Translate this page into:
Effects of Wuweizi syrup on brain aging mice model induced by d-galactose
⁎Corresponding author. miaomingsan@163.com (Mingsan Miao)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objective
To observe the effect of Wuweizi syrup on brain aging mice model induced by d-galactose in mice.
Methods
72 mice were randomly divided into blank group, model group, naokangling group and low, middle and high dose Wuweizi syrup group. After 3 days of adaptive feeding, except for the blank group, the rest mice were subcutaneously (sc) injected with d-galactose 1.25 g/kg at neck back to replicate the aging model. Sc injection was given for 40 consecutive days, and corresponding drugs were given by gavage on the 11th day of modeling. On the 39th day, the dark experiment was carried out to measure the learning ability of mice. After the last administration for 2 h, brain, liver, thymus and spleen were taken and weighed to calculate the organ index. Blood was taken and CAT, GSH and SOD levels in whole blood were determined. Some brain and liver tissues were taken to prepare homogenates, and MDA levels in tissue homogenates and plasma were measured. Brain, liver, thymus and spleen were stained with HE, and pathological sections were made for observation under light microscope.
Results
Compared with the blank group, in the model group, the incubation period of mice was significantly reduced and the number of shuttle back and forth was significantly increased (P < 0.01); Spleen and brain indexes decreased significantly (P < 0.05), thymus indexes were decreased significantly, and liver indexes were increased significantly (P < 0.01); The levels of CAT, GSH and SOD in whole blood were significantly decreased (P < 0.01); MDA levels in liver homogenates, brain homogenates and plasma were significantly increased (P < 0.01); Pathological changes of brain, liver, thymus and spleen were observed under microscope. Compared with the model group, naokangling group and low, middle and high dose Wuweizi syrup group can significantly improve the incubation period of mice and reduce the number of shuttle back and forth (P < 0.01), can decrease or increase the organ index with different degree (P < 0.01 or P < 0.05), can significantly increase the levels of CAT, GSH and SOD in whole blood (P < 0.01), and significantly reduce the levels of MDA in plasma, liver homogenate and brain homogenate (P < 0.01), as well as can improve pathological changes of brain, liver, thymus and spleen with different degree.
Conclusion
The mice aging model was successfully replicated, and Wuweizi syrup can significantly improve the memory, biochemical indexes and pathological status of brain aging model mice.
Keywords
Brain aging
Immune organs
Oxidative stress
Pathological changes of tissues
Aging is a degenerative process of the body caused by multiple factors, which is accompanied by the decline of memory, the increase of oxide level, and the degeneration and pathological changes of brain, liver, kidney, thymus and other tissues. It is estimated that the population over 60 years old will reach 300 million in 2025, making China an aging country (Wang and Ma, 2019). The research and development of anti-aging drugs is of great significance. Wuweizi syrup was published in the Chinese pharmacopoeia in 2015. It is a Chinese medicine preparation made from the single medicinal material of Wuweizi, which can be used for the treatment of insomnia, dreaminess and neurasthenia. Modern pharmacological studies have shown that schisandrin b is the main active component of lignans, which has obvious effects of protecting liver, anti-apoptosis, improving memory, enhancing immunity and anti-oxidation (Li et al., 2014). Wuweizi polysaccharide has anti-aging and liver protection effects. It can not only enhance the immune function to resist aging, but also promote the development of nerve cells and delay the decline of immune function and nerve function caused by aging (Zhou, 2016). Previous studies in the laboratory showed that Wuweizi powder can resist the pathological progress of d-galactose induced aging mice model (Miao et al., 2018), and Wuweizi syrup has a protective effect on bronchial asthma mice (Peng et al., 2018). However, studies on the pharmacodynamic mechanism of Wuweizi syrup against aging have not been carried out. Therefore, naokangling was used as the positive drug, and d-galactose was used to establish aging mice model, to observe and evaluate the action mechanism of Wuweizi syrup on aging model mice in this experiment.
1 Experimental materials
1.1 Experimental animals
KM mice, half male and half female, weighing 18–22 g. Purchased from shandong laboratory animal center, experimental animal qualification certificate: 37009200000766, 37009200000767.
1.2 Experimental drugs and reagents
Wuweizi syrup, Shanghai haihong industry (group) chaohu jinchen pharmaceutical Co., Ltd., production batch number: 150630; Compound piracetam brain protein hydrolysate tablet (Nao kangling), Hongmei pharmaceutical (China) Co., Ltd., production batch number: 20140602; d-galactose, Sigma-Aldrich LLC., production batch number: G5388; Mouse malondialdehyde (MDA), Catalase (CAT), Superoxide dismutasee (SOD) and Reduced glutathione (GSH) ELISA Kit, Suzhou Calvin biotechnology Co., Ltd., production batch number: CK-E20347M, CK-E92636m, CK-E20348M and CK-E20064M.
1.3 Experimental apparatus
KDC-160HR high-speed refrigerated centrifuge, zhongjia branch of hkust innovation Co., Ltd.; Type 680-enzyme marker, BIO-RAD company, USA; Calibrated free running pipette, Shanghai rebo analytical instrument Co., Ltd.; BA-200 mouse darkmeter, chengdu taimeng software Co., Ltd.
2 Experimental method
2.1 Grouping
72 mice (20–23 g) were randomly divided into blank group, model group, naokangling group (0.81 g/kg) and low-dose, middle-dose and high-dose Wuweizi syrup group (1 ml/kg, 0.5 ml/kg, 0.25 ml/kg), with 12 mice in each group.
2.2 Modeling (Zhao et al., 2018; Fu et al., 2017) and administration
After 3 days of adaptive feeding, except for the blank group, the rest mice were subcutaneously (sc) injected with d-galactose 1.25 g/kg at neck back to replicate the aging model, while the blank group was injected with normal saline of the same dose for 40 consecutive days. Starting from the 11th day of modeling, the corresponding drugs were given by intragastric administration for 30 consecutive days.
2.3 Behavioral measurement
On the 38th day of the experiment, the mice were trained by using the dark avoidance behavior. The mice were placed in the bright room, the dark room were subjected to 33 V voltage, their tendency toward darkness led them to run towards the dark room, and when they were shocked, they immediately fled back to the light room. Repeat for 5 min. On the 39th day, a memory test was conducted, and the incubation period was the time required for each rat to receive an electric shock from the bright room to the dark room. The numbers of shuttle back and forth within 5 min and the incubation period of the first time entering the dark room were recorded and statistically processed.
2.4 Biochemical indexes and histopathological determination
After the last administration for 2 h, the mice was weighed. Blood was taken from orbit and heparin anticoagulant was added into EP tube to prepare whole blood. CAT, GSH and SOD levels of whole blood were measured. Partial whole blood was centrifuged, plasma was separated and MDA level was determined. Brain, liver, thymus and spleen were taken and weighed to calculate viscera index. Homogenate was prepared in the right brain and part of the liver. The left brain, liver, thymus and spleen were fixed with 10% formaldehyde, embedded with paraffin, sectioned, stained with HE, and morphological changes of each tissue were observed under light microscope.
3 Statistical method
SPSS17.0 medical statistical package was used for data analysis, measurement data were represented by mean plus or minus standard deviation (—x ± s), and one-way anova was used for comparison among groups.
4 Experimental results
4.1 Effects of Wuwrizi syrup on dark avoidance behavior of brain aging mice induced by d-galactose
Compared with the blank group, the incubation period of mice was significantly reduced, and the numbers of shuttle back and forth was significantly increased in the model group (P < 0.01). Compared with the model group, naokangling could increase the incubation period significantly (P < 0.01) and reduce the numbers of shuttle back and forth obviously (P < 0.05); And low-dose, middle-dose and high-dose Wuweizi syrup could significantly improve the incubation period of the model mice and significantly reduce the numbers of shuttle back and forth (P < 0.01), and its effect on the numbers of shuttle back and forth was better than that of naokangling group. See Table 1 for details.
Group
Dose
Incubation period (s)
Numbers of shuttle back and forth
Blank group
–
46.58 ± 3.46
11.75 ± 2.18
Model group
–
11.65 ± 2.25△△
16.17 ± 3.04△△
Naokangling group
0.81 g/kg
38.46 ± 3.26**
13.75 ± 3.33*
Wuweizi syrup group
1 ml/kg
33.46 ± 4.29**
12.08 ± 2.91**
0.5 ml/kg
26.62 ± 3.58**
12.25 ± 2.93**
0.25 ml/kg
30.55 ± 3.62**
12.00 ± 2.04**
4.2 Effects of Wuwrizi syrup on spleen, thymus, brain and liver indexes of brain aging mice induced by d-galactose
Compared with the blank group, the spleen index and brain index were obviously decreased (P < 0.05), the thymus index was significantly decreased (P < 0.01), and the liver index was significantly increased in the model group (P < 0.01). Compared with the model group, naokangling could significantly increase the spleen index and thymus index (P < 0.01); The high-dose group of Wuweizi syrup could significantly increase the spleen index, thymus index and brain index of mice (P < 0.01), the middle-dose group could significantly increase the thymus index and brain index of mice, and significantly decrease the liver index (P < 0.01), and the low-dose group could significantly increase the thymus index of mice (P < 0.01). See Table 2 for details.
Group
Dose
Organ index (g/g) %
spleen
thymus
liver
brain
Blank group
–
0.30 ± 0.03
0.26 ± 0.05
3.71 ± 0.15
0.86 ± 0.06
Model group
–
0.27 ± 0.03△
0.18 ± 0.03△△
4.22 ± 0.22△△
0.80 ± 0.09△
Naokangling group
0.81 g/kg
0.41 ± 0.05**
0.27 ± 0.04**
3.85 ± 0.31
0.82 ± 0.07
Wuweizi syrup group
1 ml/kg
0.36 ± 0.04**
0.29 ± 0.04**
4.02 ± 0.60
0.95 ± 0.08**
0.5 ml/kg
0.28 ± 0.04
0.25 ± 0.01**
3.52 ± 0.42**
0.87 ± 0.11**
0.25 ml/kg
0.27 ± 0.03
0.28 ± 0.04**
4.00 ± 0.43
0.83 ± 0.09
4.3 Effects of Wuwrizi syrup on the levels of CAT, GSH and SOD in whole blood of brain aging mice induced by d-galactose
Compared with the blank group, CAT, GSH and SOD levels in the whole blood of mice were significantly decreased (P < 0.01). Compared with the model group, CAT, SOD and GSH levels in whole blood were significantly increased in naokangling group (0.81 g/kg) and low-dose, middle-dose and high-dose Wuweizi syrup group (P < 0.01). See Table 3 for details.
Group
Dose
CAT (U/ml)
GSH (ng/ml)
SOD (U/ml)
Blank group
–
170.68 ± 7.74
260.25 ± 9.96
204.73 ± 16.81
Model group
–
113.87 ± 9.79△△
173.10 ± 13.28△△
89.02 ± 16.08△△
Naokangling group
0.81 g/kg
152.89 ± 8.50**
231.98 ± 11.25**
121.71 ± 14.60**
Wuweizi syrup group
1 ml/kg
169.16 ± 5.88**
261.81 ± 12.59**
143.77 ± 16.68**
0.5 ml/kg
153.91 ± 8.22**
231.31 ± 8.42**
127.52 ± 19.70**
0.25 ml/kg
149.51 ± 9.15**
209.72 ± 11.46**
107.11 ± 11.94**
Group
Dose
MDA (nmol/ml)
plasma
liver homogenate
brain homogenate
Blank group
–
4.89 ± 0.62
5.93 ± 1.00
5.34 ± 0.91
Model group
–
16.15 ± 0.88△△
18.38 ± 1.07△△
17.27 ± 1.01△△
Naokangling group
0.81 g/kg
8.00 ± 0.63**
12.13 ± 0.92**
8.93 ± 0.72**
Wuweizi syrup group
1 ml/kg
10.06 ± 1.03**
11.87 ± 1.07**
11.07 ± 1.14**
0.5 ml/kg
12.44 ± 1.16**
14.29 ± 1.15**
13.5 ± 1.21**
0.25 ml/kg
14.60 ± 0.96**
16.65 ± 1.06**
15.78 ± 1.05**
4.4 Effects of Wuwrizi syrup on plasma, liver homogenate and brain homogenate MDA levels of brain aging mice induced by d-galactose
Compared with the blank group, MDA levels in plasma, liver and brain homogenates of the model group were significantly increased (P < 0.01). Compared with the model group, MDA levels in plasma, brain and liver homogenates of mice were significantly reduced in naokangling group and low-dose, middle-dose and high-dose Wuweizi syrup group (P < 0.01).
4.5 Effects of Wuwrizi syrup on morphology of brain, liver, thymus and spleen of brain aging mice induced by d-galactose
4.5.1 Effects of Wuwrizi syrup on morphology of brain of brain aging mice induced by d-galactose
As shown in Fig. 1 In the blank group, the cerebral cortical nerve cells were abundant, well arranged and normally colored; The pyramidal cells in the hippocampus are closely arranged, the nuclei are round and large, and the nucleoli are clear. In the model group, the number of cerebral cortical nerve cells was significantly reduced, the arrangement was loose and the staining was light. Remaining nerve cells pyknotic, round, and vacuolar changes were observed in cytoplasm; In the hippocampal area, the number of pyramidal cells decreased, the density of neurons decreased, and the arrangement was sparse and irregular. The cell volume becomes smaller, and the phenomenon of nuclear pyknosis can be seen. In the naokangling group, the number of neurons in the cerebral cortex increased, and the nuclei were larger and more orderly, and the staining was deeper than that in the model group; The hippocampal vertebral cells are closely arranged and regular in shape, the nuclei of neurons are round and large, and the nucleoli are clearly discernible. In the high-dose Wuweizi syrup group, the number of neurons in cerebral cortex of mice increased obviously, and the nuclei became larger and more orderly. In the low-dose and middle-dose Wuweizi syrup group, the number of nerve cells in the cerebral cortex of mice increased significantly, and the nucleus of nerve cells became larger, the cytoplasm decreased and the staining deepened; The number of vertebral cells in the hippocampal area was significantly increased, and the arrangement was neat. The nucleus of nerve cells became larger, and the cytoplasm was less.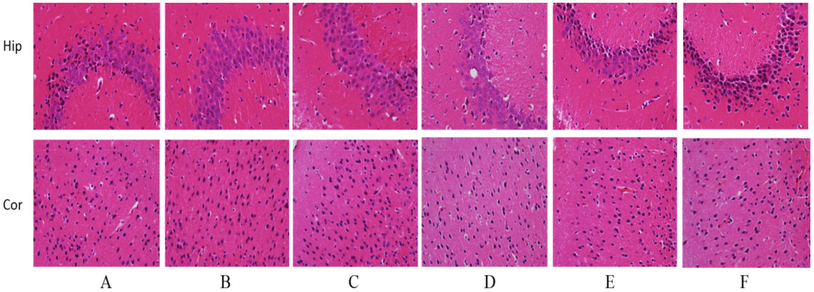
Effects of Wuwrizi syrup on morphology of brain of brain aging mice induced by d-galactose Note: A, Blank group; B, Model group; C, Naokangling group; D, High-dose Wuweizi syrup group; E, Middle-dose Wuweizi syrup group; F, Low-dose Wuweizi syrup group; Fig. 2, Fig. 3 and Fig. 4 are the same as Fig. 1.
4.5.2 Effects of Wuwrizi syrup on morphology of liver of brain aging mice induced by d-galactose
As shown in Fig. 2 In the blank group, there was no degeneration or necrosis of liver cells, and no inflammatory cell infiltration. In the model group, hepatocyte were highly edema, inflammatory cell infiltration was severe, hepatic cords were disordered, and focal necrosis was observed. In the naokangling group, the hepatocyte lesions were not obvious, and the hepatocyte cords were arranged regularly, with a small amount of inflammatory cell infiltration and mild local edema. In the high-dose Wuweizi syrup group, the degree of hepatocyte lesion was reduced, the edema site was less, and the arrangement of hepatocyte cords was relatively regular. In the low-dose and middle-dose Wuweizi syrup group, hepatocytes were disordered, with local edema and a small amount of inflammatory cell infiltration.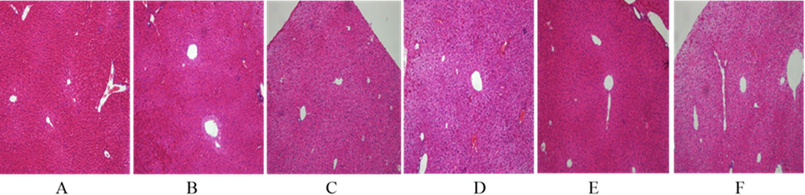
Effects of Wuwrizi syrup on morphology of liver of brain aging mice induced by d-galactose.
4.5.3 Effects of Wuwrizi syrup on morphology of thymus of brain aging mice induced by d-galactose
As shown in Fig. 3 In the blank group, the boundaries of thymus lobules were clear, medullary and cortical boundaries were clear, and lymphocytes were normal and densely arranged. In the model group, the boundary of the thymus lobule was blurred, the cortex became thinner, and the lymphocytes were normal but arranged sparsely. In naokangling group and low-dose, middle-dose and high-dose Wuweizi syrup group, the boundaries of thymus lobule were clear, the boundaries of medulla and cortex were clear, there were many lymphocytes, and no obvious pathological changes were observed.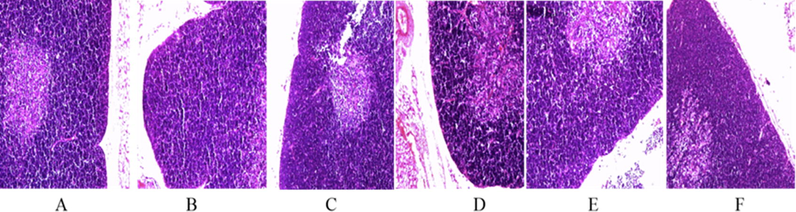
Effects of Wuwrizi syrup on morphology of thymus of brain aging mice induced by d-galactose.
4.5.4 Effects of Wuwrizi syrup on morphology of spleen of brain aging mice induced by d-galactose
As shown in Fig. 4 In the blank group, the red pulp and white pulp were clearly demarcated and the splenic nodules and lymphocytes were normal. In the model group, the boundary between red pulp and white pulp was blurred, the splenic nodules became smaller, and the lymphocytes were normal but sparse. In naokangling group and low-dose and middle-dose Wuweizi syrup group, the boundary between red pulp and white pulp were clearly demarcated, and no obvious pathological changes were observed in splenic nodules and lymphocytes. In the low-dose and middle-dose Wuweizi syrup group, the boundary between red pulp and white pulp were clearly demarcated, but the splenic nodules became smaller and lymphocytes were sparse.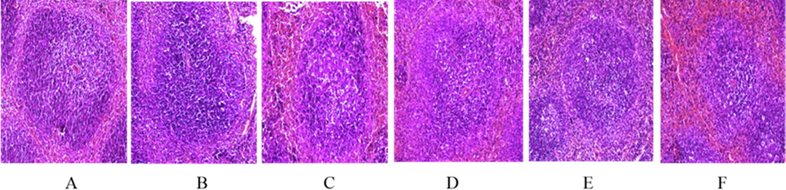
Effects of Wuwrizi syrup on morphology of spleen of brain aging mice induced by d-galactose.
5 Discussion
Aging is an inevitable law of the life process, but aging is accompanied by low immune function, low inflammatory reaction (Calder and Bosco, 2017), oxidative stress (Xiang et al., 2017), changes in microglia (Olga, 2017), etc., which can induce cognitive dysfunction diseases such as alzheimer’s disease and Parkinson’s disease (Li, 2018), so it is also regarded as a disease state. Therefore, it is of great significance to study anti-aging drugs with good effect and clear mechanism of action. Long-term injection of d-galactose in animals will lead to systemic metabolic disorder and organ degeneration, which is similar to the natural aging process of the body and is a common experimental animal model for aging research (Ding et al., 2016). Wuweizi syrup is made up of the 30% ethanol impregnating liquid of Wuweiz and the auxiliary materials, which contains lignans (Wang et al., 2014) and volatile components. Among them, the components of Wuweizi lignans have a protective effect on injured hepatocytes (Wang et al., 2017; Zhou et al., 2018). Schisandrae chinensis a can improve the pathological changes in the brain tissue of dementia mice by alleviating the degeneration and loss of neurons in brain tissue and improving the synaptic function, and play a protective role in brain tissue (Zhou et al., 2013).
The thymus is the site of T lymphocyte differentiation and maturation. The spleen is the largest peripheral immune organ of the body and an important site for the settlement of lymphocytes. And the immune organ index is a classic indicator to measure the development of immune organs in the body (Lin et al., 2019; Pang et al., 2019). Chinese medicine believes that brain aging disease is located in the brain. The neural cells in the brain are the basis of learning and memory, and the hippocampal structure and function are closely related to learning and memory. Aging or loss of cerebral cortical nerve cells and hippocampal neurons will seriously affect learning and memory (Jiang et al., 2017, Xiang et al., 2017). The liver is an important metabolic organ of the body and plays an important role in maintaining life. When stimulated by aging, liver cells will initiate the aging process through the feedback regulation mechanism, accompanied by a series of inflammatory reactions (Yang et al., 2017). Therefore, this study selected thymus, spleen, liver and brain tissues as pathological indexes to evaluate the efficacy of Wuweizi syrup. In addition, the results of the dark avoiding method showed that the learning and memory ability of mice treated with Wuweizi syrup was significantly improved, which was consistent with thememory improvement effect of total lignans of Wuweizi or Schisandrin B on aging mice induced by d-galactose reported in the existing literatures (Liu et al., 2018; Jing et al., 2016).
The aggravation of oxidative stress is one of the important causes of aging. GSH is an important antioxidant and free radical scavenger in vivo, and its content is an important index to measure the body’s antioxidant capacity. SOD scavenges superoxide radicals, but produces hydrogen peroxide, and CAT decomposes hydrogen peroxide. Only when the two cooperate, can superoxide radicals be scavenged thoroughly. MDA is one of the most important products of lipid peroxidation. Excessive accumulation of MDA can cause damage to cell structure and function. Therefore, CAT, GSH, SOD and MDA can be used as indicators to reflect the body’s antioxidant capacity and free radical scavenging capacity (Peng et al., 2014). The results showed that the levels of CAT, GSH and SOD in the whole blood of the model group were significantly decreased, while the levels of MDA in plasma, liver homogenate and brain homogenate were significantly increased, suggesting that oxidative stress injury existed in the aging mice model. Each dose of Wuweizi syrup group can improve the disordered free radical metabolism and reduce lipid peroxidation injury by increasing the activity of antioxidant enzymes (SOD, CAT, GSH) in the whole blood and inhibiting the production of free radical metabolites (MDA) in plasma, liver homogenate and brain homogenate of aging mice induced by d-galactose, thus playing an anti-aging role.
In conclusion, Wuweizi syrup has anti-aging effect. Its anti-aging effect may be related to increasing the levels of CAT, GSH and SOD in whole blood, decreasing the levels of MDA in plasma, liver homogenate and brain homogenate, improving the memory of mice, improving the pathological state of brain, liver, thymus and spleen tissues and organ index.
Acknowledgements
This research was financially supported by the Foundation item of National International Cooperation Base (2016-151) and Achievement Conversion Project in Henan Province (142201610011).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Bourdet-Sicard Raphaëlle, Capuron Lucile. Health relevance of the modification of low grade inflammation in ageing (inflammageing) and the role of nutrition. Age. Res. Rev.. 2017;40:95-119.
- [Google Scholar]
- Effects of total flavonoids of Selaginella on aging mice induced by D-galactose. Chin. J. Gerontol.. 2016;36:293-294.
- [Google Scholar]
- Effects of aerobic exercises on the expression of SYP and PSD-95 in prefrontal lobe of rats with brain aging induced by D-galactose. Chinese J. Sports Med.. 2017;36:563-570.
- [Google Scholar]
- Effect of polysaccharides of flammulina velutipes on learning and memory ability in aging mice induced by D-galactose. J. Beihua Univ. (Nat. Sci.). 2017;18:603-605.
- [Google Scholar]
- Effects of schisandra chinensis baill lignans on the learning and memory of D-GAL-induced aging mice. J. Beihua Univ. (Nat. Sci.). 2016;17:745-749.
- [Google Scholar]
- Effects of total flavonoids of astragalus on inflammatory response in brain tissue of natural aging rats and its related mechanism. J. Beihua Univ. (Nat. Sci.). 2018;19:745-749.
- [Google Scholar]
- Schisandrin B attenuates acetaminophen-induced hepatic injury through heat-shock protein 27 and 70 in mice. J. Gastroenterol. Hepatol.. 2014;29:640-647.
- [Google Scholar]
- Effects of carpesium abrotanoides extracts on immune function of mice. Herald Med.. 2019;38:415-418.
- [Google Scholar]
- Schisandrin B improves D-galactose-induced learning and memory impairment in aging mice. Food Sci.. 2018;39:201-206.
- [Google Scholar]
- Effect of Schisandrae Chinensis Fructus powder on D-galactose-induced aging model mice. Chinese Trad. Herbal Drugs. 2018;49:3074-3081.
- [Google Scholar]
- Age-related changes in microglial physiology: the role for healthy brain ageing and neurodegenerative disorders. e-Neuroforum. 2017;23:82-91.
- [Google Scholar]
- Effect of the powder of tea tree oil extract on the immune function of mice. Chinese J. Veterinary Drug. 2019;53:72-78.
- [Google Scholar]
- Protective effect of whey protein hydrolysates against oxidative stress in d-galactose-induced ageing rats. Int. Dairy J.. 2014;34:80-85.
- [Google Scholar]
- Effect of Fructus schisandrae syrup on bronchial asthma mice model. Saudi J. Biol. Sci.. 2018;25:1806-1811.
- [Google Scholar]
- Determination of mixed ZiMu lipid classes in Wuweizi Syrup. Acta Chinese Med. Pharmacol.. 2014;42:11-13.
- [Google Scholar]
- Research progress of traditional Chinese medicine with anti-aging effects and mechanisms of action. Asia-Pac. Trad. Med.. 2019;15:197-200.
- [Google Scholar]
- Comparison on the protective effects of dibenzocyclooctadiene lignans contents of Fructus Schisandrae Chinensis for the injured hepatocytes. China Med. Herald. 2017;14:35-38.
- [Google Scholar]
- Protective mechanism of ginsenoside Rg1 on hippocampus of aging mice induced by D-galactose. Chinese Trad. Herbal Drugs. 2017;48:3789-3795.
- [Google Scholar]
- Effects of total saponins of Panax japonicus on liver inflammation natural aging rats. Chinese Pharmacol. Bull.. 2017;33:848-853.
- [Google Scholar]
- Effects of schisandra fruit decoction on electrophysiological characteristics of sciatic-peroneal nerves of toad. Sichuan J. Physiol. Sci.. 2014;36:17-19.
- [Google Scholar]
- Effect of Yisui moxibustion on Klotho protein expression and insulin pathway of hippocampal tissues in aging mice model induced by D-galactose. Guangxi Med. J.. 2018;40:812-817.
- [Google Scholar]
- Advances in modern pharmacological action of Wuweizi. Strait Pharm. J.. 2016;28(03):183-184.
- [Google Scholar]
- Effect of schisandrin on expression of both SYP and α-syn in brain tissue of the APP/PS1 double transgenic dementia mice. Chinese Pharmacol. Bull.. 2013;29:1076-1079.
- [Google Scholar]
- Metabonomics study on hepatoprotective effect of Schisandrae Chinensis Fructus based on UPLC-Q-TOF-MS. China J. Chinese Materia Medica. 2018;43:3756-3763.
- [Google Scholar]







