Translate this page into:
Electron-hole recombination effect of SnO2 – CuO nanocomposite for improving methylene blue photocatalytic activity in wastewater treatment under visible light
⁎Corresponding authors at: UNESCO-UNISA Africa Chair in Nanosciences/Nanotechnology Laboratories, College of Graduate Studies, University of South Africa (UNISA), Muckleneuk Ridge, PO Box 392, Pretoria, South Africa (K. Kaviyarasu). uthraloyola@yahoo.com (R. Uthrakumar), kavi@tlabs.ac.za (K. Kaviyarasu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Heterojunction photocatalysts have gained much attraction in pollutant degradation applications due to their unique good adsorption and photocatalytic decomposition capacity and more cycle durability. Tin oxide (SnO2) doped with copper oxide (CuO) heterostructured photocatalyst was synthesized by a simple solution processes technique. X-ray diffraction and fluorescence spectroscopy were used to examine the structural properties of the SnO2 - CuO heterostructure. Analyses of FE-SEM and TEM were performed on the samples to determine their surface morphology. The photodegradation property of SnO2 - CuO heterostructure was studied using methylene blue (MB) model pollutant, where SnO2 - CuO heterostructure (90.3 % at 180 min) was shown better than the SnO2. Therefore, the n-p-junction (SnO2 - CuO) heterostructure catalysis boosted the photodegradation of MB detailed studies on the electron-hole pair recombination were conducted on n-p-junctions to observe the effect of the interface on the recombination have been published.
Keywords
SnO2 – CuO heterostructure
Photodegradation
Electron-hole pair recombination
Electron microscopy
Heterojunction photocatalysts
Visible light irradiation
1 Introduction
The fast industrialization and civilization process is creating the most hazardous pollution in the environment. Water pollution is one of the major concerns of eco-friendly environments (Kaviyarasu et al., 2012). Water pollution did not only create serious health impacts and have changed the lifestyle of water-living organisms (Perumal et al., 2022). Further, the premature deaths counts were increased day by day owing to the water pollution (Kasinathan et al., 2016). The recycling or purification of textiles, food, paint, and leather industries wastes (organic pollutant) are challenging task, because are it easily mixed with water resource (Chandrasekar et al., 2021). Research communities are much interested to develop the fast, highly efficient, and low-cost degradation method (physical, chemical, and biological). Among them, solar-driven semiconductor - based photocatalysis technology have gained much attention owing to the simple, eco-friendly, and can decomposed the organic pollutants without secondary products (Arularasu et al., 2018; Panimalar et al., 2020; Amal George et al., 2022). The different semiconductor materials were used for photocatalysts process such as metal oxide (Geetha et al., 2018; Panimalar et al., 2022; Raja et al., 2019; Perumal et al., 2022), transition metal sulfide (Panimalar et al., 2022; Chandrasekar et al., 2022; Manjula et al., 2018; Amanulla et al., 2021), carbon-based materials (Thirupathy et al., 2020; Kayalvizhi et al., 2022; Subbareddy et al., 2020; Alhaji et al., 2019), and metal-organic frameworks (MOFs). Among them, metal oxide widely investigated for photocatalytic field, which owing to the high chemical stability, low toxic nature, wide bandgap, and large exaction biding energy. Most of semiconductors metal oxide only absorbs UV lights only, which limited the practical application. The combination of two metal oxides was not only cover the visible and UV spectra, also boosted the photocatalytic properties.
The use of metal oxide nanostructures in electronics, optics, and photonics systems has gained wide attention because of their wide bandgaps (3.7 eV) and large exciton binding energies (60 meV) (Kennedy et al., 2017; Fang et al., 2014; Murmu et al., 2021). Among them, SnO2 and CuO heterojunction were formed the n-p-junction, which was boosted the photocatalytic properties due to their high photosensitivity, reduce the recombination rate, non-toxic nature, and large bandgap. SnO2 is wide bandgap n-type semiconductor (3.7 eV) and CuO is a p-type semiconductor (1.5 eV). In n-p-heterojunction, CuO was acts as a sink, where it helps to sperate the electron-hole pair (reduced the recombination) and the photogenerated electrons-holes pair move in the opposite direction, get gather in the valence band of SnO2 and efficiently pairs of electrons and holes recombine less frequently. Due to the low recombination of (high movement of electron in the opposite direction) SnO2 - CuO heterostructure, the photocatalytic property of SnO2 - CuO heterostructure will be improved (George et al., 2022; Kumar et al., 2016). In this study, SnO2 - CuO heterostructured photocatalyst was synthesized by a simple solution processes method. The as structural, optical, morphology were thoroughly characterized by XRD, UV–vis spectroscopy, FESEM and EDX. As MB dye degradation was illuminated with UV light, the catalysts were measured for their activity. The photocatalytic activities in the degradation of high concentrated malachite green dyes have been investigated in detailed.
2 Experimental
2.1 Materials
Copper acetate tetrahydrate (Cu(CO2CH3)2·4H2O), tin chloride pentahydrate (SnCl4·5H2O), sodium hydroxide (NaOH), Polyvinylpyrrolidone (PVP), Ethanol (C2H5OH) purchasing was done through Sisco Research Laboratories Pvt. ltd, India. No further purification was performed on the chemicals.
2.2 Synthesis method of SnO2 - CuO heterostructured photocatalyst
SnO2 - CuO heterostructured photocatalyst was synthesized by a simple solution processes method. Initially, 1:1 ratio of copper acetate tetrahydrate and tin chloride pentahydrate were added in 100 mL deionized (DI) water and stirrer 1 h. In addition to the clear solution, PVP was added in 0.1 g and stirred for 15 min. Then, 1.5 g of sodium hydroxide was dissolved 50 mL and sodium hydroxide solution gradually added the above homogeneous solution. Several times, DI water and ethanol were used to wash the precipitate. A final annealing at 400 °C for 3 hrs in an air atmosphere was followed by a drying at 80 °C overnight. The product was noted as SnO2 - CuO. For comparison, SnO2 and CuO were also synthesized under the same reaction condition without copper acetate tetrahydrate and tin chloride pentahydrate, respectively.
2.3 Characterization techniques
The crystalline structure of as-synthesized sample was characterized by X-ray diffractometer (XRD) (AERIS high-resolution bench top) with CuKα radiation. The morphology and energy dispersive X-ray spectroscopy (EDX) of SnO2 - CuO was investigated by FE-SEM (Thermo scientific Apreo S) with an acceleration voltage of 20 kV and TEM (JEOL Japan, JEM-2100 Plus) with an acceleration voltage of 200 kV. μ-Raman vibration modes of as synthesized samples were examined by Raman spectrometer (HORIBA France, LABRAM HR Evolution) at green (532 nm) laser light. The optical absorption properties of the samples were performed by UV–vis-DRS SHIMADZU, UV 3600 PLUS analysis, to evaluate the optical properties of the samples.
2.4 Photocatalytic studies
By degrading organic pollutants under visible light, SnO2 - CuO heterostructured catalysts were evaluated for their photocatalytic properties. We stirred 50 mL of DI water for 15 min with 10 ppm of methylene blue (MB) dye and 10 ppm of magnesium blue (MB) dye. Then, 50 mg of SnO2 - CuO heterostructured catalyst was added the above solution and stirred 20 min under the dark condition. An under visible light irradiated 10 cm away from the surface of the solution was then used to expose the model pollutant with catalyst. The organic pollutant solution was collected each 30 min and studied the UV–vis absorption spectrum. The decomposition rate of each catalyst was studied by following equation (Abinaya et al., 2018).
3 Results and discussion
3.1 XRD pattern
The SnO2 sample (Fig. 1) shows the diffraction peaks at 25.38°, 33.81°, 38.91°, 51.55°, 54.37°, 57.93°, 61.46°, 64.75° and 65.61° are assigned to (1 1 0), (1 0 1), (2 0 0), (2 1 0), (2 1 1), (2 2 0), (0 0 2), (3 1 0), (1 1 2) and (3 0 1) planes, respectively. It was matched with tetragonal rutile structure (JCPDS card no. 41–1445). It appears that CuO diffraction peaks are located at 32.51°, 35.55°, 38.72°, 48.75°, 53.48°, 58.25°, 61.51°, 66.21°, and 68.07°, which correspond to planes as follows: (1 1 0), (0 0 2), (1 1 1), ( −2 0 2), (0 2 0), (2 0 2), ( −1 1 3), ( −3 1 1), and (2 2 0). All the peaks are well-matched with the monoclinic CuO (JCPDS card no. 48–1548). Further composite the SnO2 and CuO, there was no significant changes in the diffraction pattern, which could be attributed to the formation SnO2 - CuO nanocomposite (Mariammal et al., 2012).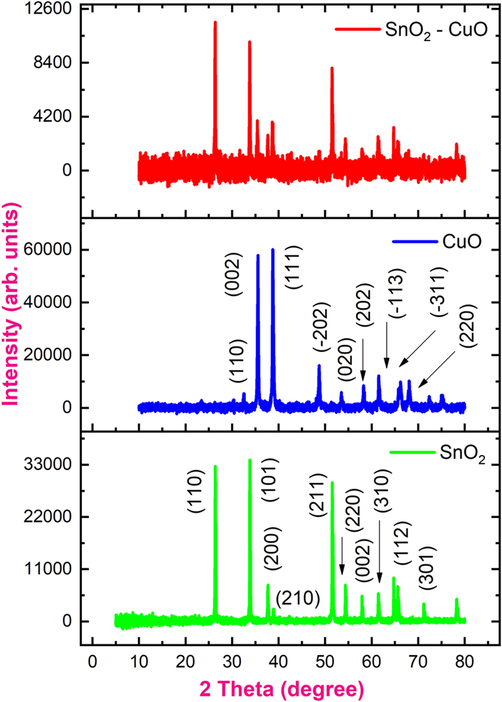
XRD pattern of samples containing SnO2, CuO, and SnO2 - CuO nanocomposites.
3.2 μ-Raman spectrum
The vibration mode of SnO2 - CuO composite was analyzed by μ-Raman analysis as shown in Fig. 2. The peaks located at 633 cm−1 and 779 cm−1 could be assigned to the (A1g) symmetric and (B2g) asymmetric stretching of Sn-O bonds, respectively. The peak at ∼ 569 cm−1 represent (Eg) doubly degenerate mode of oxygen ions in SnO2. In CuO sample was observed with three peaks at 295, 345, and 625 cm−1, which are assigned to the Ag mode (oxygen vibrations along the crystallographic b-axis), B1g mode (a-axis vibrations of oxygen), and B2g mode (oxygen vibrations perpendicular to the b-axes). Thus, the presence of SnO2 and CuO in the composites were confirmed from the μ-Raman spectroscopy (Kumar et al., 2021).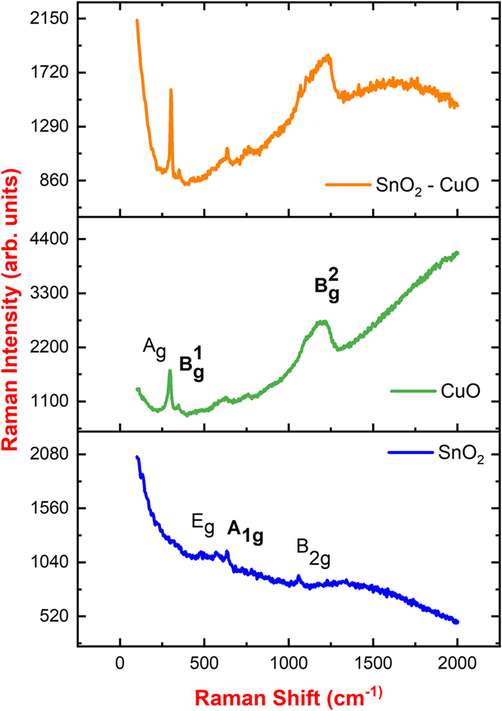
μ-Raman spectrum of SnO2, CuO, and SnO2 - CuO nanocomposite samples.
3.3 FTIR analysis
The functional properties of SnO2, CuO and SnO2 - CuO heterostructure sample were studied by FTIR analysis as shown in Fig. 3. At 3433 cm−1, a broad band was observed; it was attributed to the stretching vibration of water molecules in the O—H direction. Due to the presence of PVP in the SnO2 - CuO heterostructure, two more-absorption bands were observed at 2927 cm−1 and 1388 cm−1, which correspond to N—H and C—H vibrations. There are two peaks at 620 cm−1 and 580 cm−1 corresponding to Sn-O and Cu–O vibrations, respectively. The most inorganic metal–oxygen (M−O) stretching modes were observed 700 cm−1 to 400 cm−1. From the FTIR analysis, the presence and formation of SnO2 - CuO heterostructure were confirmed (Joshi et al., 2016; Wang et al., 2018).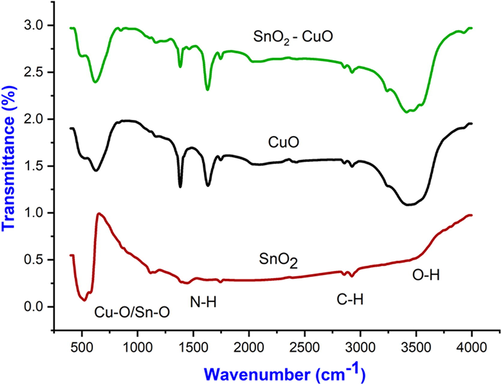
FTIR spectrum of SnO2, CuO, and SnO2 - CuO nanocomposite samples.
3.4 UV–vis analysis
The optical absorption and optical bandgap of SnO2, CuO and SnO2 - CuO heterostructure samples as shown in Fig. 4(a-c). Within the 200 nm to 800 nm wavelength range of pure SnO2, CuO, and SnO2 - CuO samples were studied for their optical absorption spectra. The SnO2 and CuO samples exhibited an absorption edge around ∼400 and 800 nm, respectively. When combination with SnO2 and CuO (heterostructure), the intermediate absorption edge was observed at 500 nm. The shift indicates that the introduction of a new energy level presence in the bandgap. The energy bandgap values have been calculated by Kubelka-Munk. At any wavelength, the Kubelka-Munk function is given by equation 2 and 3.
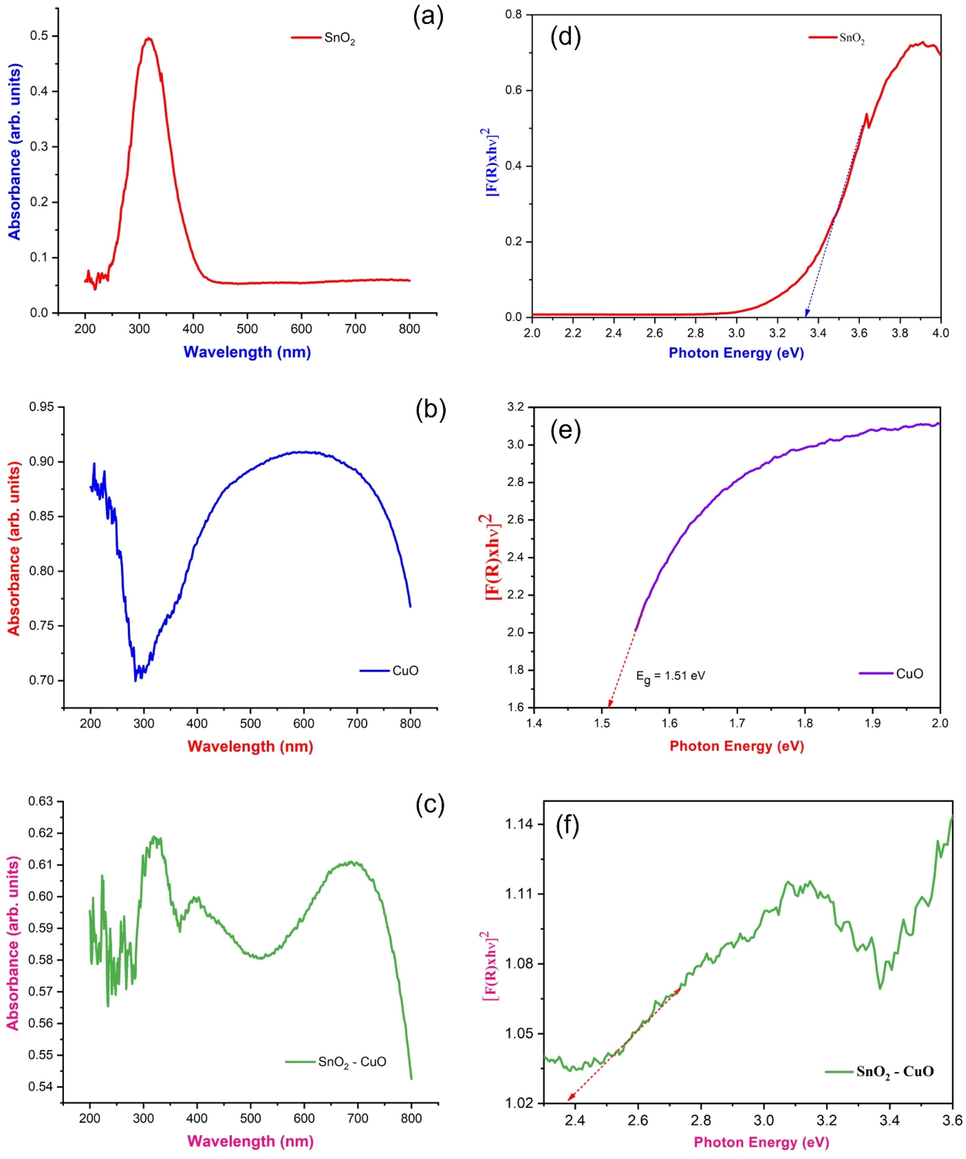
(a-c) UV–vis absorption spectrum of SnO2, CuO, and SnO2 - CuO samples and; (d-f) bandgap energy of SnO2, CuO, and SnO2 - CuO samples.
‘α’ is the absorption coefficient, ‘S’ is the scattering coefficient, and F(R) is the (K-M) Kubelka Munk function. The bandgap of SnO2, CuO and SnO2 - CuO samples were found to be 3.36 eV, 1.51 eV, and 2.3 eV, respectively as shown in inset Fig. 4(d-f). This new energy level was increasing the photocatalytic properties (Harish et al., 2016).
3.5 Morphology analysis
The morphology of SnO2 - CuO heterostructures were analyzed by FESEM techniques. As shown in Fig. 5(a-f), SnO2, CuO and SnO2 - CuO heterostructure samples exhibit different morphologies. The FESEM image of SnO2 in Fig. 5(a-b) shows rock-like morphology and it agglomerated. In Fig. 5(c-d) shows the FESEM image of CuO, which was nanoparticles matrix. In fact, the FESEM images of SnO2 - CuO heterostructures revealed that the sample was rock-like, and nanoparticles morphology was bunch of rock-like and nanoparticles aggregated together to form an irregular morphology as shown in Fig. 5(e-f) respectively.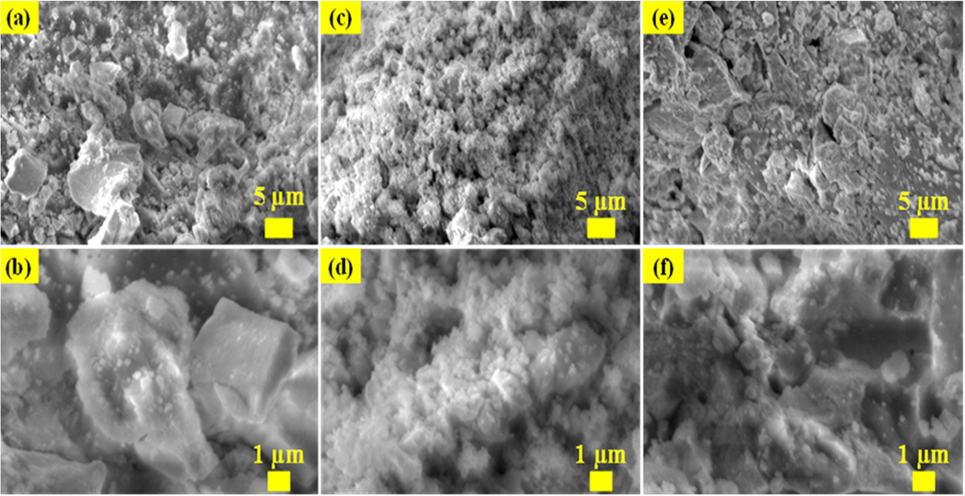
(a-b) FESEM images of SnO2; (c-d) CuO; and (e-f) SnO2 - CuO nanocomposite samples.
The illustration shows TEM images of Fig. 6(a-b) SnO2 - CuO heterostructure, the TEM results were in good agreement with FESEM (rock-like and nanoparticles morphology). Based on the TEM images of SnO2 - CuO, the clear lattice fringes were observed, which is due to the good crystalline nature of SnO2 - CuO nanocomposite. Nevertheless, smaller particles contain grain boundaries, which work as barriers to the passage of free carriers and traps for carriers. Furthermore, the elemental distribution mapping of SnO2 - CuO was characterized by FESEM analysis with EDX mapping as shown in Fig. 7(a-d). The Sn, Cu and O elements were uniform distribution of rock-like SnO2 - CuO heterostructure, where it confirmed SnO2 - CuO in (rock-like and nanoparticles morphology structure) (Panimalar et al., 2022; Magdalane et al., 2021; Subash et al., 2022).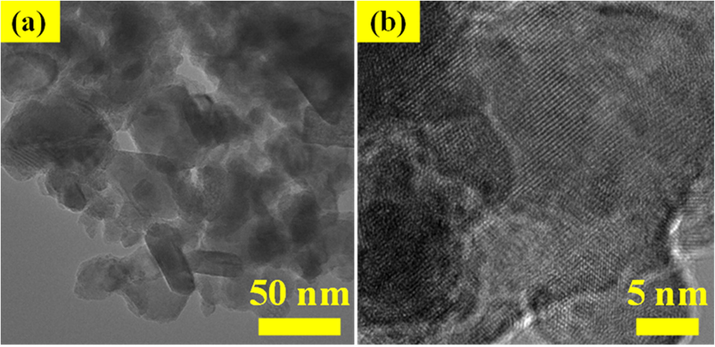
(a-b). TEM images of SnO2 - CuO nanocomposite sample.
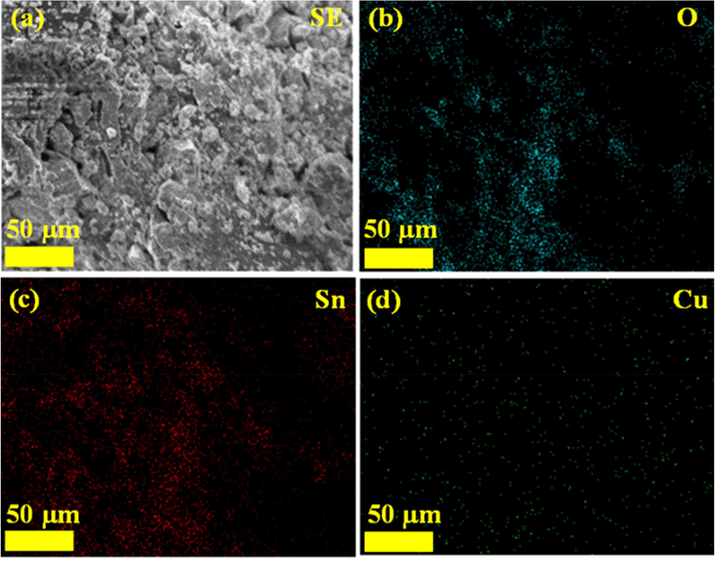
(a-d). FESEM and EDX mapping of SnO2 - CuO nanocomposite sample.
3.6 Photocatalytic activity
The photocatalytic property of SnO2 - CuO heterostructures were analyzed using MB model pollutant. The UV absorption spectrum of model pollutant was revealed in the Fig. 8(a-d). The degradation rate of MB was calculated from the UV–vis absorption spectra and the calculated percentage of SnO2, CuO, and SnO2 - CuO was 58 %, 62.2 %, and 90.3 %, respectively as shown in Fig. 8(a). In the dark condition, a negligible amount of decomposition was observed, and it as shown in Fig. 8(b). The SnO2 - CuO heterostructure shows much better performance under light irradiation when compared with other samples, which is owing to heterojunction as shown in Fig. 8(c). The optimum concentration of Cu has formed the heterojunction, which increases the trap state in the heterojunction and reduced the recombination rate of electrons (e-) and holes (h+). The Fig. 8(d) (energy band diagram of the SnO2 - CuO heterostructure) shows possible photodegradation mechanism of MB. From photodegradation mechanism scheme, the SnO2 - CuO heterostructure absorbed the photons from the electromagnetic radiation and electron - hole (e-/h+) pair create in the heterojunction. The electrons generated by photogeneration were transferred from the CB of CuO to the CB of SnO2. It is important for dye solution degradation to produce highly reactive radical ions, including hydroxyl radicals (OH–) and superoxide radical ions (•O2).
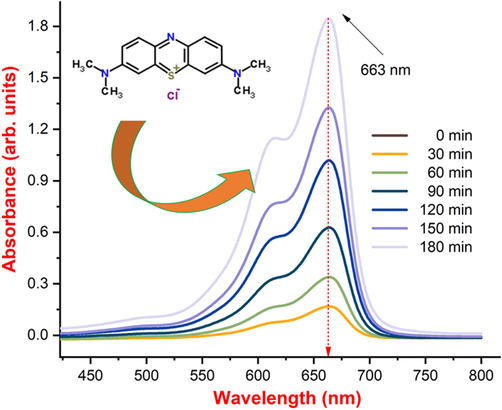
Absorption spectrum of SnO2 - CuO heterostructure samples as a function of MB dye degradation efficiency.
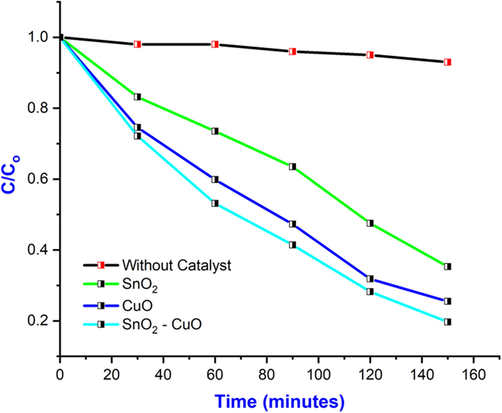
Degradation efficiency of C/Co vs time (different concentration) of SnO2, CuO, and SnO2 - CuO heterostructure samples.
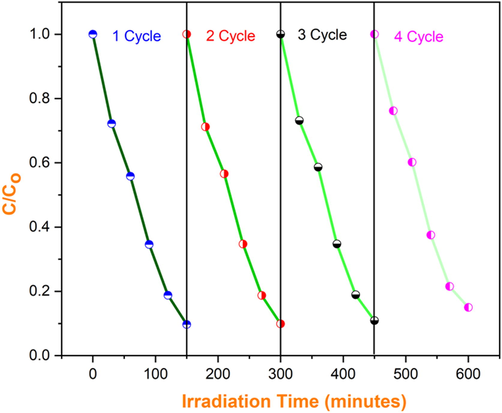
Reusability of SnO2 - CuO photocatalyst under light irradiation
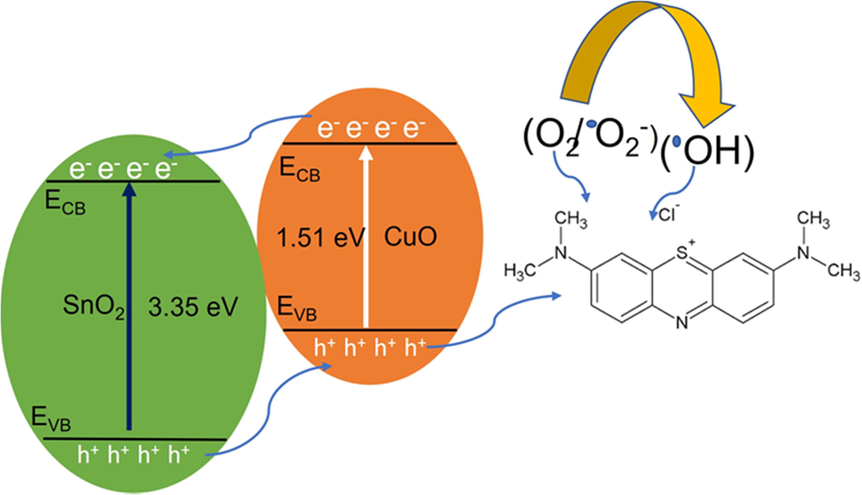
A schematic illustration of the photocatalytic mechanism for the heterojunction SnO2 - CuO nanoparticles
These defect levels operate as active sites for the photocatalytic response because photo-generated electrons and holes can be trapped by oxygen and surface hydroxyl species. In CuO, electron-hole pairs were separated after the hole was transferred from the VB of SnO2. Hydroxide radicals (OH•) were formed from OH• as OH• was being reduced. In this reaction, the super radicals reacted with each other and gave off H2O as a yield. These highly active species ultimately oxidize CO2 and H2O by generating either photogenerated electrons or holes from reactive hydroxide radicals with high oxidation abilities (Roguai and Djelloul, 2022; Begum et al., 2022).
4 Conclusion
The SnO2 - CuO heterostructure was synthesized by a simple solution processes technique. The physical properties of SnO2 - CuO heterostructure were deliberate by XRD and μ-Raman analysis, where the exhibits of SnO2 and CuO were confirmed. The presences of SnO2 and CuO in heterojunction were confirmed from the FESEM with EDX analysis. In fact, the photodegradation activity of SnO2 - CuO heterostructure were studied by using MB model pollutant, where SnO2 - CuO heterostructure was degrades 90.3 % amount of highly reactive with MB in 180 min and convert into inactive some other radicals. Though, the degradation percentage of SnO2 - CuO heterostructure was high when compared with other sample due to the high catalytic and low recombination rate of n-p-heterojunctions was calculated to be 90.3 %. Water and wastewater treatment can utilize the SnO2 - CuO heterostructure to decompose emerging pollutants using visible light.
Acknowledgements
In their acknowledgement, the authors express their gratitude to the management team. The authors also appreciate the support and encouragement of Yeungnam University, Gyeongsan, Republic of Korea.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ultrathin layered MoS2 nanosheets with rich active sites for enhanced visible light photocatalytic activity. RSC Adv.. 2018;8:26664.
- [Google Scholar]
- A comparative study of structural and photocatalytic mechanism of AgGaO2 nanocomposites for equilibrium and kinetics evaluation of adsorption parameters. Surf. Interfaces. 2019;17:100375
- [Google Scholar]
- Photocatalytic effect of CuO nanoparticles flower-like 3D nanostructures under visible light irradiation with the degradation of methylene blue (MB) dye for environmental application. Environ. Res.. 2022;203:111880
- [Google Scholar]
- Selectivity, stability and reproducibility effect of CeM-CeO2 modified PIGE electrode for photoelectrochemical behaviour of energy application. Surf. Interfaces. 2021;22:100835
- [Google Scholar]
- Structural, optical, morphological, and microbial studies on SnO2 nanoparticles prepared by co-precipitation method. J. Nanosci. Nanotechnol.. 2018;18(5):3511-3517.
- [Google Scholar]
- Ahmaruzzaman, Facile synthesis of NiO-SnO2 nanocomposite for enhanced photocatalytic degradation of bismarck brown. Inorg. Chem. Commun.. 2022;143:109721
- [Google Scholar]
- Preparation and characterization studies of pure and Li+ doped ZnO nanoparticles for optoelectronic applications. Materials Today: Proceedings. 2021;36:228-231.
- [Google Scholar]
- Kaviyarasu Specific charge separation of Sn doped MgO nanoparticles for photocatalytic activity under UV light irradiation. Sep. Purif. Technol.. 2022;294:121189
- [Google Scholar]
- Investigations of near infrared reflective behaviour of TiO2 nanopowders synthesized by arc discharge. Opt. Mater.. 2014;36:1260-1265.
- [Google Scholar]
- High performance photo-catalyst based on nanosized ZnO - TiO2 nanoplatelets for removal of RhB under visible light irradiation. J. Adv. Microsc. Res.. 2018;13(1):12-19.
- [Google Scholar]
- Regeneration study of MB in recycling runs over nickel vanadium oxide by solvent extraction for photocatalytic performance for wastewater treatments. Environ. Res.. 2022;211:112970
- [Google Scholar]
- Enhanced visible light induced photocatalytic activity on the degradation of organic pollutant by SnO nanoparticles decorated hierarchical ZnO nanostructures. RSC Adv.. 2016;6:89721.
- [Google Scholar]
- Convenient Architectures of Cu2O/SnO2 Type II p-n Heterojunctions and their Application in Visible Light Catalytic Degradation of Rhodamine. Blue RSC Adv.. 2016;6:43672.
- [Google Scholar]
- Photodegradation of organic pollutants RhB dye using UV simulated sunlight on ceria based TiO2 nanomaterials for antibacterial applications. Sci. Rep.. 2016;6(1):1-12.
- [Google Scholar]
- One pot synthesis and characterization of cesium doped SnO2 nanocrystals via a hydrothermal process. J. Mater. Sci. Technol.. 2012;28(1):15-20.
- [Google Scholar]
- Adsorption of copper and nickel by using sawdust chitosan nanocomposite beads–A kinetic and thermodynamic study. Environ. Res.. 2022;203:111814
- [Google Scholar]
- Synthesis and enhanced field emission of zinc oxide incorporated carbon nanotubes. Diam. Relat. Mater.. 2017;71:79-84.
- [Google Scholar]
- Enhanced electrocatalytic activity of CuO-SnO2 nanocomposite in alkaline medium. Appl. Phys. A Mater. Sci. Process.. 2021;127:1.
- [Google Scholar]
- Design of Binary SnO2-CuO Nanocomposite for Efficient Photocatalytic Degradation of Malachite Green Dye. AIP Conf. Proc.. 2016;12:1724.
- [Google Scholar]
- Synthesis and characterization of TiO2 doped cobalt ferrite nanoparticles via microwave method: investigation of photocatalytic performance of Congo red degradation dye. Surf. Interfaces. 2021;25:101296
- [Google Scholar]
- Structural, morphological and methanol sensing properties of jet nebulizer spray pyrolysis effect of TiO2 doped SnO2 thin film for removal of heavy metal ions. Journal of Nanoelectronics and Optoelectronics. 2018;13(10):1543-1551.
- [Google Scholar]
- On the enhancement of ethanol sensing by CuO modified SnO2 nanoparticles using fiber-optic sensor. Sensors Actuators, B Chem.. 2012;169:199.
- [Google Scholar]
- Role of phase separation in nanocomposite indium-tin-oxide films for transparent thermoelectric applications. Journal of Materiomics. 2021;7:612-620.
- [Google Scholar]
- Studies of MnO2/g-C3N4 hetrostructure efficient of visible light photocatalyst for pollutants degradation by sol-gel technique. Surf. Interfaces. 2020;20:100512
- [Google Scholar]
- Reproducibility and long-term stability of Sn doped MnO2 nanostructures: Practical photocatalytic systems and wastewater treatment applications. Chemosphere. 2022;293:133646
- [Google Scholar]
- Europium-doped MnO2 nanostructures for controlling optical properties and visible light photocatalytic activity. Mater. Today:. Proc.. 2022;56:3394-3401.
- [Google Scholar]
- Effect of Ag doped MnO2 nanostructures suitable for wastewater treatment and other environmental pollutant applications. Environ. Res.. 2022;205:112560
- [Google Scholar]
- Hierarchical nanorods of graphene oxide decorated SnO2 with high photocatalytic performance for energy conversion applications. Fuel. 2022;324:124599
- [Google Scholar]
- Enhancing the photocatalytic performance of surface - Treated SnO2 hierarchical nanorods against methylene blue dye under solar irradiation and biological degradation. Environ. Res.. 2022;209:112821
- [Google Scholar]
- Colloids Surf., A. 2019;564(5):23-30.
- Elaboration, characterization and applications of SnO2, 2 %Gd-SnO2 and 2 %Gd-9 %F-SnO2 thin films for the photocatalytic degradation of MB by USP method. Inorg. Chem. Commun.. 2022;138:109308
- [Google Scholar]
- Pseudo-first kinetics model of copper doping on the structural, magnetic, and photocatalytic activity of magnesium oxide nanoparticles for energy application. Biomass Conversion and Biorefinery. 2022;12:1-11.
- [Google Scholar]
- A facile approach of adsorption of acid blue 9 on aluminium silicate-coated Fuller's Earth - Equilibrium and kinetics studies. Surf. Interfaces. 2020;19:100503
- [Google Scholar]
- Equilibrium synthesis and magnetic properties of BaFe12O19/NiFe2O4 nanocomposite prepared by co precipitation method. Journal of King Saud University-Science. 2020;32(2):1612-1618.
- [Google Scholar]
- Wang, K., Zhang, W., Lou, F., Wei, T., Qian, Z., Guo, W. J. Solid State Electrochem. Preparation of electrospun heterostructured hollow SnO2/CuO nanofibers and their enhanced visible light photocatalytic performance 22, (2018) 2413.







