Translate this page into:
Ellagic acid nanoparticles attenuate oxidative stress and testicular damage in high fat Diet/Streptozotocin-Induced diabetic rats
⁎Corresponding authors at: Special Infectious Agents Unit, King Fahd Medical Research Center, King Abdulaziz University, Saudi Arabia (S. Harakeh). Pharmaceutical Research Institute, Albany College of Pharmacy and Health Sciences, NY 12144 USA (S. Mousa). sharakeh@gmail.com (Steve Harakeh), shaker.mousa@acphs.edu (Shaker Mousa)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
Both forms of diabetes mellitus have several detrimental effects on the male reproductive system, including damage to the testicles and impaired spermatogenesis. This study aimed to compare the probable protective effect of ellagic acid nanoformulation (NEL), regular ellagic acid (EL), metformin (MET), and their combination on testicular damage induced by diabetes in rats.
Methods
Diabetes was induced in 6 groups (n = 6) of BALB/c male rats by high-fat diet (HF) plus streptozotocin (STZ). The groups were treated with EL, NEL, MET, EL plus MET, and NEL plus MET. A healthy set of rats served as a control group.
Results
The results showed that EL possesses no antihyperglycemic activity in contrast to the NEL, which showed a significant hypoglycemic activity comparable to MET. Adding NEL to MET showed no benefit concerning the glucose-lowering activity. Both EL and NEL prevented diabetes-induced testis weight loss. Only NEL increased the testis-bodyweight ratio. Adding EL or NEL to MET improved MET therapeutic activity regarding the testis weight and testis-bodyweight ratio. Both EL and NEL and their combination with MET preserved the testicular germ cells layer and spermatogenesis with the best preservation in the combination therapy groups. EL showed no antioxidant activity, while NEL had (increased total antioxidants and decreased lipid peroxidation). Besides, adding EL and NEL to MET improved its antioxidant activity. Both EL and NEL increased PCNA immunoexpress ion in the testis, indicating spermatogenesis’s promotion with a superior activity of NEL and the combination groups. Both EL and NEL decreased caspase-3 immunoexpression in the testis, indicating inhibition of apoptosis with an equal activity of all treatment regimens.
Conclusion
These results indicated that nano formulation of EL improved the antihyperglycemic activity. NEL showed antioxidant activity. NEL more effectively prevented diabetes-induced testicular damage, apoptosis, and impaired spermatogenesis. These results recommended NEL as a single or combined with MET to protects against male testicular injury associated with diabetes.
Keywords
Diabetes
Testis
Ellagic acid
Nanoformulation
Antioxidant
Caspase 3
PCNA
1 Introduction
Diabetes mellitus (DM) is on the rise globally, with an estimated 578 million people affected by 2030 (Saeedi et al., 2019). About 18.5 percent of Saudi Arabia’s population is afflicted (Robert and Al Dawish, 2019). The disease is linked to hyperglycemia. Many structural and functional organ complications are associated with DM, including testis, retina, brain, kidney, and heart (Long et al., 2018).
It is well established that diabetes may lead to male erectile dysfunction due to compromised testicular function, which has been the case of laboratory animals with hyperglycemia (Maresch et al., 2017; Maresch et al., 2018). Studies have reported that humans with diabetes have impotence and have lower testosterone levels, sperm count and motility, and reduced volume of seminal fluid and spermatogenesis in comparison to healthy persons (Mallidis et al., 2011). Both diabetic and experimentally induced diabetes showed that either type 1 or type 2 diabetes could negatively impact male fertility, especially sperm quality (Ding et al., 2015). For this reason, there is an urgent need for need therapies for this problem to protect human reproductive health.
Such dysfunctions are most likely due to an increase in the male reproductive system oxidative stress. Diabetes causes an offset of the de-oxidation of the released reactive oxygen species (ROS) (Laleethambika et al., 2019). Excessive ROS may be responsible for all the abnormalities listed above and may cause DNA damage and affect the protein structures leading to an impairment in the cellular function (Alahmar 2019). Studies reported that oxidative stress resulting from diabetes has led to DNA testicular damage, impairment of spermatogenesis, and the reproductive cell’s survival, resulting in male infertility (Laleethambika et al., 2019). For these reasons, antioxidants are crucial for avoiding diabetes complications in the male reproductive system.
Many plants, fruits, berries contain high concentrations of ellagic acid (EL). Pomegranates contain EL, known for many activities, including antidiabetic, antioxidant, and anti-inflammatory potentials due to their polyphenolic characteristics (Amor et al., 2020, Kandylis and Kokkinomagoulos 2020, ALTamimi et al., 2021, Lin et al., 2021). The effect of EL was evaluated on testicular tissue damage caused by the consumption of monosodium glutamate. The results indicated that EL enhanced male reproduction (Hamza and Al-Baqami 2019). In another study, EL showed a protective effect against oxidative damage of sperms caused by cigarette smoking and it efficiently reduced the number of abnormal sperms (Kaya et al., 2017, Akarca Dizakar et al., 2021).
Recently, several studies conducted in our laboratory showed that the NEL showed a more potent antioxidant effect than the regular EL against cisplatin’s hepatic and nephro toxicity, as well as an antidiabetic impact on the type II diabetes model (El-Shitany et al., 2019, Harakeh et al., 2020, ALTamimi et al., 2021, Lin et al., 2021).
This study aimed to investigate the probable protective effect of EL on the testicular damage induced by diabetes in rats. Besides, comparing the impact of regular and nano EL with metformin (MET) and examining the effect of their combination.
2 Materials and methods
2.1 Chemicals
Streptozotocin (STZ) and EL were purchased from Sigma Chemicals Co. (St. Louis, MO, USA). Glucophage tablets (500 mg MET, Merck, Santa, France) was purchased from Al-Nahid Pharmacy, Jeddah, Saudi Arabia. ELN was prepared and characterized as previously published in our work (El-Shitany et al., 2019).
2.2 Animals
The rats were purchased from Theodor Bilharz research institute in Giza, Egypt with average weight (25 ± 2 gm) and housed at animal house of faculty of medicine at Assiut University. The rats were kept at room temperature (25 ± 2 °C), and the light-dark cycle was considered. Rats were able to eat and drink as much as they wanted. The rats were kept in these conditions for one week to acclimate. Animals were handled according to both the Declaration of Helsinki's recommendations for using experimental animals and the NIH protocol (Touitou et al., 2004) and All experimental protocols were approved from animal ethical committee with at faculty of medicine at Assiut University, with ethical approval number 17300570.
2.3 Initiation of diabetes
The control rats (n = 6) were offered the conventional diet while the rest (n = 36) were established on a high-fat diet (HF) for 2 months. The HF contains 58% lipid, 17% carbs, and 25% protein as a percentage of the entire kcal. A month after the experiment’s start, a single dose of STZ (35 mg/kg dissolved in 10 mM citrate buffer, pH 4.5) was administered intraperitoneally (i.p.) to all rats except the control ones. The control rats were injected with the citrate buffer. The blood glucose concentrations were assessed 24 h after STZ injection, and diabetes was verified when the glucose level exceeded 220 mg/dl. The weights of diabetic rats were registered, and they were then randomly divided into six group, each with six rats (Guo et al., 2018).
2.4 Exprimental groups
Every day for 9 weeks, all diabetic rats received the prescribed medication doses.
-
Control group (Con): This group includes the normal saline-treated rats.
-
Diabetes group (HF + STZ): This group includes the HF + STZ treated rats.
-
MET group (HF + STZ + MET): This group includes diabetic rats treated with MET (300 mg/kg) (Katakam et al., 2000).
-
EL group (HF + STZ + EL): This group includes diabetic rats treated with EL (10 mg/kg) (Amin and Arbid 2017).
-
NEL group (HF + STZ + NEL): This group includes diabetic rats treated with NEL (10 mg/kg) (Harakeh et al., 2020).
-
EL + MET group (HF + STZ + MET + EL): This group includes diabetic rats treated with both MET and EL simultaneously.
-
NEL + MET group (HF + STZ + MET + EL): This group includes diabetic rats treated with both MET and NEL simultaneously.
2.5 Measurement of fasting blood glucose (FBG) concentration
A glucometer GX (Ames, USA) was used to measure FBG concentration. The tail was placed in a 45 °C water bath, and 1 ml of its end was cut to obtain the blood, with one drop of the blood used to measure the blood level.
2.6 Testes collection
After two months, rats were weighted, euthanatized using deep ether anesthesia, and the testes were removed and washed with normal saline. The testes’ weight was assessed. Testes were stored either frozen (−80 °C) for biochemical analysis or in a 10% buffered formalin for histopathological and immunohistochemical analysis.
2.7 Calculation of testis-bodyweight ratio
Testis-bodyweight ratio was calculated by dividing testes weight by bodyweight and multiplying by 100 (Dkhil et al., 2016).
2.8 Analysis of testis histopathological alteration
The formalin-preserved testes were stained with hematoxylin and eosin (H & E) and examined under the light microscope for any histopathological alterations.
2.9 Immunohistochemical analysis of testicular proliferating cell nuclear antigen (PCNA) and caspase 3.
PCNA and caspase-3 antibodies from Lab Vision (Fremont, CA, USA) were utilized in an immunoperoxidase (PAP, peroxidase/anti peroxidase) reaction. A pathologist studied the slides under a light microscope and photographed them in a blinded way.
2.10 Statistics
All information is represented as a mean with standard error. Data was entered using IBM's SPSS 22.0 statistical program (New York, USA). A one-way analysis of variance (ANOVA) test was used for statistical analysis. At the 95 percent confidence level, statistically significant differences were found.
3 Results
3.1 Impact of EL, NEL, and their mixture with MET on FBG levels investigated in HF/STZ-induced diabetes model
Diabetic rats showed significantly increased FBG levels relative to the control rats (p < 0.001). Administration of Met alone and NEL alone to diabetic rats produced a significant decline in FBG concentrations relative to the diabetic rats (p < 0.001). Diabetic rats treated with EL and NEL combined with MET showed a significant reduction in the FBG concentrations relative to the diabetic rats (p < 0.05 and p < 0.001, respectively). Diabetic rats treated with EL and EL combined with MET showed significantly higher FBG concentrations relative to the MET-treated diabetic rats (p < 0.001 and p < 0.05, respectively). There was no significant difference between diabetic rats treated with MET alone, NEL alone, and NEL combined with MET (Fig. 1).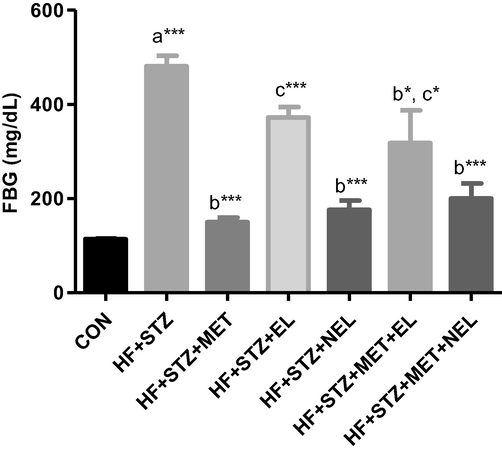
Impact of ellagic acid (EL), nano ellagic acid (NEL); and their mixture with metformin (MET) on fasting blood glucose (FBG) levels investigated in high fat diet (HF)/streptozotocin (STZ)-induced diabetes model. Findings are offered as mean ± SE (n = 6). asignificant against CON; bsignificant against HF + STZ; csignificant against MET. *p < 0.05; **p < 0.01; ***p < 0.001.
These results showed that the regular EL possesses no antihyperglycemic activity contrasted to the nano formula (NEL), which showed a significant hypoglycemic activity comparable to the standard oral hypoglycemic drug (MET). Adding NEL to MET showed no benefit concerning the glucose-lowering activity.
3.2 Impact of EL, NEL, and their combination with MET on testis weight and testis-bodyweight ratio investigated in HF/STZ-induced diabetes model
Diabetic rats showed significantly decreased testis weight and testis-bodyweight ratio relative to the control rats (p < 0.001). Administration of Met alone, EL alone, NEL alone, EL plus MET, and NEL plus MET to diabetic rats produced a significant increase in testis weight relative to the diabetic rats (p < 0.001). Administration of EL alone, NEL alone, EL plus MET, and NEL plus MET to diabetic rats produced a significant increase in testis-bodyweight ratio relative to the diabetic rats (p < 0.01, p < 0.001, p < 0.001, and p < 0.001, respectively). Diabetic rats treated with EL and NEL combined with MET showed a significant increase in the testis weight and testis bodyweight ratio relative to MET-treated diabetic rats (p < 0.001) (Table 1). Findings are offered as mean ± SE (n = 6). asignificant against CON; bsignificant against HF + STZ; csignificant against MET. *p < 0.05; **p < 0.01; ***p < 0.001.
Experimental groups
Testis weight (g)
Testis-bodyweight ratio
CON
1.41 ± 0.02
0.74 ± 0.01
HF + STZ
0.64 ± 0.01a***
0.36 ± 0.01a***
HF + STZ + MET
1.04 ± 0.05b***
0.50 ± 0.03
HF + STZ + EL
1.09 ± 0.07b***
0.56 ± 0.02b**
HF + STZ + NEL
1.22 ± 0.05b***
0.59 ± 0.01b***
HF + STZ + MET + EL
1.37 ± 0.10b***, c**
0.70 ± 0.06b***, c**
HF + STZ + MET + NEL
1.33 ± 0.07b***, c*
0.66 ± 0.04b***, c*
These results showed that both regular EL and the nano formula (NEL) prevents the loss of testis weight induced in diabetic rats. Adding EL or NEL to the conventional diabetes drug (MET) improved MET therapeutic activity regarding the testis weight and testis-bodyweight ratio.
3.3 Impact of EL, NEL, and their mixture with MET on testis histopathology investigated in HF/STZ-induced diabetes model
Control sections from rats testis stained by H & E showed normal size seminiferous tubules (ST), most are of full-thickness germ layers with mature sperms and a normal population of interstitial Leydig cells. Diabetic sections (HF + STZ) showed a marked decrease in size of ST, disorganization, degeneration, and loss of germ cells. Some tubules contain seminal fluid and congested blood vessels. However, interstitial Leydig cells are of the nearly normal population. Diabetic rats treated with MET sections (HF + STZ + MET) showed potential preservation of most germ cell layers with mature sperms; however, few ST is of decreased thickness with an absence of mature sperm. Also, interstitial Leydig cells are of a normal population. Diabetic rats treated with EL sections (HF + STZ + EL) showed better preservation of germ cell layers, mature sperms, and interstitial Leydig cells which looked healthier than in MET sections. Diabetic rats treated with NEL sections (HF + STZ + NEL) showed that most ST looked larger in size, with regular outlines and full-thickness germ cell layers, most tubules contain mature sperms. Diabetic rats treated with EL plus MET sections (HF + STZ + MET + EL) and NEL plus MET sections (HF + STZ + MET + NEL) showed the best preservation relative to their respective single treatment sections (Fig. 2).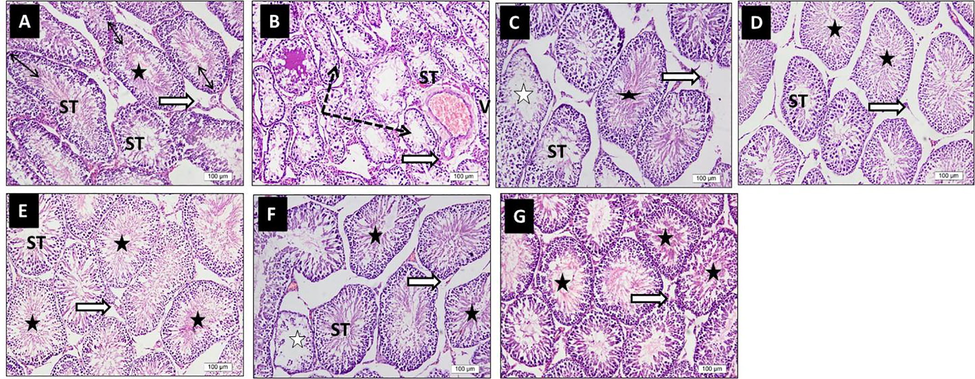
Impact of ellagic acid (EL), nano ellagic acid (NEL); and their mixture with metformin (MET) on testis histopathology investigated in high fat diet (HF)/streptozotocin (STZ)-induced diabetes model. Sections from rats testis stained by H & E (x 20 magnification). Photo A (CON) shows normal size seminiferous tubules (ST), most are of full thickness germ layers (double head arrows) with mature sperms with their tails extend to the lumina (star). Interstitial Leydig cells are of normal population (white arrow). Photo B (HF + STZ) shows marked decrease in size of ST, disorganization, degeneration, and loss of germ cells. Some tubules contain seminal fluid (dotted arrows), and congested blood vessels (V). Interstitial Leydig cells are of nearly normal population (white arrow). Photo C (HF + STZ + MET) shows potential preservation of most germ cell layers of ST with mature sperms (black star); however, few ST are of decreased thickness with absence of mature sperm (white stars). Interstitial Leydig cells are of normal population (white arrow). Photo D (HF + STZ + EL) shows better preservation of ST germ cell layers, mature sperms (black stars) and interstitial Leydig cells (white arrow) which looked more healthier than in MET photo. Photo E (HF + STZ + NEL) shows that most ST looked larger in size, with regular outlines and full thickness germ cell layers, most tubules contain mature sperms (black stars). Photo F (HF + STZ + MET + EL) and photo G (HF + STZ + MET + NEL) show the best preservation relative to their respective single treatment photos.
These results revealed that HF + STZ diabetes produced a marked decrease in germ cells and mature sperms. However, interstitial Leydig cells are of the nearly normal population. All treatments including MET, EL, NEL, MET + EL, and MET + NEL preserved the germ cells and the mature sperms with the best preservation in the combination therapy groups (MET + EL and MET + NEL). The individual treatments’ (MET, EL, and NEL) protection in descending order was: NEL, EL, and MET.
3.4 Impact of EL, NEL, and their mixture with MET on testis TAC, and LPO contents investigated in HF/STZ-induced diabetes model
Diabetic rats showed a significantly decreased TAC relative to the control rats (p < 0.001). Administration of MET alone, NEL alone, EL plus MET, and NEL plus MET to diabetic rats produced a significant increase in testicular TAC relative to the diabetic rats (p < 0.05, p < 0.01, p < 0.001, and p < 0.001, respectively). Diabetic rats treated with EL and NEL combined with MET showed a significant increase in testicular TAC relative to MET-treated diabetic rats (p < 0.001) (Table 2). Findings are offered as mean ± SE (n = 6). asignificant against CON; bsignificant against HF + STZ; csignificant against MET. *p < 0.05; **p < 0.01; ***p < 0.001.
Experimental groups
TAC (mM/mg protein)
LPO (nmol/mg protein)
CON
0.015 ± 0.001
0.50 ± 0.12
HF + STZ
0.005 ± 0.001a***
2.28 ± 0.34a***
HF + STZ + MET
0.008 ± 0.001b*
1.24 ± 0.16b**
HF + STZ + EL
0.007 ± 0.001
1.55 ± 0.16
HF + STZ + NEL
0.009 ± 0.001b**
1.33 ± 0.11b**
HF + STZ + MET + EL
0.011 ± 0.001b***, c**
1.13 ± 0.03b***
HF + STZ + MET + NEL
0.012 ± 0.001b***, c***
0.71 ± 0.002b***
Diabetic rats showed a significantly increased LPO relative to the control rats (p < 0.001). Administration of MET alone, NEL alone, EL plus MET, and NEL plus MET to diabetic rats produced a significant decrease in testicular LPO relative to the diabetic rats (p < 0.01, p < 0.01, p < 0.001, and p < 0.001, respectively). Diabetic rats treated with EL and NEL combined with MET showed a significant decrease in testicular LPO relative to MET-treated diabetic rats (p < 0.001) (Table 2).
These results showed that in comparison to EL, which had no antioxidant activity, these findings revealed that NEL had antioxidant activity. Besides, adding EL and NEL to MET improved its antioxidant activity in HF + STZ diabetic rats model.
3.5 Impact of EL, NEL, and their mixture with MET on testis PCNA protein expression investigated in HF/STZ-induced diabetes model
CON sections showed high PCNA protein immunoexpression. Diabetic sections (HF + STZ) showed decreased PCNA immunoexpression in nuclei of damaged tubules relative to the CON sections. Diabetic rats treated with MET sections (HF + STZ + MET) and diabetic rats treated with EL sections (HF + STZ + EL) showed preserved PCNA immunoexpression compared to the diabetic (HF + STZ) sections. Diabetic rats treated with NEL sections (HF + STZ + NEL), diabetic rats treated with MET plus EL sections (HF + STZ + MET + EL), and diabetic rats treated with MET plus NEL sections (HF + STZ + MET + NEL) showed increased PCNA immunoexpression compared to diabetic rats sections (HF + STZ), diabetic rats treated with MET sections (HF + STZ + MET), and diabetic rats treated with EL sections (HF + STZ + EL) (Fig. 3).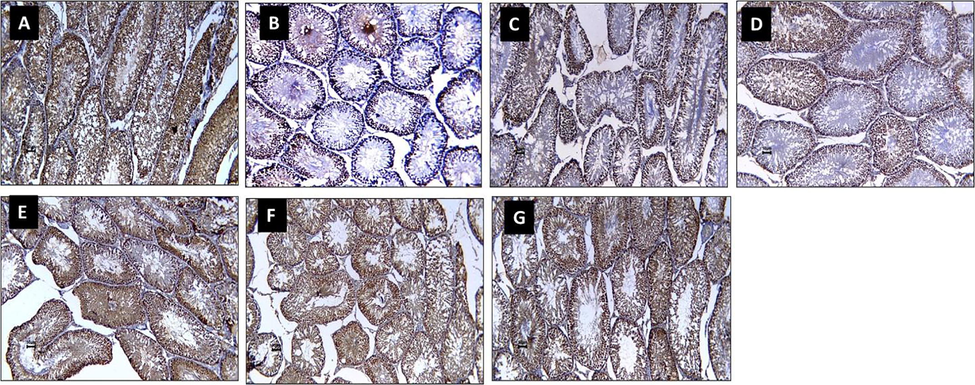
Impact of ellagic acid (EL), nano ellagic acid (NEL); and their mixture with metformin (MET) on testis PCNA protein expression investigated in high fat diet (HF)/streptozotocin (STZ)-induced diabetes model. Sections from rats testis immunohistochemically stained (x 20 magnification). Photo A (CON) shows high PCNA immunoexpression. Photo B (HF + STZ) shows decreased PCNA immunoexpression in nuclei of damaged tubules compared to CON photo. Photo C (HF + STZ + MET) and photo D (HF + STZ + EL) show preserved PCNA immunoexpression compared to HF + STZ photo. Photo E (HF + STZ + NEL), Photo F (HF + STZ + MET + EL), and photo G (HF + STZ + MET + NEL) show increased PCNA immunoexpression compared to HF + STZ, HF + STZ + MET, and HF + STZ + EL.
These results indicated that diabetes is associated with impaired spermatogenesis. Administration of EL alone, MET alone, NEL alone, EL plus MET, and NEL plus MET to diabetic rats promote spermatogenesis relative to the diabetic rats. Diabetic rats treated with NEL alone and EL and NEL combined with MET showed better spermatogenesis protection relative to diabetic, MET alone, and EL alone.
3.6 Impact of EL, NEL, and their mixture with MET on testis caspase-3 protein expression investigated in HF/STZ-induced diabetes model
CON sections showed reduced testicular caspase-3 immunoexpression. Diabetic sections (HF + STZ) showed high caspase-3 immunoexpression in nuclei of damaged tubules compared to CON sections. Diabetic rats treated with MET sections (HF + STZ + MET), diabetic rats treated with EL sections (HF + STZ + EL), diabetic rats treated with NEL sections (HF + STZ + NEL), diabetic rats treated with MET plus EL sections (HF + STZ + MET + EL), and diabetic rats treated with MET plus NEL sections (HF + STZ + MET + NEL) show moderate decrease in caspase-3 immunoexpression compared to diabetic (HF + STZ) sections (Fig. 4).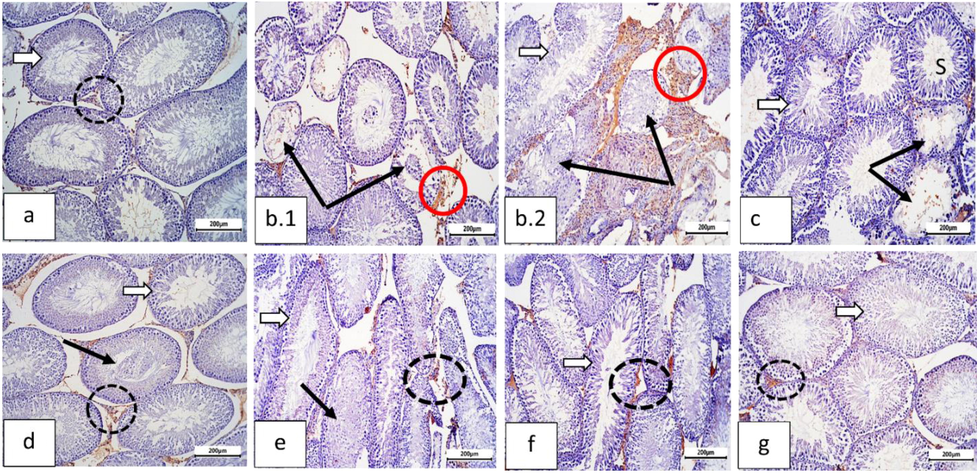
Sections of rat testis immunostained against caspase-3 (Cas-3, the apoptotic marker) and photographed at ×100 bar=200 µm showing: (a) Normal control group: with negative immunostaining for cas-3 in seminiferous tubule germ cells (white arrow) and interstitial cells (dotted black circle). (b) Diabetic group (b.1, b.2): showing higher activity of cas-3 in degenerated seminiferous tubules cells (thin black arrows) and more in the interstitial cells (red circles). (c) Metformin (Met) treated group: nearly normal cas-3 level in both seminiferous tubules (white arrow) and interstitial cells (dotted black circle), and some tubules still showed degenerated germ cells with some positive reaction (thin black arrows). (d) Ellagic acid group: moderate reduction in cas-3 activity. Interstitial cells, still, displayed positive reaction (dotted black circle). Some tubules displayed desquamated germ cells (thin black arrow). (e) Ellagic acid + Met group: showing decreased of Cas-3 activity in seminiferous tubules (white arrow), moderate level in interstitial cells (dotted circle); However, some tubules still showing degenerated cells (thin black arrow). (f) Nano-Ellagic acid group: Showing the same expression of cas-3 activity as compared to the control. Few interstitial cells still revealed positive staining of Cas-3 (circle). Nano-Ellagic acid treated group displayed the same cas-3 expression as compared to the control and few interstitial cells still revealed positive Cas-3 staining (doted circle). (g) Nano ellagic + Met group: marked improvement in cas-3 immunostaining in both seminiferous tubules (white arrow) and interstitial cells (circle).
These results showed that HF + STZ induced diabetes is associated with caspase-3 mediated apoptosis in rats testis. Administration of MET alone, EL alone, NEL alone, EL plus MET, and NEL plus MET to diabetic rats protects the testis against diabetes-induced apoptosis.
4 Discussion
In the present study, HF plus STZ were used to induce diabetes in experimental rats. Previous studies confirmed that HF prompts insulin resistance, and consequently, a small STZ dose will produce pancreatic beta-cells damage (De MagalhÃes et al., 2019). A established model for type 2 diabetes is a HF fed and low-dose STZ-treated rodent (Long et al., 2018). The most significant HF- and STZ-induced testicular disruption mechanisms have been identified as increased oxidative stress, reduced antioxidant capability, inflammation, and programmed cell death (Dkhil et al., 2016, Al-Megrin et al., 2020, Saeedan et al., 2021).
Testicular weight and testis histopathological changes are the most sensitive markers of male reproductive injury (Çeribaşi et al., 2012). The significant loss of testis weight and lower testis-to-bodyweight ratio observed in HF plus STZ diabetic rats in this study were consistent with several previous studies (Wang et al., 2018; Wang et al., 2019, Al-Megrin et al., 2020). This study showed that HF plus STZ-induced diabetes produced a marked decrease in testicular germ cell layers and mature sperms. Sertoli cells play a crucial role in spermatogenesis, and using energy from sources apart from glucose metabolism can disrupt their organelle structures, leading to cellular injury and imperfect sperm maturation (Almeer et al., 2020). Notably, EL, NEL, and their combination with MET were able to reduce testicular destruction by reducing testis weight loss and raising the testis-bodyweight ratio. The combination therapy has superior activity. The results indicated that both EL and NEL have a protective effect and that they should be used in conjunction with MET. In agreement with our results, a recently published study reported that EL improved testis histopathological structure in STZ-induced diabetic rats (Akarca Dizakar et al., 2021). EL has also been shown to protect testicles from Adriamycin (Çeribaşi et al., 2012), monosodium glutamate (Hamza and Al-Baqami 2019), arsenic (Mehrzadi et al., 2018), acetic acid (Kaya et al., 2017), and cisplatin (Ates xs xahin et al., 2008)-induced testicular damage.
It appears from the results of this study that other mechanisms lie behind the testicular protective effect of EL, as it did not show a comparable hypoglycemic effect to the MET and NEL regarding FBG. At the same time, they were all similar in improving testicular weight. This also appears with MET, which did not affect the testis-bodyweight ratio, while it lowered FBG to near the normal level. A growing body of evidence shows that high blood glucose levels may trigger oxidative stress and apoptosis by increasing the formation of reactive oxygen species (ROS) and decreasing the effectiveness of the antioxidant defense system. ROS are produced early during diabetes and play a pivotal function in its complications, including male reproductive complications (Long et al., 2015). The data indicated that inducing diabetes in rats increased LPO levels while decreasing TAC levels, contributing to increased oxidative stress in the testis (Dkhil et al., 2016). Lipid peroxidation, a chemical process able to disrupt testicular structure and function, which may explain the histopathological changes found in the testis sections in this study (Çeribaşi et al., 2012). These findings revealed that NEL, MET, and their mixture have an antioxidant effect, while EL alone has no antioxidant effect, but when combined with MET, it does. The low antioxidant activity of EL may be attributed to its weak biopharmaceutical properties, such as low solubility and bioavailability, which was improved with nano formulation (NEL) (Harakeh et al., 2020).
PCNA is an excellent marker used for studying cell kinetics. It is an inexpensive, convenient, and accurate method for determining germ cell proliferative activity (Altay et al., 2003). Examining the impact of HF plus STZ-induced diabetes on the expression of the PCNA in rats testicular tissue revealed a reduced number of immunostained basal germ cells in the seminiferous tubule. An earlier study found an inverse correlation between the number of suppressed PCNA nuclei in germ cells and the duration of type 2 diabetes in rats (Salama et al., 1998, Altay et al., 2003). They reported that the longer the diabetic state, the reduced PCNA index (Salama et al., 1998). These results may explain diabetes associated impaired spermatogenesis. In diabetic rats, administration of EL alone, NEL alone, and their combination with MET promotes spermatogenesis with superior activity to NEL and the combination regimens.
Testicular apoptosis is triggered by increased ROS and oxidative stress, which is accompanied by elevated blood glucose levels (Nna et al., 2019). EL alone, NEL alone, and their combination with MET significantly decreased caspase-3-mediated apoptosis in this HF plus STZ diabetes model. Similar results are reported recently by (Akarca Dizakar et al., 2021) who reported that EL inhibits testicular programmed cell death (TUNEL) during STZ-induced diabetes in rats. EL exerted also a protective effect on adriamycin-induced testicular apoptosis (Bax and Bcl-2) in rats (Çeribaşi et al., 2012).
5 Conclusion
Nano formulation of EL (NEL) improved EL bioavailability and improved its antihyperglycemic activity to reach MET activity. Antioxidant activity was observed with NEL, not EL. Both EL and NEL protect against diabetes-induced testicular damage and apoptosis with NEL more effective. Both EL and NEL promote normal spermatogenesis during diabetes. These results recommended NEL as a single or combined with MET to protects against male reproductive diabetic complications.
5.1 Animal rights
The protocol of the current study was in accordance to both the Declaration of Helsinki's recommendations for using experimental animals and the NIH protocol (Touitou et al., 2004) and All experimental protocols were approved from animal ethical committee with at faculty of medicine at Assiut University, with ethical approval number 17300570.
Acknowledgments
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under grant no. G: 742-140-1441. The authors, therefore, acknowledge with thanks The DSR for their technical and financial support.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Effects of ellagic acid in the testes of streptozotocin induced diabetic rats. Drug and Chemical Toxicology. 2021;1–8
- [CrossRef] [Google Scholar]
- Green Coffea arabica Extract Ameliorates Testicular Injury in High-Fat Diet/Streptozotocin-Induced Diabetes in Rats. Journal of Diabetes Research. 2020;2020:6762709.
- [CrossRef] [Google Scholar]
- Role of oxidative stress in male infertility: An updated review. Journal of Human Reproductive Sciences. 2019;12:4-18.
- [CrossRef] [Google Scholar]
- Ziziphus spina-christi leaf extract attenuates mercury chloride-induced testicular dysfunction in rats. Environmental Science and Pollution Research. 2020;27(3):3401-3412.
- [CrossRef] [Google Scholar]
- Ellagic acid improved diabetes mellitus-induced testicular damage and sperm abnormalities by activation of Nrf2. Saudi Journal of Biological Sciences. 2021;28(8):4300-4310.
- [CrossRef] [Google Scholar]
- Streptozotocin-induced diabetic effects on spermatogenesis with proliferative cell nuclear antigen immunostaining of adult rat testis. Fertility and Sterility. 2003;80:828-831.
- [CrossRef] [Google Scholar]
- Estimation of ellagic acid and/or repaglinide effects on insulin signaling, oxidative stress, and inflammatory mediators of liver, pancreas, adipose tissue, and brain in insulin resistant/type 2 diabetic rats. Applied Physiology, Nutrition and Metabolism. 2017;42(2):181-192.
- [CrossRef] [Google Scholar]
- Ellagic acid as a tool to limit the diabetes burden: Updated evidence. Antioxidants. 2020;9:1-26.
- [CrossRef] [Google Scholar]
- Improvement of cisplatin-induced injuries to sperm quality, the oxidant-antioxidant system, and the histologic structure of the rat testis by ellagic acid. Fertility and Sterility. 2008;89(5):1474-1481.
- [CrossRef] [Google Scholar]
- Impact of ellagic acid on adriamycin-induced testicular histopathological lesions, apoptosis, lipid peroxidation and sperm damages. Experimental and Toxicologic Pathology. 2012;64(7-8):717-724.
- [CrossRef] [Google Scholar]
- The effects of diabetes on male fertility and epigenetic regulation during spermatogenesis. Asian Journal of Andrology. 2015;17:948-953.
- [CrossRef] [Google Scholar]
- Selenium nanoparticles attenuate oxidative stress and testicular damage in streptozotocin-induced diabetic rats. Molecules. 2016;21:1517.
- [CrossRef] [Google Scholar]
- Nanoparticles Ellagic Acid Protects Against Cisplatin-induced Hepatotoxicity in Rats Without Inhibiting its Cytotoxic Activity. International Journal of Pharmacology. 2019;15(4):465-477.
- [CrossRef] [Google Scholar]
- Guo X, Wang Y, Wang K, Ji B, Zhou F (2018) Stability of a type 2 diabetes rat model induced by high-fat diet feeding with low-dose streptozotocin injection. Journal of Zhejiang University: Science B 19: 559–569. https://doi.org/10.1631/jzus.B1700254.
- Testicular protective effects of ellagic acid on monosodium glutamate-induced testicular structural alterations in male rats. Ultrastructural Pathology. 2019;43(4-5):170-183.
- [CrossRef] [Google Scholar]
- Antidiabetic effects of novel ellagic acid nanoformulation: Insulin-secreting and anti-apoptosis effects. Saudi Journal of Biological Sciences. 2020;27(12):3474-3480.
- [CrossRef] [Google Scholar]
- Food applications and potential health benefits of pomegranate and its derivatives. Foods. 2020;9:122.
- [CrossRef] [Google Scholar]
- Metformin Improves Vascular Function in Insulin-Resistant Rats. Hypertension. 2000;35(1):108-112.
- [CrossRef] [Google Scholar]
- Antioxidant effects of ellagic acid on testicular tissue of rats exposed to tobacco smoke. Journal of Turgut Ozal Medical Center. 2017;24:381-386.
- [CrossRef] [Google Scholar]
- Diabetes and Sperm DNA Damage: Efficacy of Antioxidants. SN Comprehensive Clinical Medicine. 2019;1(1):49-59.
- [CrossRef] [Google Scholar]
- Ellagic acid inhibits high glucose-induced injury in rat mesangial cells via the PI3K/Akt/FOXO3a signaling pathway. Experimental and Therapeutic Medicine. 2021;22:1017.
- [CrossRef] [Google Scholar]
- Protective effects of scutellarin on type II diabetes mellitus-induced testicular damages related to reactive oxygen species/Bcl-2/Bax and reactive oxygen species/microcirculation/staving pathway in diabetic rat. Journal of Diabetes Research. 2015;2015:1-11.
- [CrossRef] [Google Scholar]
- 9 Oncotarget Hyperglycemia induced testicular damage in type 2 diabetes mellitus rats exhibiting microcirculation impairments associated with vascular endothelial growth factor decreased via PI3K/Akt pathway. Available from: www.impactjournals.com/oncotarget (April. 2018;9(4):5321-5336.
- [Google Scholar]
- High-fat diet and streptozotocin in the induction of type 2 diabetes mellitus: A new proposal. Anais da Academia Brasileira de Ciencias. 2019;91:e20180314
- [CrossRef] [Google Scholar]
- The influence of diabetes mellitus on male reproductive function. A poorly investigated aspect of male infertility. Der Urologe. Ausg. A. 2011;50:33-337.
- [CrossRef] [Google Scholar]
- Diabetes-induced hyperglycemia impairs male reproductive function: A systematic review. Human Reproduction Update. 2018;24:86-105.
- [CrossRef] [Google Scholar]
- Hyperglycemia is associated with reduced testicular function and activin dysregulation in the Ins2Akita+/− mouse model of type 1 diabetes. Molecular and Cellular Endocrinology. 2017;446:91-101.
- [CrossRef] [Google Scholar]
- Ellagic acid: A promising protective remedy against testicular toxicity induced by arsenic. Biomedicine and Pharmacotherapy. 2018;103:1464-1472.
- [CrossRef] [Google Scholar]
- Oxidative Stress, NF-κB-Mediated Inflammation and Apoptosis in the Testes of Streptozotocin-Induced Diabetic Rats: Combined Protective Effects of Malaysian Propolis and Metformin. Antioxidants. 2019;8:465.
- [CrossRef] [Google Scholar]
- The Worrying Trend of Diabetes Mellitus in Saudi Arabia: An Urgent Call to Action. Current Diabetes Reviews. 2019;16(3):204-210.
- [CrossRef] [Google Scholar]
- Artemisia judaica L. diminishes diabetes-induced reproductive dysfunction in male rats via activation of Nrf2/HO-1-mediated antioxidant responses. Saudi Journal of Biological Sciences. 2021;28(3):1713-1722.
- [CrossRef] [Google Scholar]
- Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Research and Clinical Practice. 2019;157:107843.
- [CrossRef] [Google Scholar]
- Impact of Aging and Diabetes Mellitus on the Expression of the Proliferating Cell Nuclear Antigen in Rat Testicular Tissue. Archives of Andrology. 1998;40:95-107.
- [CrossRef] [Google Scholar]
- Ethical Principles and Standards for the Conduct of Human and Animal Biological Rhythm Research. Chronobiology International. 2004;21(1):161-170.
- [CrossRef] [Google Scholar]
- Grape seed proanthocyanidin extract alleviates high-fat diet induced testicular toxicity in rats. RSC Advances. 2019;9(21):11842-11850.
- [CrossRef] [Google Scholar]
- Fish oil ameliorates high-fat diet induced male mouse reproductive dysfunction via modifying the rhythmic expression of testosterone synthesis related genes. International Journal of Molecular Sciences. 2018;19:1325.
- [CrossRef] [Google Scholar]







