Translate this page into:
Efficacy of cytokine-induced killer therapy following operation of renal cell carcinoma in north China
⁎Corresponding author. 15866600623@sina.cn (Shuai Liu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Purpose: The purpose of the study was to evaluate the antitumor activity of CIK cells in vitro and investigate the clinical efficacy of cytokine-induced killer (CIK) therapy following operation of renal cell carcinoma patients in North China. Method: In vitro experiments, the secretion of IFN-γ and IL-2 in CIK cells were detected by ELISA and the degranulation was detected by flow cytometry. From January 1, 2012 to October 1, 2015, 202 patients were enrolled in the study and were followed up for at least 36 months. The information of 101 patients who received CIK treatment following nephrectomy and other 101 patients who only received nephrectomy was retrospectively collected. In addition, the correlation of CIK treatment to prognosis of patients following nephrectomy, including disease-free survival (DFS) and overall survival (OS), was analyzed by Kaplan-Meier analyses. COX proportional hazards analyses were used to investigate the benefited population. Results: In vitro, DC-CIK group exhibited the highest cytokines secretion and cytotoxicity followed by the CIK group. In addition, survival analysis showed that patients in North China who received CIK therapy has better prognosis compared to the control group. And the COX proportional hazards analyses demonstrated that in North China, patients with different characteristics can benefit from CIK treatment at the same. Conclusions: CIK immunotherapy is an effective and safety adjuvant for the patients with ccRCC following nephrectomy in North China.
Keywords
Cytokine-induced killer cells
Clear cell renal carcinoma
Prognosis
Survival analysis
Adjuvant therapy
1 Introduction
According to the National Cancer Institute, kidney and renal pelvis cancer accounts for 3.8% of cancer cases in the US. Among all the subtypes of kidney cancers, clear cell renal cell carcinoma (ccRCC) comprises 70% of the renal cancers and remains insensitive to radiotherapy and chemotherapy (Yang et al., 2017). The surgical resection is the main type of treatment used for ccRCC. However, the prognosis of surgery is unsatisfactory. Even after renal resection, several patients are likely to relapse at a rate of 20–50% (Gbormittah et al., 2014). To date, the recurrence of renal cancer has become the main factor that affects the survival of patients and particular focus has been made to prevent this process. Further studies are required to develop a novel therapeutic strategy that can improve the prognosis of patients and prolong their 36-month survival rate.
The characteristic nature of renal cancer renders the use of radiotherapy and chemotherapy ineffective in preventing its recurrence. Recently, targeted-therapy has been employed to prevent these types of cancer. However, targeted-therapy shows significant side effects and may lead to resistance (Liu and Kurzrock 2014, Priya et al., 2017, Sun et al., 2018). Moreover, the financial state of the patients in North China does not enable the prolonged use of this type of therapy and it is usually administered when the recurrence or metastasis has occurred.
In addition to surgery, radiotherapy and chemotherapy, immunotherapy has been recognized as the fourth major treatment of cancer (Zhang et al., 2013). The common types of immunotherapy used in renal cancer include cytokines, immune checkpoint inhibitors (ICIs) and cellular immune therapy. The traditional IL-2 and IFN-α therapy is not currently used due to serious side effects, such as fever. In addition, medical treatment based on PD-1 targeting has not been approved in China and the highly mortality rate with severe ICI myocarditis, which is more frequent with PD-1 blockade, limited the use of PD1 inhibitor. Therefore, CIK therapy is considered the only appropriate therapy to improve the prognosis of ccRCC patients. CIK cell immunotherapy exerts its effects by the cytotoxic effects of T lymphocytes. These cells possess non-MHC-restricted activity and can cause cytolysis of the cancer cell. As an emerging type of immunotherapy, cytokine-induced killer (CIK) cell immunotherapy can reduce tumor recurrence and metastasis of specific types of cancer, such as non-small cell lung cancer (Schmidt-Wolf et al., 1993), lung adenocarcinoma (Wongkajornsilp et al., 2016), breast cancer (Li et al., 2018)and gastric cancer (Jiang et al., 2010). However, the clinical data are not sufficient to establish a confirmed therapeutic effect of this CIK treatment and the public opinion of the human populations with ccRCC, with regard to the efficacy of CIK therapy, which is controversial.
The purpose of the present retrospective study was to evaluate the antitumor activity of CIK cells in vitro and to determine the clinical efficacy of DC-activated CIK cells compared with that of the auto-induced lymphocyte-activated CIK cells in patients with renal cell carcinoma following radical or partial nephrectomy. In addition, we aimed to identify the characteristics of the human population that could benefit from the type of therapy.
2 Material and methods
2.1 Patients
The retrospective study enrolled a total of 202 patients who were diagnosed clear cell renal cell carcinoma and received partial or radical nephrectomy at Shandong provincial hospital affiliated to Shandong university from January 1, 2012 to October 1, 2015. The cohort was divided by different adjuvant therapies into two groups. One subgroup consists of 101 patients who received CIK cell immunotherapy after nephrectomy, the other group consists of 101 patients who received nephrectomy. Among the control group, 15 patients received IL-2 as adjuvant therapy. All the patients were followed up for 36 months with detailed clinical information about gender, age, site of tumor, degree of differentiation, surgical method and tumor diameter. Prognosis and adverse reactions after CIK cell immunotherapy are also collected.
All patients met the inclusion criteria: pathology confirmed ccRCC; having an expected survival duration of longer than 3 months; a Karnofsky performance status score higher than 40%; adequate bone marrow, liver and heart function. The exclusion criteria: had malignancy other than RCC; immunodeficiency; pregnant; transplant recipient. The Fuhrman (1982) renal cell carcinoma morphologic stage system was used to evaluate the staging of patients with RCC.
The study was approved by the Ethics Committee of Shandong provincial hospital affiliated to Shandong University, according to the guidelines of the Declaration of Helsinki. All patients signed informed consent before CIK cell immunotherapy.
2.2 Treatment
Before CIK transfusions, the peripheral blood mononuclear cells (PBMCs) were isolated from the patient and cultured for 2 weeks with the detecting standard levels of bacteria, fungus and endotoxin. One course of treatment that patient received includes two intravenous transfusion with an interval of one day. The frequency of treatment varies from person to person due to the economic conditions and wishes of patients. The number of CIK cells transfused each time was at least 3 × 109.
2.3 CIK cells preparation
The preparation of immune cells was performed compliance with GMP (Good Manufacturing Practice). In brief, PBMC was isolated from peripheral blood of patients by density centrifugation method, and then cultured in fresh serum-free medium under 37 °C and 5% CO2 with different cytokines (Fig. 1). Dendritic cells were stimulated by 786-O lysate and mixed with CIK cells at the ratio of 1:5. Cells had been carried out quality inspection to ensure the microbiological safety of the cell products at the eleventh day, and phenotypic examination performed at Day 14.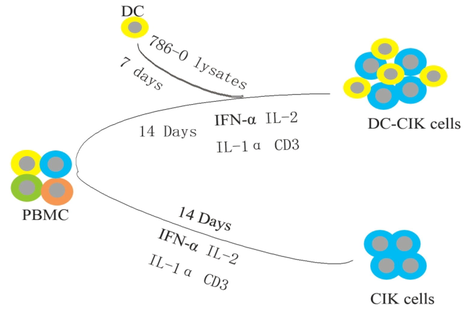
Preparation of immune cells.
2.4 IL-2 and IFN-γ level analysis by ELSIA
The secretion of IFN-γ and IL-2 were detected by ELASA. Antigen loading of DC was required freeze-thaw lysate of 786-O cell, while for functional test, integrated cells had been used to incubate with immune cells at the ratio of 20:1 for 5 h. The expressions of IL-2 and IFN-γ in supernatant of each group were detected. Specific methods of operation refered to ELASA instruction (BioLegend. Cat.431804. and Cat.430104), and Thermo microplate reader was utilized for data analysis.
2.5 CD107a degranulation assay
In order to detect the CD107a degranulation of DC-CIK and CIK, DC-CIK and CIK were set up as effector cells, and then incubated with autologous tumor cells or 786-O cells respectively at the ratio of 20:1 for 5 h. Meanwhile, we established the positive control group by PAM and ionomycin stimulation and the negative control without antigenic stimulation. All experimental groups had added the BFA and anti-CD107a mAb and cultured in 37 °C for 4–6 h. Cells were labeled for FITC-anti-CD3, PE- anti-CD56 and APC-anti-CD107a mAbs, and analyzed by flow cytometry.
2.6 Real time cellular analysis
Real time cellular analysis was performed to evaluate the cytotoxicity to tumor cells in vitro. Briefly, CIK or DC-CIK serves as effector cells and 786-O cells serves as target cells. The whole monitoring process was set to 70 h, 786-O cells had been adherent seeded with a concentration of 1 × 104 cells/well. Effector cells were added at 1:1 ratio of effective cells to target cells. Wells which contain target cells alone were set as control.
2.7 Response and side effects assessment
The side effects were evaluated according to the World Health Organization criteria.
2.8 Cell immunologic assessment
T lymphocytes subpopulations’ changes were used to assess the immune functions. The ratio of various T lymphocytes subsets was detected in the PBMCs of 15 patients before and after CIK treatment. The phenotype of T lymphocytes include CD3+, CD3+CD4+, CD3+CD8+, CD16+CD56+, CD19+ and CD4+/CD8+.
2.9 Statistical analysis
The data were analyzed using SPSS 21.0 software. The measurement data and numeration data were statistically analyzed with t test and Pearson χ2 test respectively. In addition, the adverse reaction of two groups was compared by Fisher’s exact test. The rate of DFS and OS were analyzed by the Kaplan-Meier method. The log-rank test was used to compare differences in Kaplan–Meier estimates for each group. A COX proportional hazards regression approach was used in univariable and multivariable analyses by using the forward-LR method. The level for entering variables was 0.15. A value of P < 0.05 was considered statistically significant.
3 Results
3.1 In vitro antitumor activity
The in vitro antitumor effects of the immune cells were examined via the assessment of cytokine production and the evaluation of their cytotoxic activity in 786-O cells. IFN-γ secretion was considerably low for each group in the absence of antigenic stimulation, while it was increased during co-culture with autologous tumor cells or 786-O cells. DC-CIK group exhibited the highest secretion of IFN-γ, followed by the CIK group. The T cell + IL-2 group was considered as a control that was used to simulate therapeutic effects in vivo in patients treated with cytokine therapy. The DC-CIK group was co-cultured with 786-O cells and exhibited the highest antitumor potential (Fig. 2A). This may be caused by the preparation of DC-CIK, which was once stimulated by the 786-O cell lysate. The production of IL-2 was similar to that noted for IFN-γ (Fig. 2B).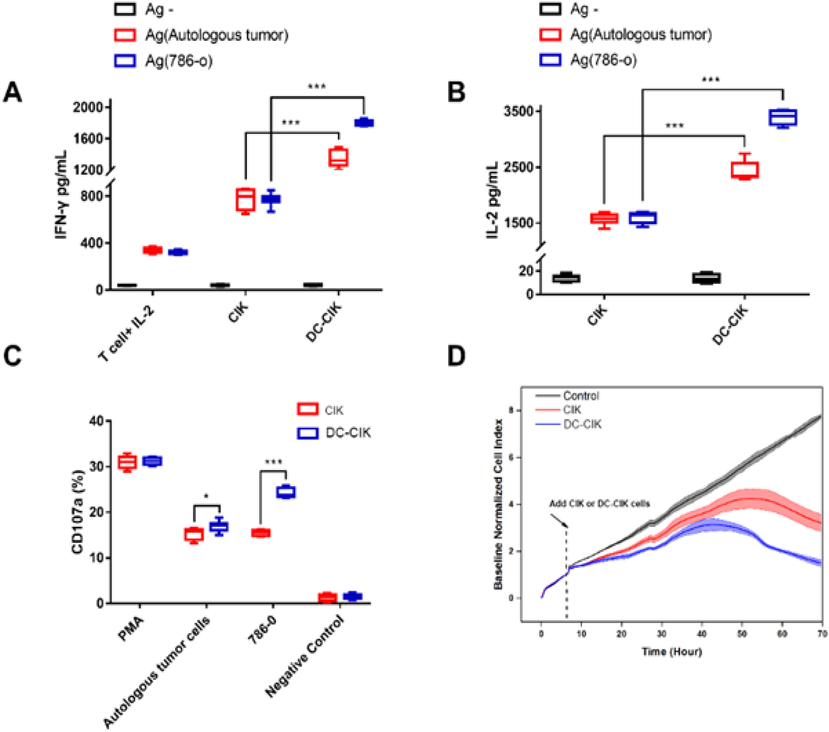
Secretion and cytotoxicity of T, CIK and DC-CIK cells. (A, B) The secretion of IFN-γ was detected by ELISA. T cell + IL-2 group was set as control (n = 8, ***P < 0.001). (C) CD107a degranulation of CIK and DC-CIK were detected by flow cytometry (n = 8, *P < 0.05, ***P < 0.001). (D) The antitumor activities of CIK and DC-CIK were monitored by RTCA. The translucent area represents the error bands.
Degranulation was detected by flow cytometry and CD107a was used as a marker to detect the efficacy of treatment compared to that noted in the CIK group (Fig. 2C). The cytotoxicity of the DC-CIK group indicated significant improvement in vitro both in the presence of autologous tumor cells and/or in the presence of 786-O cells. CIK and DC-CIK cells were monitored by RTCA. CIK or DC-CIK cells were added to 786-O cells at the 6 h time point, and the isometric culture was added to the medium of the control group. The inhibitory rates of the CIK and DC-CIK groups in 786-O cells at the 70 h time point were 58.65% and 80.61%, respectively (Fig. 2D). The results indicated that CIK exhibited higher cytotoxicity in DC-CIK cells than that noted in 786-O and CIK cells in vitro.
3.2 Patient characteristics
The 202 patients enrolled in the present study were pathologically diagnosed as clear cell renal carcinoma cases and were scheduled to undergo nephrectomy. A total of 101 out of 202 patients were treated with CIK cell immunotherapy. All patients were followed up for at least 36 months. The detailed information of the patients is listed in Table 1. The differences noted between the two groups were not significant (P > 0.05).
Characteristics
CIK groups
control groups
P values
Number of patients
101
101
1.000
Male
71
66
0.372
Female
30
35
Age(year)
<40
17
14
0.833
40 ≤ age < 60
58
60
≥60
26
27
Site of tumor
Left
54
53
0.944
Right
46
47
Both
1
1
Pathologic type
Renal clear cell carcinoma
101
101
1.000
Degree of differentiation
High
87
88
0.751
Mediumorlow
14
13
Surgical method
partial nephrectomy
43
40
0.887
radical nephrectomy
58
61
Tumor diameter
≤4 cm
52
61
0.160
>4 cm
49
40
3.3 Clinical outcome
In 202 postoperative patients, the 36-month DFS rate of the CIK group was 94.1%, which was significantly higher than that of the control group (82.2%, P = 0.018, Fig. 3A). In 202 postoperative patients, the 36-month OS rate of the CIK group was 99.0% and was significantly higher than that of the control group (86.1%, P = 0.001, Fig. 3B).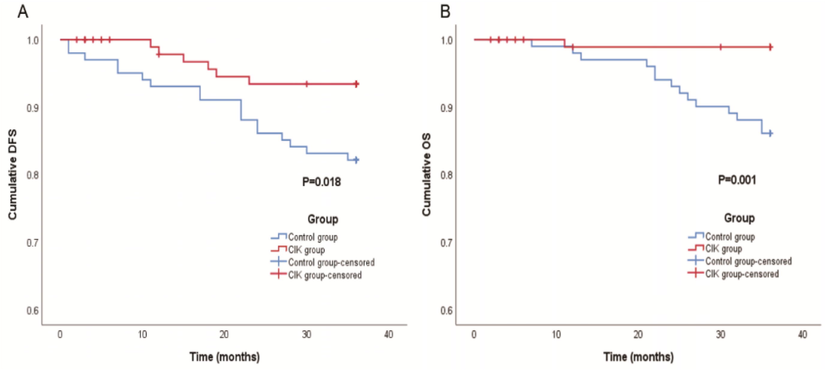
Survival analysis of the CIK and control group. (A) The DFS of the patients received CIK therapy after surgery (P = 0.018). (B) The OS of patients received CIK or DC-CIK (P = 0.001).
3.4 CIK group prognostic indicator
The favorable DFS outcome of the CIK treatment for patients with ccRCC was further supported by univariate and multivariate Cox proportional hazards regression analyses (Table 2). According to the univariate analysis, factors including: age (p = 0.686), tumor diameter (p = 0.377), pathologic grade (p = 0.150), type of CIK (p = 0.611), therapy cycles (p = 0.686) or scale of nephrectomy (p = 0.686) didn’t significantly influence the DFS of patients. Further multivariate survival analysis indicated that well-differentiated tumor may associated with improved DFS, although the difference was not significant (P = 0.053). *Cox regression’; HR: hazard ratio; Cl: Confidence interval. cell immunotherapy exhibited temporary fever, while all the patients experienced fever following IL-2 treatment, which indicated that CIK-treated cells were less susceptible to the induction of fever compared with the IL-2- treated cells following transfusion (P < 0.01).
Univariate analysis
Multivariate analysis
Variables
HR(95%Cl)
p value*
HR(95%Cl)
p value*
Age
1.295(0.371–4.522)
0.686
(<40,40 ≤ age < 60 or ≥ 60)
Tumor diameter
2.149(0.394–11.732)
0.377
1.808(0.244–13.417)
0.562
(≤4cm vs. 4 cm)
Pathologic grade
0.287(0.053–1.568)
0.15
0.287(0.053–1.568)
0.053
(Ⅰ, Ⅱ vs.III,IV)
Nephrectomy
0.719(0.145–3.564)
0.686
(partial vs. radical)
Type of CIK
1.553(0.285–8.482)
0.611
1.994(0.348–11.433)
0.439
(CIK vs. DC-CIK)
Therapy cycles
1.391(0.281–6.892)
0.686
1.548(0.249–9.625)
0.639
(≤2 vs.>2)
3.5 Change of T-cell subsets
The change in the T lymphocyte subpopulations was used to assess the immune functions of the patients. Following transfusion, the ratio of CD3+, CD3+CD4+,CD16+CD56+ and CD19+ T lymphocytes was slightly increased, although the difference was not significant (Table 3). CIK
CIK group(n = 14)
P value
Before transfusion
After transfusion
CD3+ (%)
70 ± 8.280
74 ± 9.227
0.221
CD3 + CD4+ (%)
43 ± 7.739
45 ± 10.272
0.479
CD3 + CD8+ (%)
24 ± 8.445
25 ± 8.696
0.635
CD16 + CD56+ (%)
16 ± 8.327
13 ± 8.085
0.438
CD19+ (%)
12 ± 3.904
11 ± 3.785
0.410
CD4+/CD8+ Ratio
2 ± 0.992
2 ± 1.017
0.995
In addition, 2 patients exhibited digestive discomfort in the CIK group, which was not significantly different compared with that noted in the IL-2 group (Table 4).
Adverse reaction
CIK group (n = 101)
IL-2 group (n = 15)
P value
Fever
6(5.94%)
15(100%)
<0.001
Rash
0
0
1.000
Digestive discomfort
2(1.98%)
0
1.000
Allergy
0
0
1.000
Headache
0
0
1.000
Cardiovascular discomfort
0
0
1.000
4 Discussion
Renal cell carcinoma (RCC) is the one of most common cancers encountered worldwide. It was estimated that 63,990 cases with RCC were reported worldwide in the United States, resulting in 14,400 deaths (Chen et al., 2015). Among all the RCC types, clear cell RCC (ccRCC) accounts for approximately 70%. As RCC is not sensitive to radiotherapy or chemotherapy, surgical resection remains the gold standard for its management. Despite the successful application of surgery, relapse is possible in this type of malignancy. It is of great significance to further improve the prognosis of patients following nephrectomy. A limited number of adjuvant therapies have been found to prevent RCC recurrence following surgery, although the recurrence following nephrectomy is a challenge in clinical practice. Previous studies have proposed that the treatment of metastatic RCC, by targeted therapy, such as inhibitors of VEGF and mTOR, vaccines and cytotoxic agents (IL-2 and IFN-α/γ), as well as immune check point PD-L1-targeted therapy, may exert beneficial effects on the reduction of the recurrence of metastatic RCC. Unfortunately, due to the high cost of treatment, side effects and drug approval, none of the aforementioned therapeutic strategies have been widely recognized viability for ccRCC patients following operation in North China. Until now, PD-L1 antagonist has not been approved in China and researchers had found it can lead to severe myocarditis (Moslehi et al., 2018). In addition, the use of IL-2 in immune therapy has demonstrated adverse effects such as fever (Lenis et al., 2018, Passalacqua et al., 2014). As one kind of immunotherapy, CIK therapy has a promising future. Accumulating studies have revealed the cytotoxic activity of CIK cells in various types of cancers. In metastatic RCC, CIK therapy comprises a therapeutic regimen (Zhao et al., 2015; Yang et al., 2017). Therefore, we speculated that CIK immunotherapy could prevent recurrence and prolong the prognosis of ccRCC patients undergoing renal surgery.
CIK cells are a heterogeneous population of T lymphocytes and comprise two major subsets, namely CD3+/CD56+ and CD3+/CD8+. Therefore, they possess both non-MHC-restricted cytotoxicity and T lymphocyte anti-tumor activity (Qin et al., 2018). During the application of CIK cell immunotherapy, CIK cells are expanded in vitro from PBMCs using anti-CD3, interferon (IFN)-gamma and IL-2 (Iudicone et al., 2016). They express leukocyte function associated antigen-1 (LFA-1) and activating NK receptors, including NKG2D, DNAX accessory molecule-1 (DNAM-1) and NKp30 that allow them to form stable conjugations with targeted tumor cells facilitating degranulation and ultimately cell death. Activated CIK cells further secrete cytokines, such as IL-2 and IFN-gamma in order to regulate the immune system function. Dendritic cells are responsible for antigen presentation and can enhance the efficiency of CIK cells. It has been reported that dendritic cell–cytokine-induced killer cells (DC-CIK) secrete higher levels of specific cytokines compared with those of CIK cells in order to promote the induction of immunity (Li et al., 2015). Furthermore, DC-CIK cells can cause selective tumor cell death by the down regulation of Treg cells, which are related to tumor escape, and can kill the residual tumor cells effectively (Buzzonetti et al. 2014, Song et al. 2017). In the present study, we further compared the in vitro proliferation, in vitro secretory ability and in vivo clinical outcome of CIK and DC-CIK in order to identify the beneficial effects of this type of therapy to the prognosis of the patients.
We detected the secretion of cytokines in CIK cells, DC-CIK cells and T cells + IL-2 with ELISA assays, and the results demonstrated that the DC-CIK group exhibited the highest secretion of IFN-γ and IL-2, followed by the CIK group. The T cell + IL-2 group was considered as a control and was used to simulate the in vivo response of patients treated with cytokine therapy. CD107a was used for the degranulation assay of CIK and DC-CIK and it indicated that the DC-CIK group exhibited significant improvement either in the presence of autologous tumor cells or in the presence of 786-O cells. The data demonstrated that autologous tumors could promote the degranulation of DC-CIK and CIK cells more efficiently than 786-O cells. In addition, real time cellular analysis indicated that the cancer cell inhibitory rates of the CIK and DC-CIK groups at the 70 h time period in 786-O cells were 58.65% and 80.61% respectively, indicating that DC-CIK cells were more susceptible to the immune-mediated cytotoxic activity compared with that noted for CIK cells. Taken collectively, the in vitro experiments demonstrated the immune cytotoxic function of CIK and DC-CIK cells. This effect was higher when DC-CIK cells were examined.
In this study, the DFS and OS time of patients compared with the value of the patients administered nephrectomy alone. This could be attributed to the elimination of potential or residual tumor cells, including cancer stem cells (Torabi-Rahvar et al., 2020). Previous reports demonstrated that DC-CIK was more efficient for patients compared to the CIK (Zhao et al., 2015), which was not consistent to the results in this study. In this study, efficacy of the DC-CIK therapy was not significantly different than that noted in the CIK therapy with regard to the DFS. Although in vitro experiment, DC-CIK cells can secrete more cytokines than CIK cells, the environment in vivo is complex and CIK therapy may exert effect in many other ways. Some study has revealed that DC-CIK cells can activate the TNFRI-ASK1-AIP1 path way to kill autologous ovarian cancer stem cells (Qin et al., 2018). This suggests that DC-CIK may kill ccRCC cells by various methods. In addition, large-scale clinical trials are essential to identify whether CIK treatment or DC-CIK treatment is better.
The risk of post-operative tumor recurrence varies and the predictive factors that determine the selection of the type of adjuvant treatment are not clear. A limited number of reports have demonstrated the successful application of CIK immunotherapy. Therefore, we analyzed certain characteristics of the present study subjects that could be related to the therapeutic effects of CIK cells in order to provide suggestions for patients and medical staff. The present study demonstrated that in North China, patients with moderate-to-poor differential RCC exhibited improved DFS than patients with well-differentiated RCC (P = 0.053). This indicated that patients with medium or low RCC differentiation status may benefit more from CIK immunotherapy. The reason for this result may be due to patients with well differentiation already possessing better prognosis, and hence might derive some benefit from CIK therapy, but the benefit would be statistically significant. This conclusion is consistent with the findings reported in previous studies (Mesiano et al., 2012). It is noteworthy that the recent advances in histology have facilitated the grading of renal cancer, indicating that CIK immunotherapy could be a promising adjuvant therapy for patients with moderate-to-poor differential RCC following nephrectomy. People in North China have fewer physical examinations than those in the West, which lead to the late detection of ccRCC. According to this study, CIK therapy is an appropriate therapy for patients in North China.
Recent studies have indicated that increased cycles of DC/CIK immunotherapy can contribute to the decreased recurrence by regulating the percentage of CD4 + CD25+ Tregs and CD3+CD56+ cells, but the cut-off value is controversial (Mohsenzadegan et al., 2020). In this study, we use two cycles as a cut-off value. However, patients treated with two or more cycles didn’t show better DFS that those with fewer than two cycles. The data presented cannot fully support the conclusion that increased cycles of DC/CIK immunotherapy result in better prognosis. The reason may be that fewer people can receive four courses of treatment in full, which may lead to the uneven distributed data and influence the results.
The diameter of the solid tumors, age of patients and the type of surgical approaches exhibited no effect on patient prognosis. This could be attributed to the fact that these parameters could not influence the progression of residual tumor cells or T lymphocyte proliferation, and therefore they could not be included as selection criteria for CIK cell immunotherapy.
Patients accepted CIK cells always appear improved immune status (Cappuzzello et al., 2017). We further assessed the cell immunity function of the patients prior to and following the therapy. Unfortunately, since the size of sample is too small, the differences noted were not significant, which also possibly due to the inability to collect timely peripheral samples following treatment administration and further studies are required to evaluate the influence of CIK cells to the function of cell immunity. A minimal CIK-associated toxicity was noted in the present study, which suggested that CIK immunotherapy is safer than IL-2 therapy.
In conclusion, the present study suggested that CIK immunotherapy is an effective and safety adjuvant for the patients with early-stage RCC following nephrectomy in North China. The in vitro experiments suggested the potential proliferative activity and strong non-MHC-restricted tumor killing ability of the CIK cells, while the clinical outcomes demonstrated the benefits of CIK treatment in improving prognosis and prolonging the survival period of the patients. In addition, the CIK treatment could be further improved by increasing the specificity of CIK cells or combined with other immunotherapy. Finally, an essential step in improving the treatment efficacy of RCC in North China is the identification of a specific type of population that will be more responsive to CIK treatment before large-scale clinical applications of this therapy are utilized.
Acknowledgements
We thank the patients for participating in this study and all the investigators involved in the study. This study was supported by the National Natural Science Foundation of China (Grant No. 81602227), the Natural Foundation of Shandong Province (Grant No. ZR2014HP015), the Shandong Provincial Key Research and Development Program (Grant No. 2017GSF218070 and No. 2015GSF118055).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Immunological response induced by abagovomab as a maintenance therapy in patients with epithelial ovarian cancer: relationship with survival-a substudy of the MIMOSA trial. Cancer Immunol. Immunother.. 2014;63(10):1037-1045.
- [Google Scholar]
- Cytokines for the induction of antitumor effectors: The paradigm of Cytokine-Induced Killer (CIK) cells. Cytokine Growth Factor Rev. 2017 S1359610117300746
- [Google Scholar]
- Comparative studies of the proteome, glycoproteome, and N-glycome of clear cell renal cell carcinoma plasma before and after curative nephrectomy. J. Proteome Res.. 2014;13(11):4889-4900.
- [Google Scholar]
- Interleukin-15 enhances cytokine induced killer (CIK) cytotoxic potential against epithelial cancer cell lines via an innate pathway. Hum. Immunol.. 2016;77(12):1239-1247.
- [Google Scholar]
- Increasing the frequency of CIK cells adoptive immunotherapy may decrease risk of death in gastric cancer patients. World J. Gastroenterol.. 2010;16(48):6155-6162.
- [Google Scholar]
- Adjuvant Therapy for high risk localized kidney cancer: emerging evidence and future clinical trials. J. Urol.. 2018;199(1):43-52.
- [Google Scholar]
- Comparison of the proliferation, cytotoxic activity and cytokine secretion function of cascade primed immune cells and cytokine-induced killer cells in vitro. Mol. Med. Rep.. 2015;12(2):2629-2635.
- [Google Scholar]
- Efficiency of cytokine-induced killer cells in combination with chemotherapy for triple-negative breast Cancer. J. Breast Cancer. 2018;21(2):150-157.
- [Google Scholar]
- Toxicity of targeted therapy: Implications for response and impact of genetic polymorphisms. Cancer Treat. Rev.. 2014;40(7):883-891.
- [Google Scholar]
- Cytokine-induced killer (CIK) cells as feasible and effective adoptive immunotherapy for the treatment of solid tumors. Expert Opin. Biol. Ther.. 2012;12(6):673-684.
- [Google Scholar]
- Dendritic cell/cytokine-induced killer cell-based immunotherapy in lung cancer: What we know and future landscape. J. Cell. Physiol.. 2020;235(1):74-86.
- [Google Scholar]
- Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet. 2018;391(10124):933.
- [Google Scholar]
- Adjuvant low-dose interleukin-2 (IL-2) plus interferon-alpha (IFN-alpha) in operable renal cell carcinoma (RCC): a phase III, randomized, multicentre trial of the Italian Oncology Group for Clinical Research (GOIRC) J. Immunother.. 2014;37(9):440-447.
- [Google Scholar]
- DC-CIK cells derived from ovarian cancer patient menstrual blood activate the TNFR1-ASK1-AIP1 pathway to kill autologous ovarian cancer stem cells. J. Cell Mol. Med.. 2018;22(7):3364-3376.
- [Google Scholar]
- Phenotypic characterization and identification of effector cells involved in tumor cell recognition of cytokine-induced killer cells. Exp. Hematol.. 1993;21(13):1673-1679.
- [Google Scholar]
- Increased cycles of DC/CIK immunotherapy decreases frequency of Tregs in patients with resected NSCLC. Int. Immunopharmacol. 2017:52197-52202.
- [Google Scholar]
- Adjuvant vascular endothelial growth factor-targeted therapy in renal cell carcinoma: a systematic review and pooled analysis. Eur. Urol.. 2018;74(5):611-620.
- [Google Scholar]
- Antigen-independent killer cells prepared for adoptive immunotherapy: One source, divergent protocols, diverse nomenclature. J. Immunol. Methods 2020:477112690.
- [Google Scholar]
- Effects of the Ayurved Siriraj Wattana recipe on functional and phenotypic characterization of cytokine-induced killer cells and dendritic cells in vitro. BMC Complement. Altern. Med.. 2016;16(1):489.
- [Google Scholar]
- Combination of sorafenib and cytokine-induced killer cells in metastatic renal cell carcinoma: a potential regimen. Immunotherapy. 2017;9(8):629-635.
- [Google Scholar]
- Autologous CIK Cell Immunotherapy in Patients with Renal Cell Carcinoma after Radical Nephrectomy. Clin. Dev. Immunol. 2013:20131-20212.
- [Google Scholar]
- Cytokine induced killer cell-based immunotherapies in patients with different stages of renal cell carcinoma. Cancer Lett.. 2015;362(2):192-198.
- [Google Scholar]







