum.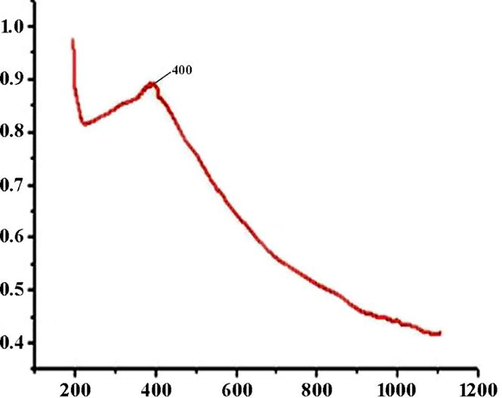
UV –Visible Spectroscopic analysis of Cellulase/Iron oxide magnetic composites (CMNPS) from 200 to 1200 nm.
3.2.2 Fourier transform infrared spectrometer (FTIR)
The FTIR spectrum of the CMNPS is shown in Fig. 4. The transmittance band at 3409 cm−1 corresponds to the OH stretching vibration of cellulose. The band at 2922 cm−1 denotes the C–H asymmetric and symmetric tensile vibration in the pyranoid ring and the peak at 1634.01 cm−1 is formed due to the OH bending vibration. The CH2 symmetric scissoring in the pyranoid ring, C–O anti-symmetric bridge stretching, the crystal absorption peak of cellulose, C–O–C pyranoid ring skeletal vibration, and the n-glycosidic linkages are attributed to the absorption peaks at 1420.27, 1111.85, 1033.87, and 1057.53, respectively. The peak at 1634.01 cm−1 formed from the bending mode of the absorbed water. In the CMNPS, a new peak develops at 447 cm−1, which can be attributed to the vibration of γ-Iron oxide due to the overlapping bands around 583.05 cm−1 in cellulose and the CMNPS.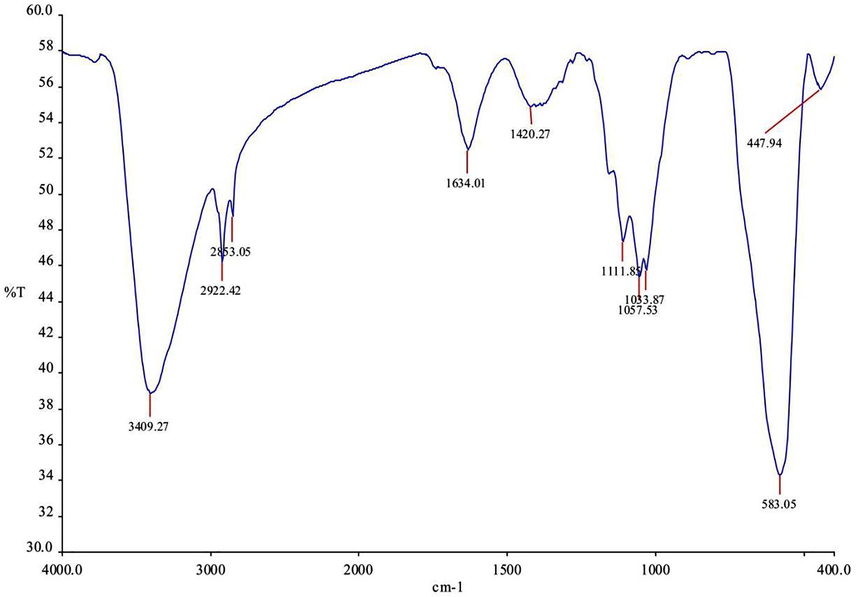
Fourier transform infrared Spectroscopic analysis of Cellulase/Iron oxide magnetic composites (CMNPS) and the indicative peaks are labelled appropriately.
3.2.3 X-ray diffraction (XRD)
The XRD curve of the CMNPS is shown in Fig. 5. The diffraction peaks for cellulose at 15.20° and 22.70° are assigned to native cellulose. Moreover, the characteristic peaks at 30.20°, 35.50°, 43.20°, 53.70°, 57.20°, and 62.70° planes of Iron oxide (JCPDS card no. 39–1346), also appear for the resultant composites, suggesting that Iron oxide has been successfully prepared in the cellulose matrix. The CMNPS size range between 2 and 18 nm.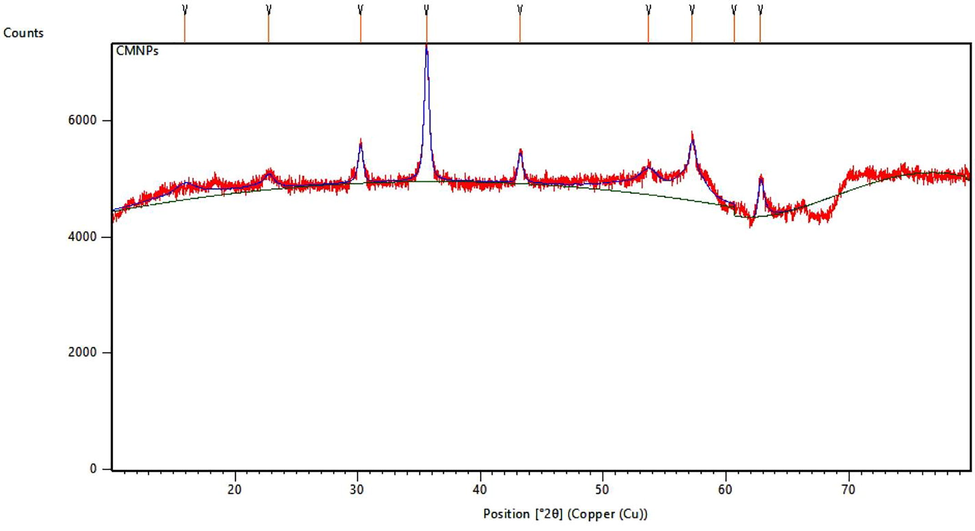
X-Ray Diffraction analysis of Cellulase/Iron oxide magnetic composites (CMNPS).
3.3 Enzyme production
3.3.1 Cellulase assay
In a 250 ml conical flask, Aspergillus niger was inoculated into Czapek dox media and incubated for 1–12 days at 25 °C. At three-day intervals, the cellulase activity was assessed (Fig. 6). However, after 9 days, the maximal yield of endoglucanase activity in the CMNPS (0.82 mg/mL) was attained. After 3–6 days, however, minimum β-glucosidase activity (0.74 mg/mL) was seen. The largest amount of glucose was observed on the sixth day, which was used to identify the best incubation period for Aspergillus sp. enzyme production. Throughout the incubation period, A. niger was the most active cellulolytic species. The isolate A. niger required 4 and 6 days of incubation to obtain maximal cellulase activity, which was adequate for commercial use.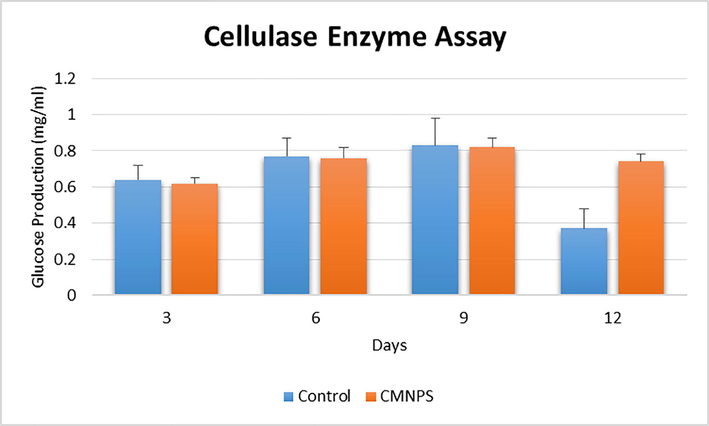
Changes in the activity of cellulase enzyme in control group and Cellulase/Iron oxide magnetic composites (CMNPS) group for 12 days.
3.3.2 Filter paper assay (FPA)
In a 250 ml conical flask, Aspergillus niger was inoculated into Czapek dox media and incubated at 25 °C for 1–12 days. At three-day intervals, the filter paper assay was measured (Fig. 7). However, after 9 days, the maximal yield of FPA activity in the CMNPS (0.81 mg/mL) was attained. After 3–6 days, however, the Minimum FPA activity (0.79 mg/mL) activity was visible. The largest amount of glucose was observed on the sixth day, which was used to identify the best incubation period for Aspergillus sp. enzyme production. Throughout the incubation period, A. niger was the most active cellulolytic species. The isolate A. niger required 4 and 6 days of incubation to obtain maximal cellulase activity, which was adequate for commercial use.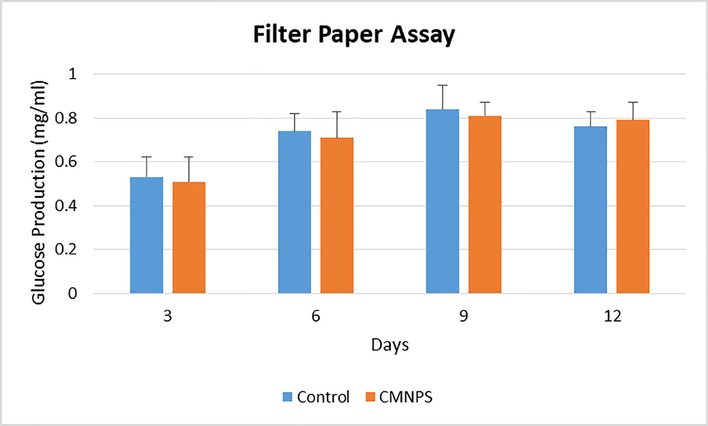
Changes in the cellulase activity as indicated by the Filter paper assay by the formation of glucose (mg/mL) over different time periods.
4 Discussion
Microbial organisms are important agents in genetic engineering¸ especially in industrial microbiology (Vitorino and Bessa, 2017). They are widely utilized for the production of various biologically important molecules including enzymes, proteins, or other bioactive compounds (Abdel-Aziz et al., 2017; Ditu and Gheorghe, 2017). Fungal enzymes are widely used in industries and the most prominent among them is the cellulase enzyme (Singh et al., 2021b). This enzyme is of great significance due to its ability in cleaving the beta 1–4 glycosidic linkages in cellulose. It is highly utilized in the textile industry, pharmaceutical production, biofuel production and so on (Ejaz et al., 2021; Kuhad et al., 2011b). The commonly utilized microorganisms for cellulase production are Cellulomonas, Clostridium, Trichoderma and Thermomonospora. Apart from these, Aspergillus sp. is also emerging as an important producer of cellulase enzymes (Li et al., 2020).
Recent reports have indicated the use of metal nanocomposites for the production and enhancement of cellulase production (Rotaru et al., 2018). A study by Khalilzadeh et al. (2020) indicated the possible use of green synthesized cellulose nanocrystals using iron oxide for various purposes. Among the various models, iron oxide-based cellulose nanocomposites are promising in biotechnological applications (Yadav et al., 2015).
The results indicated the potential of CMNPS in enhancing the cellulase enzyme production by the Aspergillus niger in the Czapek dox medium. Previously it has been found that the iron oxide/ grapheme oxide nanocomposite modified by chitosan has been shown to increase the production of cellulase in Trichoderma reesei (Asar et al., 2020). Similarly, Han et al. (2018) has reported a similar increase in the production of cellulase and the production of sugars during the application of grapheme/ iron oxide nanocomposite. Reports have also indicated that the cellulase produced by the nanocomposite mediated method have better reusability and storability. Apart from using the magnetic nanocomposites, there are alternative methods like solid-state or submerged fermentation methods based on different substrates; however compared to the methods described the results of the present study has a significantly higher yield of cellulase and its activity (Mrudula and Murugammal, 2011; Singh et al., 2021a). In this study, the highest amount of cellulase enzyme produced using the CMNPS as substrate was 0.800 mg/ml of cellulase enzyme production. And the magnetic nanoparticle is reused and the enzyme is produced in a high amount (Islam and Roy, 2018). The use of molasses as substrate and the highest enzyme activity is 0.90 µmol ml−1 min. In this study, the CMNPS produces the highest amount of cellulase enzyme activity at 0.148 µmol ml−1 min.
Aspergillus species are more effective in terms of cellulase activity compared to Alternaria, Rhizopus, and Penicillium, which had moderate cellulase activity, while Trichoderma and Fusarium had low cellulase activity (Famurewa and Olutiola, 1991; Zhang et al., 2017). Besides, the Aspergillus isolates have been found to have endoglucanase activity and cotton degrading abilities (an indicator of the polygalacturonase activity), which is in corroboration with the previous reports of Anuradha et al. (2010) and El Bergadi et al. (2016). Overall, the study indicated the possible use of cellulase/ iron oxide nanocomposites in the production and enhancement of cellulase activity from A. niger strains. It may be useful for the industrial production of the enzyme as an easier and cheaper method.
5 Conclusion
Microorganisms are widely employed for the production of industrially important molecules such as enzymes. The present study utilized the soil fungus, Aspergillus niger, for the production of cellulase enzyme and its enhancement by using the cellulase/ iron oxide nanocomposite. Overall the study concludes that the substrate, CMNPS, is easily separated and reused 1–5 times and also found to enhance the production of cellulase enzyme. Hence, the study suggests the use of CMNPS or similar nanocomposites for the modulation of cellulase enzyme production in Aspergillus sp. and which can bring revolutionary changes in the industrial applications of the fungi.
Acknowledgements
The authors acknowledge King Saud University, Riyadh, Saudi Arabia for funding this research through Researchers Supporting Project No: RSP 2021/11.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Chapter 2 – microbial biosynthesis: a repertory of vital natural products. In: Food Biosynthesis. Academic Press; 2017. p. :25-54.
- [Google Scholar]
- Response surface optimization of cellulase production from Aneurinibacillus aneurinilyticus BKT-9: an isolate of urban Himalayan freshwater. Saudi J. Biol. Sci.. 2020;27(9):2333-2343.
- [Google Scholar]
- Fungal isolates from natural pectic substrates for polygalacturonase and multienzyme production. Indian J. Microbiol.. 2010;50(3):339-344.
- [Google Scholar]
- Chitosan modified Fe(3)O(4)/graphene oxide nanocomposite as a support for high yield and stable immobilization of cellulase: its application in the saccharification of microcrystalline cellulose. Prep. Biochem. Biotech.. 2020;50(5):460-467.
- [Google Scholar]
- Chapter 1 – introduction in soft chemistry and food fermentation. In: Grumezescu A.M., Holban A.M., eds. Soft Chemistry and Food Fermentation. Academic Press; 2017. p. :1-19.
- [Google Scholar]
- Characterisation of microorganisms used for the production of food enzymes. EFSA J.. 2019;17(6)
- [CrossRef] [Google Scholar]
- Cellulases: from bioactivity to a variety of industrial applications. Biomimetics. 2021;6(3):44.
- [CrossRef] [Google Scholar]
- Determination of endoglucanase activity of paper decaying fungi from an old library at the ancient Medina of Fez. Microbiology. 2016;85(1):47-55.
- [Google Scholar]
- Comparison of growth and cellulolytic enzyme production in Aspergillus chevalieri and Penicillium steckii from mouldy cacao beans. Folia Microbiol.. 1991;36(4):347-352.
- [Google Scholar]
- A fast, sensitive and easy colorimetric assay for chitinase and cellulase activity detection. Biotechnol. Biofuels. 2014;7:37.
- [Google Scholar]
- Biochemical and structural characterization of a novel halotolerant cellulase from soil metagenome. Sci. Rep.. 2016;6:39634.
- [Google Scholar]
- The development of nanobiocatalysis via the immobilization of cellulase on composite magnetic nanomaterial for enhanced loading capacity and catalytic activity. Int. J. Biol. Macromol.. 2018;119:692-700.
- [Google Scholar]
- Simple synthesis of bacterial cellulose/magnetite nanoparticles composite for the removal of antimony from aqueous solution. Int. J. Environ. Sci. Technol.. 2019;16(3):1433-1448.
- [Google Scholar]
- Screening, purification and characterization of cellulase from cellulase producing bacteria in molasses. BMC Res. Notes. 2018;11:445.
- [Google Scholar]
- Isolation and characterization of Aspergillus niger NBC001 underlying suppression against Heterodera glycines. Sci. Rep.. 2019;9:591.
- [Google Scholar]
- Cellulose nanocomposites: Fabrication and biomedical applications. J. Bioresour. Bioproducts. 2020;5(4):223-237.
- [Google Scholar]
- Green synthesis of magnetic nanocomposite with iron oxide deposited on cellulose nanocrystals with copper (Fe3O4@CNC/Cu): investigation of catalytic activity for the development of a venlafaxine electrochemical sensor. Ind. Eng. Chem. Res.. 2020;59(10):4219-4228.
- [Google Scholar]
- Microbial cellulases and their industrial applications. Enzyme Res.. 2011;2011:1-10.
- [Google Scholar]
- Recent updates on different methods of pretreatment of lignocellulosic feedstocks: a review. Bioresour. Bioprocess.. 2017;4:7.
- [Google Scholar]
- Li, J.-X., Zhang, F., Jiang, D.-D., Li, J., Wang, F.-L., Zhang, Z., Wang, W., Zhao, X.-Q., 2020. Diversity of cellulase-producing filamentous fungi from Tibet and transcriptomic analysis of a superior cellulase producer Trichoderma harzianum LZ117. Front. Microbiol., 11.
- Industrial applications of cellulases and hemicellulases. In: Fang X., Qu Y., eds. Fungal Cellulolytic Enzymes: Microbial Production and Application. Singapore: Springer Singapore; 2018. p. :267-282.
- [Google Scholar]
- Laboratory detection and identification of Aspergillus species by microscopic observation and culture: the traditional approach. Med. Mycol.. 2005;43(s1):125-128.
- [Google Scholar]
- Production of cellulose by Aspergillus niger under submerged and solid state fermentation using coir waste as a substrate. Braz J Microbiol. 2011;42:1119-1127.
- [Google Scholar]
- Cellulase production by Trichoderma longi, Aspergillus niger and Saccharomyces cerevisae cultured on waste materials from orange. Pak. J. Biol. Sci.. 2008;11(20):2382-2388.
- [Google Scholar]
- FTIR spectroscopy combined with partial least square for analysis of red fruit oil in ternary mixture system. Int. J. Spectroscopy. 2014;2014:1-5.
- [Google Scholar]
- Ferromagnetic iron oxide–cellulose nanocomposites prepared by ultrasonication. Polym. Chem.. 2018;9(7):860-868.
- [Google Scholar]
- Cellulases immobilization on chitosan-coated magnetic nanoparticles: application for Agave Atrovirens lignocellulosic biomass hydrolysis. Bioprocess Biosyst. Eng.. 2017;40(1):9-22.
- [Google Scholar]
- Evaluation of cellulase production from Aspergillus niger and Aspergillus heteromorphus under submerged and solid-state fermentation. Environ Sustainability. 2021;4(2):437-442.
- [Google Scholar]
- An overview on the recent developments in fungal cellulase production and their industrial applications. Bioresour. Technol. Rep.. 2021;14:100652.
- [Google Scholar]
- Nanotechnology innovations, industrial applications and patents. Environ. Chem. Lett.. 2017;15(2):185-191.
- [Google Scholar]
- Vitorino, L.C., Bessa, L.A., 2017. Technological microbiology: development and applications. Front. Microbiol. 8, 827–827.
- Synthesis and characterization of iron oxide/cellulose nanocomposite film. Int. J. Biol. Macromol.. 2015;74:142-149.
- [Google Scholar]
- Cellulose digestion and metabolism induced biocatalytic transitions in anaerobic microbial ecosystems. Metabolites. 2013;4(1):36-52.
- [Google Scholar]
- Rapid determination of the content of carboxymethyl cellulose sodium in aqueous solution by a color indicator-assisted spectroscopy. Polym. Test.. 2021;93:106990.
- [Google Scholar]
- Measurement of filter paper activities of cellulase with microplate-based assay. Saudi J. Biol. Sci.. 2016;23(1):S93-S98.
- [Google Scholar]
- One-step synthesis of magnetic composites of cellulose@iron oxide nanoparticles for arsenic removal. J. Mater. Chem. A. 2013;1(3):959-965.
- [Google Scholar]
- Production of cellulases by Rhizopus stolonifer from glucose-containing media based on the regulation of transcriptional regulator CRE. J. Microbiol. Biotechnol.. 2017;27(3):514-523.
- [Google Scholar]







