Translate this page into:
Efficacy of Carnosine administration along with or without Coenzyme Q10 on sodium valproate induced testicular toxicity in vivo models
⁎Corresponding author at: 1-Department of Community Health Sciences, College of Applied Medical Sciences, King Saud University, P.O.Box 10219, Riyadh 11433, Saudi Arabia. mfbadr@ksu.edu.sa (Mohamed Farouk Elsadek),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Background and aim
In the present study, the effect of carnosine along with or without Coenzyme Q10 was tested on the sodium valporate induced testicular toxicity in male rats. Methods: The five different treatments were designed and its biological, relative weight of reproductive organs, serum lipid profiles, reproductive hormones levels and sperm motility factors were evaluated to provide a possible alternative to meet the problem of infertility in males. Results: the experimental rats showed a significant decrease in body weight gain and no difference was recorded feed intake and feed efficiency ratio in different treatments. The treatment of Carnosine along with CoQ10 does not produce any significant change in the relative weight of prostate in SVA induced rats. Further, the SVA induced male rats treated with the combination of Carnosine and CoQ10 showed significant decrease in total cholesterol, triglycerides and a significant increase in HDL-c, LDL-C, VLDL-C levels as compared to control. The same treatment resulted in the significant improvement in the reproductive hormones like testosterone, FSH and LH levels in the serum. Also, the biochemical parameters such as SOD, GST, GPX and catalase levels increased and decreased MDA serum antioxidant level when treated with both Carnosine and CoQ10. Conclusions: The combination of Carnosine and CoQ10 showed a succeeding increase in sperm motility even after 6 h of sperm storage. Thus, the obtained results from current study proved that the carnosine along with CoQ10 may be best alternative solution to treat male infertility.
Keywords
Male infertility
Carnosine
CoQ10
Sodium valporate
1 Introduction
Globally, infertility is a major health issue well documented by the WHO and nearly 15 % of the couples affected by this problem (Hussein et al., 2021). The incidence of infertility has gradually increasing and about 50 % of cases are estimated due to male factors (Illiano et al., 2020). In reproductive biotechnology, Sperm cryopreservation is an essential process in which during storage, a 50 % decrease in sperm quality due to the generation of an excessive reactive oxygen species (ROS), post-thawing sperm quality (Farstad, 2009).
Several other reasons like osmotic changes; excessive cell dehydration and intracellular ice crystals formation cause alterations in sperm plasma membrane permeability (Watson, 2000). It was investigated that the role of seminal plasma and proteins plays an important role in sperm maintenance, viability and transportation into female reproductive tract (Viana et al., 2018) and also act as nutrient rich medium (Juyena and Stelletta, 2012). Superoxide dismutase, catalase and glutathione peroxidase (GPX) are the important enzymes that formed the antioxidant system in semen (Fraczek and Kurpisz, 2005).
Further, there are numerous non-enzymatic antioxidants like Vitamins A, E, C, and B complex, co-enzyme Q10, carnitine, and minerals such as zinc, copper, selenium, and chromium are present in semen (Safarinejad, 2011). It is found by several researchers that the extra supplementation of l-carnitine (naturally concentrated in the testis and epididymis) improve sperm motility by promoting mitochondrial function. Carnosine acts as a free radical scavenger and an endogenously synthesized di-peptide composed by β-alanine and l-histidine. It performs physiological roles such as exhibiting buffering capacity at neutral pH (Sale et al., 2010). Also, carnosine bound to aldehydes formed after lipid peroxidation and act by sacrifice the nucleophiles, sequester aldehydes and attenuate damage and succession of oxidative process (Burcham et al., 2002).
The action of CoQ10 has a profound increase in motility and fertilization rates after several weeks of CoQ10 supplementation. Safarinejad (2011) reported that there is a significant increase in sperm density and motility with CoQ-10 therapy. Lafuente et al. (2013) demonstrated a significant improvement in sperm and concentration motility in men receiving Co-Q10.
Testicular injury or toxicity using Sodium valproate (SVA) toxicity has been reported by Rossi (2013). Furthermore, SVA has the ability to disrupt the testicular by producing some oxidative stress and reproductive toxicity in male rats. Therefore, in the current study, Carnosine along with or without Coenzyme Q10 was tested on the sodium valporate induced testicular toxicity in male rats to provide a possible alternative to meet the problem of infertility in males.
2 Materials and methods
2.1 Animals
A total of forty healthy adult male albino rats at the age of 10–12 weeks were obtained from Laboratory of Animal Colony, Helwan, Egypt. All rats were initially weighed around 210 ± 5 g was used for all experiments in the study. The rat models were adapted in cages made of stainless steel and they were fed with basal diet at 25 ± 2 °C with 55–60 % humidity and a 12 h light/dark cycle for 7 days before starting experimental trials. The experimental protocol was conducted in accordance with the European Community Directive 2010/63/EU. The animal care procedures were in agreement with the National Institutes of Health (NIH) and Helwan University Guidelines.
2.2 Preparation of basal diet
Basal diet consists of 20 % protein (as casein), sucrose 10 %, corn oil 4.7 %, choline chloride 2 %, vitamin mixture 1 %, salt mixture 3.5 %, and fibers 5 %, and corn starch up to 100 % in accordance to Reeves et al. (1993).
2.3 Experimental design
The rats were divided randomly into 5 groups (n = 8 rats).
Group I: Normal control fed with basal diet without any treatment.
Group II: Induced control fed with basal diet with an oral administration of Sodium Valproate (SVA) at a dose of 500 mg/kg/BW.
Group III: Rats were Fed with basal diet and given SVA + CAR (200 mg/kg BW).
Group (IV): Rats were Fed with basal diet and given SVA + Q10 (15 mg/kg BW).
Group (V): Rats were Fed with basal diet and given a mixture of SVA+ (Q10 + CAR) at equal ratios.
2.4 Biological DETERMINATION:
The biological determinants such as Feed Intake (FI) by the rats were recorded daily and the Body Weight Gain (BWG) and Feed Efficiency Ratio (FER) were calculated every week. The Feed efficiency ratio (FER) was estimated using the equation:
The values of different treatments were assessed till the end of the experimental period and the mean of the body weight gain, feed intake and feed efficiency ratio were also taken and recorded. The relative weight of sexual organs (testis, seminal vesicles and prostate) was also taken at end of the experiment (Fernandez et al., 2011).
2.5 Biochemical estimation of serum lipid profiles:
All chemicals and Biochemical Kits used for determinations were of analytical grade and procured from Sigma Chemicals Co., USA.
The estimation of serum lipid profiles such as Serum cholesterol (CHO), triglycerides (TG), high density lipoprotein cholesterol (HDL-C), Low density lipoprotein cholesterol (LDL-C) and Very Low density lipoprotein cholesterol (VLDL-C) were done using enzymatic colorimetric methods adopted by Abell et al. (1952); Buccolo and David (1973); Kostener (1976).
2.6 Sexual hormonal level assay
The rats were slightly anesthetized with sodium pentobarbital (40 mg/Kg), weighed and killed by decapitation. Blood was collected from the ruptured cervical vessels for determination of sexual hormone levels (testosterone, follicle stimulating hormone - FSH, luteinizing hormone - LH). For the evaluation of sperm motility and sperm morphology, semen was collected from the ducts of right and left deferens respectively. The in vitro assay for the evaluation of testosterone level was done by collecting the right testes. According to methods described by Maruyama et al. (1987), the concentrations of serum testosterone, FSH and LH were estimated.
2.7 Serum lipid peroxidation, enzymatic and non-enzymatic antioxidant biomarkers estimation:
The activity of Serum lipid peroxidation, enzymatic and non-enzymatic antioxidant biomarkers such as superoxide dismutase (SOD), glutathione transferase (GST), glutathione peroxidase (GPX), and catalase, enzymes were determined using commercial kits according to the methods described by Sun et al. (1988); Moron et al. (1979); Tappel (1978) and Cohen (1970), malondialdehyde (MDA) by Ohkawa et al. (1979) respectively.
2.8 Computer assisted semen analysis (casa) for sperm motility test
The sperm suspension was collected from the droplets after 6 h of sperm storage and the sperm motility was analyzed using the CASA system (SMAS, Ver.3, DITECT Digital Image Technology, Japan). The glass slides pre coated with 2 % BSA was warmed at 39 °C on a hot plate. Then, the sperm suspension was placed on the warmed glass slide and covered carefully with cover glass. The slide was observed microscopically using Nikon ECLIPSE E200 (Nikon, Tokyo, Japan). The sperm motility parameters such as straight line velocity (VSL μm/s), curvilinear velocity (VCL μm/s), average path velocity (VAP μm/s), linearity (LIN) (LIN = VSL /VCL), straightness (STR) (STR = VSL/VAP), wobble (WOB) (WOB = VAP/VCL), amplitude of lateral head displacement (ALH μm), beat cross frequency (BCF Hz) and motility rate (%) were recorded and assessed.
2.9 Statistical analysis:
The data obtained were analyzed statistically using SPSS version 11 (SPSS, Chicago, IL) in accordance to Almajwal and Elsadek (2015). The results were investigated using one-way analysis of variance (ANOVA) followed by Duncan's Multiple Range Test (DMRT) and P < 0.05 was selected as statistical significance level.
3 Results
3.1 Biological effect of Carnosine and CoQ10 in normal and SVA induced reproductive toxicity in male rats
The biological effect on the parameters such as the body weight gain, feed intake and feed efficiency ratio (FER) of experimental rats were presented in Table 1. Among the different treatments assessed at the end of the experiment showed a significant decrease in body weight gain in induced control followed by other treatments compared to normal control. Further, there was no significant difference was recorded feed intake and feed efficiency ratio in normal control and other treatments as compared to induced control group. * FER = feed efficiency ratio; Data (n = 5 independent experiments) is expressed as mean ± SD. a, b and c indicates the significant differences at P < 0.05 among treatments.
Parameters
Groups
Body weight gain
(g)
Feed intake
(g/d)
FER*
Normal control
65.43 ± 0.3a
18.31 ± 1.3 a
0. 325 ± 0.04a
Induced control
46.40 ± 0.4c
12.43 ± 1.4b
0.242 ± 0.02b**
SVA + CAR (200 mg/kg BW)
57.26 ± 0.2b
16.53 ± 1.1 a
0.309 ± 0.03 a
SVA + Q10 (15 mg/kg BW)
57.29 ± 0.3b
15.39 ± 1.6 a
0.297 ± 0.02 a
SVA+(Q10 + CAR)
58.31 ± 0.2b
16.38 ± 1.3 a
0.319 ± 0.02 a
3.2 Effect of Carnosine and CoQ10 in normal and SVA induced reproductive toxicity on the relative weight of reproductive organs in male rats
The results of Carnosine and CoQ10 in normal and SVA induced reproductive toxicity on the relative weight of reproductive organs in male rats are represented in the Fig. 1. Moreover, a significant decrease (P < 0.05) was observed in the relative weight of testicular vice versa, seminal vesicles of SVA induced rats under treatment with Carnosine and CoQ10 enzyme compared to normal control ranged between 25.1 % and 31.4 % testes weight. Also, there is a drastic decrease in the relative weight of testicular and seminal vesicles of SVA induced control. There is no significant change in the relative weight of prostate of SVA induced rats under treatment with Carnosine and CoQ10 enzyme and between normal SVA induced controls.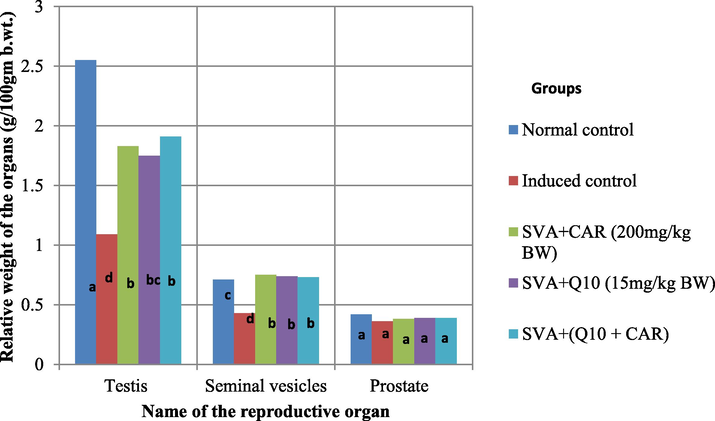
Effect of different treatments of Carnosine and CoQ10 on the relative weight of reproductive organs of male rats. Data (n = 5 independent experiments) is expressed as mean ± SD. a, b,c and d indicate the significant differences at P < 0.05 among treatments.
3.3 Lipid profile in serum of SVA induced male rats treated withCarnosine and CoQ10
The Serum lipid profiles (CHO, TG, HDL-C, LDL-C and VLDL-C) of normal and SVA induced reproductive toxicity in male rats treated with Carnosine and CoQ10 are illustrated the Table 2. The SVA induced male rats administered both with Carnosine and CoQ10 showed a significant decrease of total cholesterol value of 131.46 mg/dl at P < 0.05 compared to other treatments and induced control. In normal control, a total cholesterol value of only 124.35 mg/dl was recorded. The presence of Triglycerides also showed a significantly decrease value of 124.31 mg/dl in SVA induced male rats administered both with Carnosine and CoQ10 compared to the values obtained for the serum homogenates in the other treatments and induced control. But in normal control, TG value of only 112.54 mg/dl was recorded. Data (n = 5 independent experiments) is expressed as mean ± SD. a, b and c indicates the significant differences at P < 0.05 among treatments.*,**,*** indicates the level of significance.
Parameters
Groups
Total CHO
(mg/dl)
TG
(mg/dl
HDLc
(mg/dl)
LDLc
(mg/dl)
VLDLc
(mg/dl)
Normal control
124.35 ± 2.2c
112.54 ± 4.1c
51.34 ± 2.8a
68.23 ± 3.1c
20.55 ± 4.21c
Induced control
197.62 ± 3.1a**
166.47 ± 6.8a***
38.34 ± 3.2c**
97.19 ± 0.4.2a***
33.74 ± 5.42a**
SVA + CAR (200 mg/kg BW)
141.50 ± 2.5b*
134.37 ± 3.7b
46.21 ± 2.5ab*
79.36 ± 3.4b**
28.45 ± 4.27b
SVA + Q10 (15 mg/kg BW)
149.31 ± 2.8b
130.42 ± 4.1b
47.71 ± 3.1ab
77.16 ± 2.9b**
26.54 ± 3.89b
SVA+(Q10 + CAR)
131.46 ± 3.3bc*
124.31 ± 5.5bc
49.11 ± 2.9a
81.31 ± 4.6b**
25.64 ± 3.78ab
The high density lipoprotein cholesterol (HDL-C) levels increased in the groups treated both with Carnosine and CoQ10 recorded 49.1 mg/dl whereas in normal control it was 51.34 mg/dl. The other treatment groups also have significant increase in HDL-c level compared to induced control. There is also a significant increase in Low density lipoprotein cholesterol (LDL-C)level up to 81.31 mg/dl in treatment groups compared to normal control. The SVA induced control has recorded very high level of LDL of 97.19 mg/dl.
The level of very low density lipoprotein cholesterol (VLDL-C) has showed a significant difference (P < 0.05). The data represented that the administration of Carnosine and CoQ10 there is a slight increase in VLDL-C level compared to control. The SVA induced control has recorded high level of VLDL of 33.74 mg/dl.
3.4 Effect of Carnosine and CoQ10 in reproductive hormones level in serum
The results in the Fig. 2 indicated that the sodium valporate caused significant decrease in all the hormones tested like testosterone, LH and FSH levels compared to normal control group. There was significant increase in testosterone, FSH and LH levels up to 24.01, 156.60 and 3.57 mg/dl respectively in all treatment groups compared to SVA induced control. However, the hormones testosterone, LH and FSH levels was very low in SVA induced control by recording 13.73, 92.17 and 1.8 mg/dl respectively.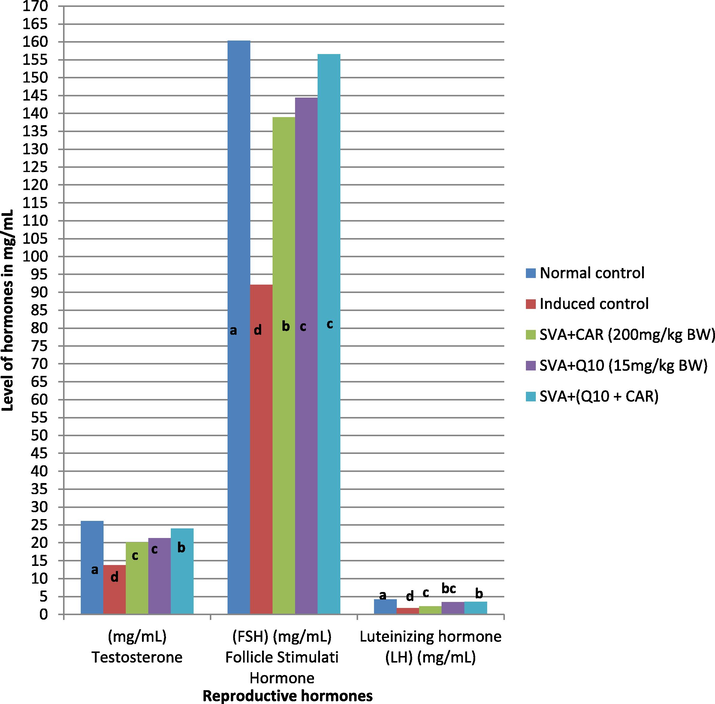
Effect of Carnosine and CoQ10 on the level of different reproductive organs hormones in SVA induced male rats. Data (n = 5 independent experiments) is expressed as mean ± SD. a, b,cand d indicates the significant differences at P < 0.05 among treatments.
3.5 Determination of serum lipid peroxidation, enzymatic and non-enzymatic antioxidant biomarker of the experimental rat groups
The effects of Carnosine and CoQ10 on serum antioxidant parameters (SOD,GST,GPX, Catalase and MDA) are represented in the Table 3.The serum antioxidant parameters such as SOD, GST, GPX and Catalase levels in the groups treated with both Carnosine and CoQ10 has increased to the high level of 57.31, 156.44, 48.36 and 141.53 mMol/L and decreased level of MDA of 11.83 mMol/L respectively as compared to SVA induced Control. The normal control has recorded SOD, GST, GPX, Catalase and MDA levels of 66.45, 170.88, 53.81, 148.17 and 9.34 mMol/L respectively. Data (n = 5 independent experiments) is expressed as mean ± SD. a, b and c indicates the significant differences at P < 0.05 among treatments.
Parameters
Groups
SOD
(mmol/l)
GST
(mmol/l)
GPX
(mmol/l)
Catalase
(u/l)
MDA
(mmol/l)
Normal control
66.45 ± 3.17a
170.88 ± 5.7a
53.81 ± 3.6a
148.17 ± 4.1a
9.34 ± 1.1b
Induced control
31.16 ± 2.40c**
59.82 ± 4.4c**
22.61 ± 2.4c**
80.16 ± 5.8c**
19.33 ± 1.5a**
SVA + CAR (200 mg/kg BW)
52.22 ± 3.21b*
141.63 ± 4.3b*
42.33 ± 311a
130.36 ± 3.6a
12.19 ± 1.2b
SVA + Q10 (15 mg/kg BW)
49.43 ± 3.18b*
134.34 ± 5.3b*
38.41 ± 3.6ab
128.62 ± 4.4a
12.27 ± 1.3b
SVA+(Q10 + CAR)
57.31 ± 3.15ab
156.44 ± 6.2ab
48.36 ± 4.1a
141.53 ± 3.9a
11.83 ± 1.3b
3.6 Effect of Carnosine and CoQ10 on sperm motility parameters
The effect of Carnosine and CoQ10 were tested on the various sperm motility parameters like VSL, VCL, VAP, LIN, STR and WOB and the results were presented in the Table 4. The CASA analysis showed a successive increase in sperm motility in Carnosine and CoQ10 treatment compared to normal and induced control after 6 h of sperm storage. The combined effect of Carnosine and CoQ10 has drastically increased the sperm motility parameters like VSL, VCL, VAP, LIN, STR and WOB by showing the values of 11.4 μm/s, 62.8 μm/s, 24.6 μm/s, 0.18, 0.46 and 0.39 respectively. Also there was a significant increase in the treatment with COQ10 alone followed by carnosine alone. The SVA induced control shown very low values of all the sperm motility parameters tested. Data (n = 5 independent experiments) is expressed as mean ± SD. a, b and c indicates the significant differences at P < 0.05 among treatments. Whereas Linearity (LIN) calculated by (LIN = VSL/VCL), Straightness (STR) calculated by (STR = VSL/VAP), Wobble (WOB) calculated by (WOB = VAP/VCL).
Parameters
Groups
VSL (μm/s)
VCL (μm/s)
VAP (μm/s)
LIN
STR
WOB
Normal control
5.7 ± 2.2a
41.2 ± 5.3a
11.9 ± 2.4a
0.14 ± 0.4
0.48 ± 0.9
0.29 ± 0.4
Induced control
1.1 ± 0.1b
7.7 ± 0.6b
2.1 ± 1.1b
0.14 ± 0.17
0.52 ± 0.1
0.27 ± 1.8
SVA + CAR (200 mg/kg BW)
8.7 ± 1.6a
53.6 ± 7.4a
19.3 ± 2.3a
0.16 ± 0.2
0.45 ± 0.7
0.36 ± 0.3
SVA + Q10 (15 mg/kg BW)
9.5 ± 2.7a
57.8 ± 6.3a
22.4 ± 3.7a
0.16 ± 0.4
0.42 ± 0.7
0.39 ± 0.6
SVA+(Q10 + CAR)
11.4 ± 2.4a
62.8 ± 5.8a
24.6 ± 4.5a
0.18 ± 0.4
0.46 ± 0.5
0.39 ± 0.7
4 Discussion
Male infertility is due to variation in sperm motility and /or morphology or an alteration in sperm concentration in humans so, in many studies it is highlighted that these reasons accounts for 40–50 % of infertility and also in 7 % of all men (Lotti and Maggi, 2015). There are many clinical reports that men have high prevalence of infertility. A higher prevalence of infertility amongst 38-year-old men of about 18.3 % was reported by Van Roode et al. (2015). At present there is inevitable evidence that a key role is played by the oxidative stress for the problem of male infertility and sperm dysfunction (Aitken et al., 2014). A very best alternative to address the problem is the use of antioxidants or herbal therapies which are highlighted in many literatures. But there is also studies reporting the failure of these treatments (Showell et al., 2014). Consequently, to meet out this problem, in the present study, Carnosine along with or without Coenzyme Q10 was tested on the sodium valporate induced testicular toxicity in male rats.
Using carnitines as antioxidant to improve the reproductive performance of female infertility in human and animal models was applied. For example, the supplementation of CoQ10 has improved the functions of seminal parameters has been reported by Littarru and Tiano (2007). Though, in the present study, the carnosine and CoQ10 was tested individually and in combination in male rats as animal models. The experimental rats were treated with sodium valproate to induce the cytotoxic effort, sodium valproate decreases the sperm count significantly in a linear fashion up to 7 weeks.
With regard to the biological effect on the body weight gain, feed intake and feed efficiency ratio (FER) of experimental rats showed a significant decrease in body weight gain and no difference was recorded feed intake and feed efficiency ratio in different treatments in the current study. These results were in accordance with the results obtained by Brandsch et al. (2002) who tested l-carnitine in male rats that did not modify any biological changes in body weight and body composition. Likewise, the treatment of Carnosine and CoQ10 does not produce any significant change in the relative weight of prostate of SVA induced rats under treatment with Carnosine and CoQ10 enzyme and between normal SVA induced controls. Rahim et al. (2013) also reported that the treatment of hydrogen peroxide induced male rats with Cymbopogon citratus (lemon grass) did not shown differences in the body testicular and epididymal weight compared to control rats.
In our study, the Serum lipid profiles (CHO, TG, HDL-C, LDL-C and VLDL-C) of normal and SVA induced reproductive toxicity in male rats treated with Carnosine and CoQ10 are evaluated. This resulted in a significant decrease of total cholesterol, Triglycerides levels and a significant increase in HDL-C, LDL-C, VLDL-C levels in SVA induced male rats treated with the combination of Carnosine and CoQ10 as compared to control. The results obtained were in relation with many studies that investigated that there is a close association between lipid profile and semen quality. For example, Liu et al. (2017) reported that the sperm morphology changes by showing a low percentage of spermatozoa with intact acrosomes and a smaller sperm head area and perimeter when the high serum levels of total cholesterol, free cholesterol, and phospholipids were detected. Ergün et al. (2007) detected that high serum VLDL and total triglyceride levels were statistically associated with low sperm motility and increased deleterious effect on the spermatogenesis. Similarly, Hagiuda et al. (2014) identified that the sperm morphology has associated with serum triglyceride level positively and there is no relation with sperm concentration or motility.
Further, in the present study, the effect of carnosine and CoQ10 in reproductive hormones level in serum was tested. It resulted in the significant improvement in the testosterone, FSH and LH levels up to 24.01, 156.60 and 3.57 mg/dl respectively in the combined treatment with carnosine and CoQ10 as compared to SVA induced control. Similar results were obtained by the treatment with Vitamin E and lemon grass treated male rats induced with hydrogen peroxide (Rahim et al., 2013). Also, in our study, the sodium valporate caused significant decrease in testosterone, LH and FSH levels compared to normal control group. This may be due to the disruption of leydig cells in the seminiferous tubules due to the induction of sodium valporate. The leydig cells are responsible for the production of testosterone which initiates the spermatogenesis. The parameters such as SOD, GST, GPX and catalase levels increased and MDA serum antioxidant level decreased when treated with both Carnosine and CoQ10 in the present study. A decreased level of MDA proves the reduction of oxidative stress that relatively increases spermatogenesis. Also, the superoxide dismutase (Yan et al., 2014) and catalase (Macanovic et al., 2015) has positively correlated with sperm concentration and motility as they form the main antioxidant system in semen. In the current study, the combined effect of Carnosine and CoQ10 were tested on the various sperm motility parameters like VSL, VCL, VAP, LIN, STR and WOB showed a consecutive increase in sperm motility as compared to SVA induced control even after 6 h of sperm storage. These results were also obtained by Nezhad et al. (2021) who proved that the addition of Carnosine and CoQ10 reduced the number of ROS during cryopreservation process. Similar effects of the imidazole dipeptides such as carnosine and anersine were reported in various studies (Bosler et al., 2014; Sarkar et al., 2021).
5 Conclusion
In the current study, it is proved from the results that the combined mix of Carnosine and CoQ10 following by SVA + CAR mix those promote spermatogenesis even though when induced with SVA. The SVA action has been strongly ameliorated by the carnosine along with CoQ10. Thus the carnosine along with CoQ10 may be an alternative to treat male infertility. Although, further studies has been suggested to test carnosine along with CoQ10 supplementation in other species and also to find its control mechanism.
Compliance with ethical standards
The Author(s) declare(s) that the work is in compliance with ethical standards.
Availability of data and materials
All the data is contained in the manuscript.
Author contribution
MF designed the study, conceived the study and analyzed the results. MF and MI conceived an initial part of the study, performed the experiment and helped in compiling the results. MF wrote the paper with input from MI.MF and MI made substantial contribution in interpretation of data and revising the manuscript for intellectual content. All authors read and approved the final manuscript. Authors also declare that all data were generated in-house and that no paper mill was used.
Acknowledgments
The authors extend their appreciation to the Researchers Supporting Project number (RSP2022R508), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- A simplified method for the estimation of total cholesterol in serum and demonstration of its specificity. J. Biol. Chem.. 1952;195:357-366.
- [Google Scholar]
- Lipid-lowering and hepatoprotective effects of Vitis vinifera dried seeds on paracetamol-induced hepatotoxicity in rats. Nutr Res Pract.. 2015;9(1):37-42.
- [Google Scholar]
- Peptides in seminal fluid and their role in infertility: A potential role for opiorphin inhibition of neutral endopeptidase activity as a clinically relevant modulator of sperm motility: A review. Reproductive Sciences. 2014;21:1334-1340.
- [Google Scholar]
- Effect of L-carnitine on weight loss and body composition of rats fed a hypocaloric diet. Ann. Nutr. Metab.. 2002;6(5):205-210.
- [Google Scholar]
- Quantitative determination of serum triglycerides by use of enzymes. Clin. Chem.. 1973;19:476-482.
- [Google Scholar]
- Aldehyde-sequestering drugs: tools for studying protein damage by lipid peroxidation products. Toxicol.. 2002;181:229-236.
- [Google Scholar]
- Measurement of catalase activity in tissue extracts. Anal. Biochem.. 1970;34(1):30-38.
- [Google Scholar]
- Correlation of seminal parameters with serum lipid profile and sex hormones. Arch. Androl.. 2007;53(1):21-23.
- [Google Scholar]
- Cryopreservation of canine semen- new Challenges. Reprod. Domest. Anim.. 2009;44(suppl. 2):336-341.
- [Google Scholar]
- Vitamin C partially attenuates male reproductive deficits in hyperglycemic rats. Reprod Biol Endocrinol.. 2011;9(1):1-9.
- [Google Scholar]
- The redox system in human semen and peroxidative damage of spermatozoa. Postepy Hig Med Dosw (Online). 2005;59:523-534.
- [Google Scholar]
- Relationship between dyslipidaemia and semen quality and serum sex hormone levels: an infertility study of 167 Japanese patients. Andrologia.. 2014;46(2):131-135.
- [Google Scholar]
- Assessment of Serum Vitamin D Level and Seminal Vitamin D Receptor Gene Methylation in a Sample of Egyptian Men with Idiopathic Infertility. Andrologia.. 2021;53(9):e14172.
- [Google Scholar]
- Resveratrol-Based Multivitamin Supplement Increases Sperm Concentration and Motility in Idiopathic Male Infertility: A Pilot Clinical Study. J Clin Med.. 2020;9(12):4017.
- [Google Scholar]
- Seminal plasma: An essential attribute to spermatozoa. J. Androl.. 2012;33(4):536-551.
- [Google Scholar]
- Enzymatic determination of cholesterol high density lipoprotein fraction prepared by polyanion precipitation. J Clin Chem.. 1976;22(5):695.
- [Google Scholar]
- Coenzyme Q10 and male infertility: A meta-analysis. J. Assist. Reprod. Genet.. 2013;30(9):1147-1156.
- [Google Scholar]
- Bioenergetic and antioxidant properties of coenzyme Q10: recent developments. Mol. Biotechnol.. 2007;37(1):31-37.
- [Google Scholar]
- Serum lipid profiles are associated with semen quality. Asian J Androl.. 2017;19(6):633-638.
- [Google Scholar]
- Ultrasound of the male genital tract in relation to male reproductive health. Hum Reprod Update.. 2015;21(1):56-83.
- [Google Scholar]
- Correlation between sperm parameters and protein expression of antioxidative defense enzymes in seminal plasma: A pilot study. Dis. Markers. 2015;2015:436236
- [Google Scholar]
- Sex-steroid binding, Plasma protein (SBP), testosterone, estradiol and DHEA in prepuberty and puberty. Acta Endocrinol.. 1987;114(1):60-67.
- [Google Scholar]
- Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. BBA. 1979;582(1):67-78.
- [Google Scholar]
- The Effect of L-Carnitine and Coenzyme Q10 on the Sperm Motility, DNA Fragmentation, Chromatin Structure and Oxygen Free Radicals During, before and after Freezing in Oligospermia Men. Urol J.. 2021;18(03):330-336.
- [Google Scholar]
- Assay for lipid peroxidation in animal tissues by thiobarbituric acid reaction. Ann. Clin. Biochem.. 1979;95(2):351-358.
- [Google Scholar]
- Protective effect of Cymbopogon citratus on hydrogen peroxide-induced oxidative stress in the reproductive system of male rats. Syst. Biol.Reprod. Med.. 2013;59(6):329-336.
- [Google Scholar]
- AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr.. 1993;123:1939-1951.
- [Google Scholar]
- Australian Medicines Handbook (2013 ed.). Adelaide: The Australian Medicines Handbook Unit Trust; 2013. p. :978.
- Effect of Omega-3 Polyunsaturated Fatty Acid Supplementation on Semen Profile and Enzymatic Anti-Oxidant Capacity of Seminal Plasma in Infertile Men With Idiopathic Oligo asthenoterato spermia: A Double-Blind, Placebo-Controlled. Randomised Study. Andrologia.. 2011;43(1):38-47.
- [Google Scholar]
- Effect of beta-alanine supplementation on muscle carnosine concentrations and exercise performance. Amino Acids. 2010;39(2):321-333.
- [Google Scholar]
- Effect of Anserine and Carnosine on Sperm Motility in the Japanese Quail. J Poult Sci.. 2021;58(3):186-191.
- [Google Scholar]
- Antioxidants for male subfertility. Cochrane Database Syst. Rev.. 2014;12:CD007411.
- [Google Scholar]
- A simple method for clinical assay of superoxide dismutase. Clin. Chem.. 1988;34(3):497-500.
- [Google Scholar]
- Cumulative incidence of infertility in a New Zealand birth cohort to age 38 by sex and the relationship with family formation. Fertil. Steril.. 2015;103(4):1053-1058.
- [Google Scholar]
- Proteomic landscape of seminal plasma associated with dairy bull fertility. Sci. Rep.. 2018;8(1):1-13.
- [Google Scholar]
- The causes of reduced fertility with cryopreserved semen. Anim. Reprod. Sci.. 2000;60:481-492.
- [Google Scholar]
- Seminal superoxide dismutase activity and its relationship with semen quality and SOD gene polymorphism. J. Assist. Reprod. Genet.. 2014;31(5):549-554.
- [Google Scholar]







