Translate this page into:
Effects of salinity and wastewater on the growth of Synechococcus elongatus (strain PCC 7942) and some of its cellular components
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The aim of this study is to determine the effect of salinization and wastewater stresses on the growth and some cellular contents of the unicellular cyanobacterium Synechococcus elongatus (strain PCC 7942). The results indicated that the treatment of S. elongatus with NaCl to 400 mgL−1 was significantly increased the growth of this cyanobacterium and its cellular macro-molecules. However, with increasing concentration, the effect becomes inhibitory to cellular growth and cell contents. Also, in this study, the cells of S. elongatus were exposed to the lethal concentration (800 mg L−1) to study the extent of the sensitive or resistance effects of the organism to salinity stress. In this respect, treatment with NaCl at the lethal concentration (800 mg L−1) had different effects on its detected amino acids. Whereas, all amino acids exhibited stimulatory responses, except Gly., Ala., leu., and His., which significantly inhibited. On the other hand, the internal cellular structure, which was examined with TEM, showed a partial dissolution of cell wall and most cell components could be observed although not as clear as in control cell. Meanwhile, salinization treatment induced partial disorganization of the cell end contents. Although, the treatment with wastewater induce the growth and stimulate the cellular contents of S. elongatus, the cells exhibited an elevation in starch granules and disorganization of thylakoid membranes.
Keywords
Salinity
Wastewater
Synechococcus elongatus
1 Introduction
Salinization is an important ecological factor which affect the physiological and biochemical reactions of algae and consequent on its distribution (Ebrahimi and Salarzadeh, 2016). Algae living in an environment where salinities may change their physiological processes rapidly to be able to maintain their growth as constant as possible (Ding et al., 2013). Thus, in many coastal and usually nutrient-rich environments salinity might be expected to shift the species dominance in a community reaching a variable salinity area and to be responsible for the peculiar composition of resident populations. Variation in salinity is accompanied by changes in the concentration of the major ions that may be energetically coupled to nutrient uptake in algae (Liu and Yildiz, 2018). There is a natural phenomenon that some species of blue-green algae can grow or capable of osmotic adjustment which permits them to inhibit environments of widely differing salinities (El-Din, 2015). One of the factors recently shown to be important in enabling Cyanobacteria to exit at elevated salinities is higher ability to accumulate low molecular carbohydrates as internal osmotic in response to external osmotic stress (Fakhry and El-Maghraby, 2015; Monika et al., 2015; Miranda et al., 2016). Nosseir and Abouel-kheir (1970) stated that the blue-green algae were plentiful where the salinity was high as well as where it is low. On the other hand, the salinity had a danger effects on growth rate and photosynthesis of some species of algae. The reduction of photosynthetic capacity of the cells reflected a lower ability to utilize light energy and resulted in an increase in the susceptibility of the stressed cells to photoinhibition (Vonshak et al., 1996). In contrast there is a positive response from some species of algae especially Cyanobacteria with salinity. As the cyanobacterial response to salinity was very rapid varied with time and was correlated with external salt (NaCl) concentration during stress (Minhas et al., 2016). Photosynthesis in some cyanobacteria has been shown to be stimulated by elevated Na+ concentrations (Anand et al., 1994). Non marine blue-green algae are known to be extremely halotolerant, growing at salinities in excess of 200 g L−1 (Vaishya and Kaushik, 1989). The main constituents of the dry matter namely carbohydrates, proteins were affected by salinity treatments. The proteins contents depending on the algal species under treatment. Salinity induced three predominant types of modification; a) the synthesis of several proteins was inhibited especially in salt-sensitive strain, b) the synthesis of certain protein was significantly enhanced and c) synthesis of a specific set of proteins was induced de novo by salinity stress (Kumar and Bhagwat, 1989).
Although the wastewater considered as a negligent thing, it is very rich with phosphorus, nitrogen and other compounds which are very necessary for algal growth (Ibraheem, 1998; Abdel-Raouf et al., 2012; Wang et al., 2017). Increasing eutrophicity of water as a result of P and N high levels could consequently cause a pronounced enhancement of algal colonization potentiality (Abdel-Raouf et al., 2003). They have considered phosphorus and nitrogen to be the key of eutrophication. In the presence of excess nutrients, the algae are capable of rapid growth and multiplication. The calculated gain in fresh and dry weights progressively increased by longer time (Lahaniatis et al., 1991).
Wastewater borne nutrients are converted into biomass protein has gained great interest recently (Ibraheem et al., 2017). Algae which grown on wastewater recorded high protein and lipid content. Many algal species were used for wastewater treatment to become suitable for re-using so this parameter have an environmental and economic importance. Wastewater supported algal growth with incorporation of a significantly higher content of the individual amino acids (Abdel-Raouf et al., 2012). So, it was suggested that, the availability of the essential nutritional elements in the wastewater, beside the tendency of the algae for bioaccumulation and incorporation of such elements into the cellular macromolecules.
The method of TEM was applied recently to the study of algae which may add a new progress in studies concerning the taxonomy and anatomy of algae. Although, algae have received more attention in recent years, still little information are available in the local literatures. Such kind of study may enable researches to throw some lights on and follow up the environmental and physical behavior of the investigated algal forms in response to environmental stresses as salinization and wastewater. Such study will open the gates on the long road of search for new forms of algae and provide us with more information about the role of the investigated alga through estimation of algal growth. In addition, this study was designed to assess the effect of environmental stresses on some cellular macro-molecules of the investigated algae including proteins and the associated amino acids, carbohydrates, and nucleic acids. The cellular ultrastructural alterations were monitored by transmission electron microscopy.
2 Materials and methods
2.1 The biological material
In the present investigation, an axenic cultures of the unicellular blue-green alga S. elongatus (strain PCC 7942) which kindly supply from Phycological Lab, Collage of Science, King Saud University, was used.
2.1.1 Media used for culturing algal taxa (Modified Chu’s 10 medium)
This medium was recommended by Chu (1942) and modified by Gerloff et al. (1950). It was the best medium for the investigated cyanobacterium. It was selected from different tested media (data not included).
2.1.2 Maintenance of the studied algal cultures
Stock cultures were maintained in a refrigerator at 5 °C on agar slants (El-Nawawy et al., 1958). Sub-culturing should be conducted each thirty days and keep purified.
2.1.3 Algal growth conditions
Preliminary tests were conducted by using a wide range of temperature (24–30 °C), light duration (12–24 h), light intensities (1000–6000 Lux) and pH values (6–8) to obtain the optimum growth conditions for the studied cyanobacterial taxon. As a result of these experiments S. elongatus (strain PCC 7942) cultures incubated at 30 °C, continuous light at 3000 Lux and pH 7. The cyanobacterial culture was harvested on the 6th day then used in the experimental study.
2.2 Uptake test of investigated environmental stresses from culture media by S. elongatus (strain PCC 7942)
According to Wong and Pak (1992), a preliminary experiment using a wide range of different NaCl concentrations and different wastewater percentages were carried out to determine the suitable concentrations of these materials which could be tolerated by the studied alga. Selection of these concentrations was based on the response of the studied alga to it, which had a slightly or marked effects on their growth, and also to avoid the non-effective and directly lethal concentrations on the alga experimented with.
The actual experiment according to Shaaban et al. (2004) was then carried out by placing the appropriate volumes of selected concentrations of the studied NaCl or wastewater percentages (after filtration through bacterial filter) into the culture media making up to 100 mls with particle-free deionized water and cyanobacterial cells with a known density (initial inoculum): 4 × 103 cells/ml of S. elongatus (strain PCC 7942) using 250 ml measuring conical flasks as culture vessels. The pH of the media was adjusted by 1 M HCl/NaOH prior to autoclaving. The culture media were aerated (to provide CO2) through the cotton plugs. Three replicates for each concentration of the studied stresses in addition to the control were prepared. Then the culture vessels were incubated under conditions required for the growth of the studied alga for a required period of growth. At the final growth period, the cyanobacterial residue was harvested, washed three times with distilled water and subjected for determination of the investigated cellular macromolecules according to the described plan of this study.
2.3 The wastewater sources
The wastewater samples were provided from Sewage Station in Al-Kharj Governorate.
2.4 Chemical analysis
2.4.1 Optical density: (Turbidity technique)
The optical density of the homogenized blue-green or green algal suspension were measured at 760 nm (Adhikary, 1983).
2.4.2 Chlorophyll a content
Chlorophyll a content of algae was determined according to the method described by Strickland and Persons (1972). The concentration of chlorophyll a (μg L−1) was calculated using the equation of Strickland and Persons (1968).
Chl.a = 11.64 E663 − 2.16 E645 + 0.1 E630 μg L−1
2.4.3 Determination of total soluble proteins
This was carried out according to the method of Lowery et al. (1951), using bovine serum albumin as a standard protein.
2.4.4 Extraction and determination of total soluble carbohydrates
2.4.4.1 Extraction
The algal growth suspension after being dried at room temperature, were ground to a fine powder and extracted with mixture of 5 mls of 2% phenol water and 10 mls of 30% trichloroacetic acid (Said and Ramzy, 1964).
2.4.4.2 Determination
Total water-soluble carbohydrates were determined by anthrone technique according to Umbreit et al. (1969).
2.4.5 Quantitative estimation of nucleic acids (RNA-DNA)
The method applied for total RNA and DNA determination (Schmidt and Thannhauser, 1945) with some little modifications as described by Morse and Carter (1949) was applied.
2.4.5.1 Ribonucleic acid (RNA) content
It was estimated colorimetrically by the orcinol reaction as described by Dische (1953). To 0.5 ml RNA extract 3 ml of an acid reagent (0.5 ml of a 10% FeCl3·6H2O was mixed with 100 ml of concentrated HCl) were added. This was followed by adding 0.2 ml of a freshly prepared 6% solution of orcinol in 96% ethanol. The mixture was heated in a boiling water bath for 20 min and its optical density was measured at 660 O.D.
2.4.5.2 Deoxyribonucleic acid (DNA) content
It was estimated by DPA (diphenylamine) color reaction described by Burton (1956). Samples of 1.0 ml DNA extract were mixed with 2.0 ml of DPA reagent (1.5% g of steam distilled diphenylamine were dissolved in 100 ml of redistilled glacial acetic acid then 1.5 ml of concentrated H2SO4 were added and mixed well) let stand at 30 °C for 16–20 h. Their optical densities were measured at 540 mu. with pure RNA and DNA, the method gives a linear relation between the concentration of RNA and DNA and the optical density.
2.4.6 Amino acid determination
Amino acid determination was performed according to the method of Winder and Eggum (1966). Oxidation with performic acid, to protect methionine and cysteine from distraction during acid hydrolysis, followed by acid hydrolysis were carried out in closed conical flask for determining all amino acids other than tryptophan. Sample of 20–30 mg was weighed in the conical flask and 5 mls of performic acid were added. The flask was closed and placed in ice water bath for 16 h. Sodium meta bisulfate was added followed by 25 mls of HCl 6 N were added to the oxidized mixture. The flasks were placed in an oven at 110 °C for 24 h. The flasks were then opened and all HCl was removed by evaporating the samples to dryness in a rotary evaporator. A suitable volume of sodium citrate buffer (pH 2.20) was added to the dried film of hydrolyzed samples. After all soluble material completely dissolved, the samples were ready for analysis. The system used for the analysis was high performance amino acid Analyzed, Beckman 7300.
2.4.7 Transmission electron microscope
After 6 days of treatment for S. elongatus (strain PCC 7942) by NaCl (800 mg L−1) and wastewater (100%), the cells become to make ready for harvesting by centrifugation at 2500 r.p.m. for 10 min at 4 °C, immediately fixed in fresh 3% glutaraldehyde-formaldehyde at 4 °C for 18–24 h. The specimens were then washed in phosphate buffer (pH 7.4) and then postfixes in isotonic 1% osmium tetroxide for one hour at 4 °C (Mercer and Birbeck, 1966). Ultrathin sections were then prepared using the ultramicrotome glass knives, stained with uranyl acetate and lead citrate (Reynolds, 1963) and examined by Philips 400 T electron microscope at 60–80 KV.
2.5 Statistical analysis
Data obtained in the present investigation were statistically analyzed using the Least Significant Difference test (L.S.D) at 1% and 5% levels of probability (Snedecor and Cochran, 1967).
3 Results
Preliminary tests using wide range of salinization treatments (mg L−1 of NaCl) were conducted on the growth of the studied alga S. elongatus (strain PCC 7942) (as mentioned in materials and methods) to avoid the non-effective and directly lethal concentrations of NaCl. The obtained results indicated that the most suitable concentrations of NaCl for this study were: 200, 400, 600, and 800 mg L−1. The recorded results in the present study were mean values of three replicates of determinations.
3.1 Salinization treatments
The data recorded in Table 1 showed a correlation between the responses of the cyanobacterium as indicated by the growth and chlorophyll a to the experimental salinization treatments. The obtained data revealed that S. elongatus (strain PCC 7942) was resistant to some of the applied NaCl concentrations. The cyanobacterium exhibited different stimulation responses which accounted for 21, 10 and 8% at 200, 600 and 800 mg L−1 of NaCl, respectively, with maximum stimulation rate of 35% at 400 mg L−1 of NaCl. A drastic decline in growths (58, 79 and 88%) were exhibited at higher concentrations of NaCl 1000, 1200 and 1400 mg L−1, respectively (Table 1). Similar responses were detected on the chlorophyll a content. A slight stimulation of chl.a synthesis (2.6%) was observed at 200 mg L−1 of NaCl. Increasing salt level to 400 mg L−1 highly stimulated chl. a by 15% higher than control. However, there is a marked inhibitory effect of salinization on chl.a synthesis appeared at higher concentrations of NaCl above 800 mg L−1.
NaCl conc. (mg L−1)
Control
200
400
600
800
1000
1200
1400
LSD 1%
LSD 5%
Growth (O.D. at 760 nm)
0.64 ± 0.03
0.76 ± 0.01*
0.86 ± 0.04**
0.66 ± 0.02**
0.65 ± 0.04**
0.30 ± 0.01**
0.14 ± 0.01**
0.08 ±**
3.9 × 10−2
1.9 × 10−2
Chlorophyll a (μg L−1)
6.80 ± 0.2
7.00 ± 0.2*
7.8 ± 0.5**
7.00 ± 0.2**
6.9 ± 0.3**
1.00 ± 0.06**
0.01 ± 0.001**
0.01 ± 0.001**
5.8 × 10−2
0.03
The main constituents of the dry matter namely, total soluble proteins, total soluble carbohydrates and nucleic acids were also affected by salinization treatments (Fig. 1). Generally investigated cellular macro-molecules showed increase in their contents in response to certain levels of NaCl (200 and 400 mg L−1). However, further increasing of salt levels led to pronounce declines in total soluble carbohydrates and the total soluble proteins.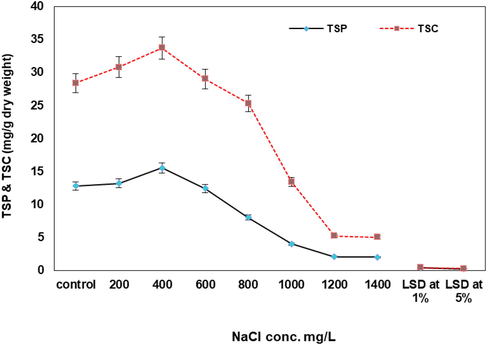
Effect of salinity (mg L−1) on total soluble proteins and total soluble carbohydrates (mg g−1 dry weight) of Synechococcus elongatus (strain PCC 7942).
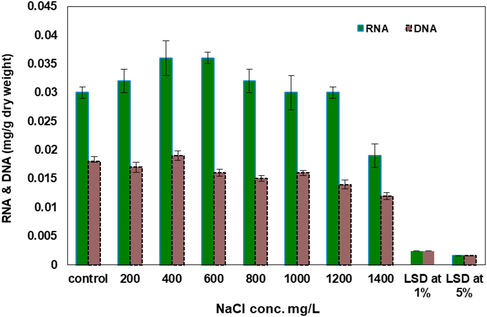
Effect of salinity (mg L−1) on RNA & DNA (mg g−1 dry weight) of Synechococcus elongatus (strain PCC 7942).
Such effect extends to comprise nucleic acids, which were injured and suppressed by rise in salinization process. RNA recorded moderate stimulation’s levels of 7, 25, 24 and 8% at 200, 400, 600 and 800 mg L−1 of NaCl, respectively. On the other hand, there is a significant inhibition of RNA values by 38% was exhibited at 1400 mg L−1 of NaCl. Similarly, DNA values were also, inhibited with increasing NaCl levels and marked fluctuations of DNA values were observed with certain levels of salt as compared with the control.
Fig. 3 revealed that there was different response in the amino acid's contents of S. elongatus (strain PCC 7942) towards salinization treatment at 800 mg L−1 of NaCl. Although, Asp, Thr, Ser, Cys, Val and Iso-leu were slightly stimulated by 800 mg L−1 NaCl, Glu, Pro, Meth, Ph.ala, Lys and Arg were highly stimulated by salinization. On the other hand, Gly, Ala and Leu were highly inhibited. It was noticeable that the amino acid Tyr was detected in the treated cultures (Fig. 3).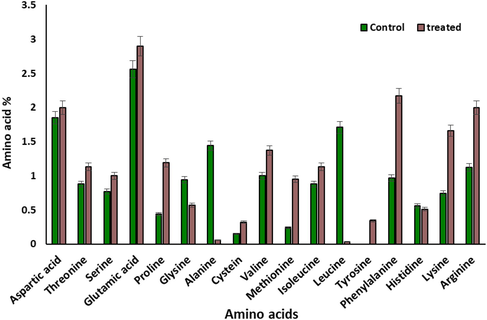
Percentages of amino acids contents in Synechococcus elongatus (strain PCC 7942) cells grown on nutrient medium under salinity level (800 mg L−1) of NaCL after 6 days.
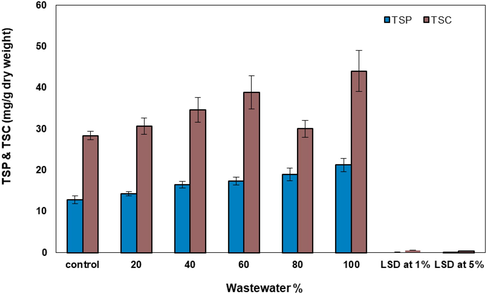
Effect of wastewater percentage on total soluble proteins and total soluble carbohydrates (mg L−1 dry weight) of Synechococcus elongatus (strain PCC 7942).
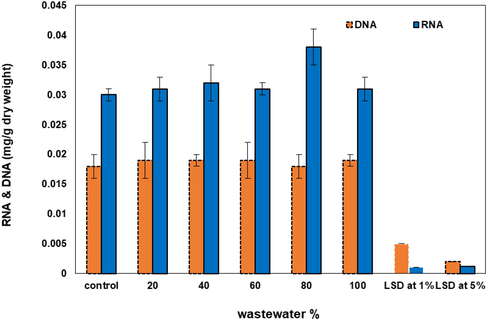
Effect of wastewater percentage on RNA and DNA (mg g−1 dry weight) of Synechococcus elongatus (strain PCC 7942).
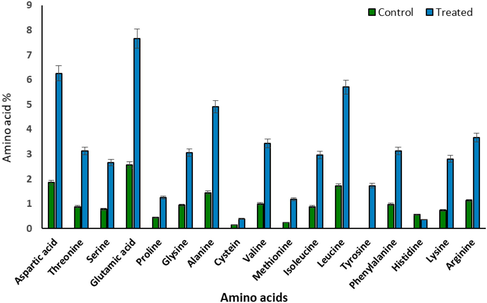
Percentages of amino acids contents in Synechococcus elongatus (strain PCC 7942) after 6 days of treatment by 100% of wastewater.
3.1.1 Ultrastructural changes of S. elongatus (strain PCC 7942) treated with 800 mg L−1 of NaCl for 6 days
The alterations in the S. elongatus (strain PCC 7942) ultrastructure caused by concentration of 800 mg L−1 of NaCl for 6 days, are illustrated (Plate 1). Cells of S. elongatus (strain PCC 7942) subjected to 800 mg L−1 of NaCl through 6 days, showed a certain dissolution of cell contents large vacuole formation was observed at the end of experimentation period (Plate 1).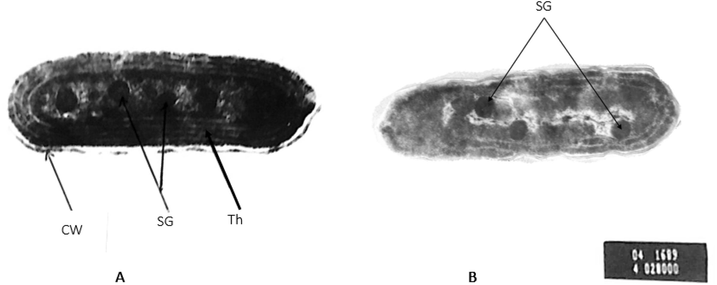
Electron micrograph of Synechococcus elongatus (strain PCC 7942); A: control with normal cell structures (CW = cell wall, SG = starch granules, Th = thylakoids) and B: cells treated with 800 mg L−1 of NaCl for 6 days, which represent a partial dissolution of cell wall. Apparently, the majority of cell components could be observed although not as clear as in control cell. Meanwhile, salinization treatment induced partial disorganization of the cell end contents, X = 42000.
3.2 Effect of wastewater
In the present investigation primary treatment carried out through the preliminary sieving step at station to get-rid of the large suspended solids, then subjected to sterilization through a Bacterial Filter in laboratory.
Wastewater quality
Physicochemical characteristics of the wastewater sample under the experimental investigation were represented in Table 2. During the study period, the pH-value measured 9.0, which lies mainly in the alkaline side. In addition, the electrical conductivity (E.C.) recorded 1.7 mohs. cm−1 and the total soluble salts (T.S.S) estimated as 590 mg L−1. The bicarbonate level was reached 6.02 mg L−1 during the investigation period. Apparently, the wastewater samples were completely depleted of any detectable carbonate. On the other hand, the chloride content was relatively low and recorded an average of 3.00 mg L−1. However, the sulphate content was very low and estimated as 0.04 mg L−1 during the investigation period. The data further revealed that the detected cations were limited to Ca++ (2.00 mg L−1), Mg++ (1.09 mg L−1), Na+ (3.00 mg L−1), and K+ (0.95 mg L−1).
-
b.
Wastewater affecting the studied alga S. elongatus (strain PCC 7942)
| Parameter | Values | |
|---|---|---|
| Physical values | Water Color | Yellowish |
| Water Odor | Unacceptable | |
| Chemical values | pH | 9.0 ± 0.2 |
| E.C | 1.7 ± 0.2 m mohs cm−1 | |
| T.S. S | 590 ± 03 | |
| Cl− | 3.00 ± 0.5 mg L−1 | |
| SO4 | 0.04 ± 0.02 mg L−1 | |
| HCO−3 | 6.02 ± 0.1 mg L−1 | |
| CO−3 | – | |
| Ca++ | 2.00 ± 0.1 mg L−1 | |
| Mg++ | 1.09 ± 0.2 mg L−1 | |
| Na+ | 3.00 ± 0.3 mg L−1 | |
| K+ | 0.95 ± 0.2 mg L−1 | |
It is note worthily that there were striking differences in growth, chl.a and the studied cellular macro-molecules contents pre and post-wastewater treatment (Table 3 and Figs. 4 and 5). The growth of the investigated cyanobacterium was stimulated by wastewater treatment. Both cell multiplication and chl.a content generally increased with subsequent increase in the wastewater concentrations. High concentrations of wastewater (60 and 80%), significantly enhanced the growth of studied cyanobacterium by 23 and 24% of control respectively. Further increase in wastewater concentration to 100% resulted in stimulation of growth to 30% over the control.
Wastewater %
Control
20
40
60
80
100
LSD at 1%
LSD at 5%
Growth (O.D. at 760 nm)
0.64 ± 0.03
0.67 ± 0.03*
0.71 ± 0.02**
0.77 ± 0.02**
0.79 ± 0.01**
0.83 ± 0.04**
0.13
6.9 × 10−2
Chlorophyll a (μgL−1)
6.80 ± 0.2
7.5 ± 0.2*
7.85 ± 0.2**
9.00 ± 0.3**
9.8 ± 0.4**
10.00 ± 0.06**
0.90
0.62
Accordingly, in pure wastewater medium (100% wastewater concentration) chl.a was stimulated by approximately 10 and 12% at low concentrations of wastewater (20 and 40%, respectively). Elevation, wastewater doses to 60 and 80% led to increasing in chl.a biosynthesis by 35 and 39%, respectively.
Similarly, the data in Fig. 4 revealed that the total soluble carbohydrates and the total soluble proteins of the treated S. elongatus (strain PCC 7942) were significantly higher than control in all studied NaCl concentrations.
Nucleic acids were slightly affected by wastewater treatments so, they recorded a fluctuations behavior upon treatment with wastewater (Fig. 5). RNA exhibited a slight stimulation by 4 and 7% at 20 and 40% of wastewater percentage respectively. Irregular fluctuations appeared at the other studied concentrations. On the other hand, DNA showed a slight stimulation and steadily elevation as concentration increased. The highly stimulations of DNA values (30%) was recorded at 80% wastewater. No further increase of DNA was recorded with the increasing of wastewater levels.
Wastewater favored algal growth with incorporation of a relatively higher content of the individual amino acids (Fig. 6). The recorded data revealed that the most amino acids were highly stimulated by wastewater treatment. However, His was the only amino acid participated with less amount inhibited by wastewater treatment.
3.2.1 Ultrastructural alterations of S. elongatus (strain PCC 7942) cell, grown on wastewater medium for 6 days
It is noteworthy that wastewater exerted no obvious cellular changes in the ultrastructure of S. elongatus (strain PCC 7942), except for an elevation in starch granules inside the cell (Plate 2).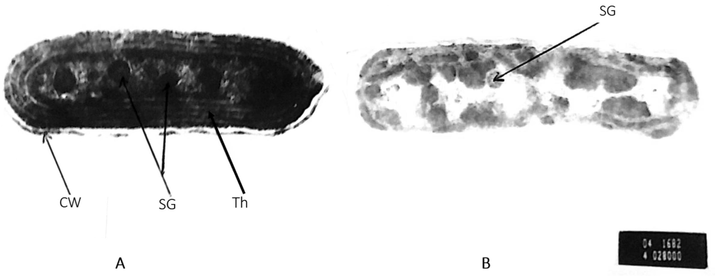
Electron micrograph of Synechococcus elongatus (strain PCC 7942) cell, A: control cells, and B: cells after 6 days of wastewater treatment showing, no obvious cellular distortions, other than a general increase in cell size and in number of starch granules (SG). X = 42000.
4 Discussion
Algae living in intertidal and estuarine environments. Frequently experience changes in external salinity. In intertidal habitats this may be due to rain fall and evaporation during exposure. Estuarine algae are subject to fairly regular changes on a diurnal and seasonal pattern depending on weather and stratification of the saline and freshwater layers (Wilkinson, 1981). Due to such changes in salinity, blue-green algae and some species of green algae have the unique capacity to adapt themselves to several ecologically extreme habitats. Their occurrence in varying saline situations has down much interest in recent years especially on their levels of halotolerance and mechanism of adaptation.
Fig. 1 revealed that growth of S. elongatus (strain PCC 7942) was highly stimulated with increasing NaCl concentrations. Meanwhile, the total soluble proteins and total soluble carbohydrates increased gradually at 600 mg L−1 of NaCl a trend that was reversed with increasing of NaCl. It is in accordance with Fakhry and El-Maghraby (2015); Monika et al. (2015) and Miranda et al. (2016) who reported that, Cyanobacteria were accumulate low molecular carbohydrates as internal osmotic in response to external osmotic stress to resist the elevated salinities.
The collected data further reveal that halotolerant organisms like S. elongatus (strain PCC 7942) possess an effective mechanism of osmotic balancing. In halotolerant blue-green algae an efficient photosynthetic system resulting in ample carbon fixation and ultimate accumulation of carbohydrates appears to be one of the important mechanisms that helps the organism to adaptation. These data are in agreement with Anand et al. (1994), who stated that accumulation of total soluble carbohydrates have now been shown to help in maintaining the osmotic balance in several blue-green algae.
It is of prime interest to note that S. elongatus (strain PCC 7942) was stimulated upon 400 mg L−1 of NaCl. Such findings are in accordance with (Trainor, 1978) who showed that the halophilic blue-green algal species which can grow at high salt concentrations (as salt marsh habitats) are probably capable to do so due to their prokaryotic organization, the absence of large sap vacuoles and adaptability. Possible explanation is that S. elongatus (strain PCC 7942) was tolerant to 800 mg L−1 of NaCl may carry unique genetic determinants encoding for salinization resistance or this strain possessed a mechanism of challenging salinization within its cell.
This result justifies the findings of (Hagemann et al., 1990), who reported that cyanobacterial cells have adopted two different types of mechanisms, one is the avoidance of toxic internal amounts of inorganic ions using active export systems. The other in the synthesis and accumulation of osmoprotective compounds to achieve an equilibrium of osmotic potential, so increasing carbohydrates proteins, RNA at certain concentrations for both S. elongatus (strain PCC 7942) may be attributed to the above previous reasons. The ability of numerous species of blue-green algae to flourish under high salinity conditions as in salt marshes has been shown by many investigators (Zhand et al., 2013).
The decrease in carbohydrate contents which was evident in S. elongatus (strain PCC 7942) at 800 mg L−1 of NaCl may be attributed to inhibition of photosynthetic activity and/or to the promotion in the rate of respiration (Rakko and Seppala, 2014). Increasing of RNA contents which displayed at 400 mg L−1 for S. elongatus (strain PCC 7942) (Fig. 2), a trend that was reversed with increasing salinity concentrations may be considered as a tool for defense mechanism against salinization while the inhibition of DNA accumulation of the studied algae may be attributed to direct or indirect interfering of salinization effect on metabolic conversion (Bemal and Anil, 2018). The obtained data indicate that most of the detected individual amino acids of S. elongatus (strain PCC 7942) were stimulated with salinization treatment (800 mg L−1). This may be attributed to salinization stimulation of the synthesis of amino acids, especially Proline which considered as indication of salinity. In this investigation the effect of 800 mg L−1 of NaCl for 6 days on intracellular structure of unicellular blue green alga S. elongatus (strain PCC 7942) was studied by employing transmission electron microscopy.
Examination of S. elongatus (strain PCC 7942) under saline stress showed a partial dissolution of certain cell contents which led to formation of a large vacuole, thylakoids mostly appeared in three layers and the majority of cell contents existed but not as clear as in control. Although wastewater was studied as an environmental stress, the obtained results revealed that wastewater appeared as a stimulatory agent for growth and the studied macro-molecules of the investigated alga, that in accordance with findings of Korosh et al. (2018). Table 3 and Figs. 4 & 5 revealed that undiluted wastewater (100%) achieved the highest levels of growth and the studied cellular macro-molecules of S. elongatus (strain PCC 7942). These results are in agreement with Lau et al. (1995), who found that wastewater is very rich in phosphorus, nitrogen and other compounds which are necessary for algal growth. Accordingly, in the presence of excess nutrients, the algae are capable of rapid growth and multiplication so, the photosynthetic activity increased which led to increasing the total soluble carbohydrates (Shetty et al., 2019).
An increase in the level of the detected individual amino acids of S. elongatus (strain PCC 7942) grown in undiluted wastewater medium (Fig. 6) was observed except for Histidine which exhibited slight inhibition in accordance with Hammouda et al. (1995). On the other hand, increasing protein content in the studied algae which were grown on pure wastewater may be attributed to the composition of wastewater which contain the major nutrient of algae (Badr et al., 2010; Ankit et al., 2020; Reno et al., 2020).
Ultrastructure examination of S. elongatus (strain PCC 7942) which grown on undiluted wastewater for 6 days, showed a general increase in starch granules inside the cells. Such observation could be explained by the increase of total soluble carbohydrates in the studied cyanobacterium. The results agree with Ibraheem (1998), who found that, load of wastewater increase the carbohydrate contents of Phormidium tenue and subsequently increase the starch granules inside the cells.
5 Conclusion
Allow discharge of untreated sewage to water sources from agricultural, domestic, and industrial waste residues well increase pollutants in water drains. This may result in increased salinity and organic compounds that may lead to the end of natural flora in these sources. The current study proved that these pollutants are dangerous to different biota in the agricultural channels and also represents an indirect risk to the biodiversity.
Consent for publication
Not applicable
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Competing interests
There are no competing interests.
Funding
Not applicable.
Authors' contributions
Not applicable.
Conflict of interest
No Conflict of interest.
Acknowledgments
This publication was supported by the Deanship of Scientific Research at Prince Sattam Bin Abdulaziz University, Alkharj, Saudi Arabia.
References
- Abdel-Raouf, N., Ibraheem I.B.M., Hammouda, O., 2003. Eutrophication of River Nile as indicator of pollution. Al-Azhar Bull. of Sci., Proceeding of 5th Int. Sci. Conf., 25-27.
- Growth measurements by monitoring light scattering of a filamentous blue-green alga which does not give uniform and stable suspension in culture vessels. Zeitschrift für Allg. Mikrobiologie. 1983;23:475-483.
- [Google Scholar]
- Responses of certain blue-green algae (Cyanobacteria) to salinity. Recent Adv. Phycol. 1994:255-259.
- [Google Scholar]
- Ankit, Bordoloi, N., Tiwari, J., Bauddh, K., 2020. Efficiency of algae for heavy metal removal, bioenergy production, and carbon sequestration. In book: Emerging Eco-friendly Green Technologies for Wastewater Treatment. 10.1007/978-981-15-1390-9_4.
- Toxicity assessment of cyanobacteria in a wastewater treatment plant, Egypt. J. Appl. Sci. Res.. 2010;6(10):1511-1516.
- [Google Scholar]
- Effects of salinity on cellular growth and exopolysaccharide production of freshwater Synechococcus strain CCAP1405. J. Plankton Res.. 2018;40(1):46-58.
- [Google Scholar]
- A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem. J.. 1956;62:315.
- [Google Scholar]
- The influence of the mineral composition of the medium on the growth of planktonic algae. J. Ecol.. 1942;30:284-325.
- [Google Scholar]
- Ding, L., Ma, Y., Huang, B., Chen, S., 2013. Effects of seawater salinity and temperature on growth and pigment contents in Hypnea cervicornis J. Agardh (Gigartinales, Rhodophyta). BioMed Research International. Volume 2013, Article ID 594308. 10.1155/2013/594308.
- Dische, E.L., 1953. J. Am. Chem. Soc., 22, 3014 (cited in physiological studies on the herbicide cotoran, S.S.; Roushdy, 1983, M.Sc. Thesis, Fac. of Sci., Ain-Shams Univ., 131.
- The effect of temperature and salinity on the growth of Skeletonema costatum and Chlorella capsulata in vitro. Int. J. Life Sci.. 2016;10(1):40-44.
- [Google Scholar]
- Effect of seawater salinity concentrations on growth rate, pigment contents and lipid concentration in Anabaena fertilissma. CATRINA. 2015;11(1):59-65.
- [Google Scholar]
- Studies on the ability of some blue-green algae to fix atmospheric nitrogen and their effect on growth and yield of paddy. Argic. Res. Rev.. 1958;36(2):308-320.
- [Google Scholar]
- Lipid accumulation in response to nitrogen limitation and variation of temperature in Nannochloropsis salina. Botanical Stud.. 2015;56:6.
- [Google Scholar]
- The isolation, purification and culture of blue-green algae. Am. J. Botany. 1950;37:216-218.
- [Google Scholar]
- Alterations of protein synthesis in the cyanobacterium synechocystis sp. PCC 4803 after a salt shock. J. Gen. Microbiol.. 1990;136:1393-1399.
- [Google Scholar]
- Utilization of certain algae in the treatment of wastewater. Egypt: Fac.of Sci. Al-Azhar Univ., Cairo; 1998. Ph.D.Thesis
- Screening of the microbial community of sewage sludge of Beni-Suef Wastewater Treatment Plant and identification of a novel actinomycetes strain. Aust. J. Basic Appl. Sci.. 2017;11(7):110-117.
- [Google Scholar]
- Korosh, T.C., Dutcher, A., Pfleger, B.F., Mcmahon, K.D., 2018. Inhibition of Cyanobacterial Growth on a Municipal Wastewater Sidestream Is Impacted by Temperature. 3(1), e00538-17.
- Salinity stress induced proteins in two nitrogen fixing Anabaena strains differentially tolerant to salt. J. Bacteriol.. 1989;171(2):909-915.
- [Google Scholar]
- Lahaniatis, E.S., Parlar, H., Lay, J.P., Pfister, G., Bergheim, W., Kotzias, D., Haritonidis, S., Nikolaidis, G., Tryfon, H., Gartsonis, K., 1991. Culture of macroalgae in wastewater treatment 1-Biomass, environmental pollution and its impact on life in the Mediterranean region 515-520.
- Effect of algal density on nutrient removal from primary settled wastewater. Environ. Pollut.. 1995;89(1):59-66.
- [Google Scholar]
- The effect of salinity concentration on algal biomass production and nutrient removal from municipal wastewater by Dunaliella salina. Int. J. Energy Res.. 2018;42:2997-3006.
- [Google Scholar]
- Electron Microscopy. A. Hand Book for Biologists (2nd ed.). Oxford: Black well Scientific publications; 1966.
- A review on the assessment of stress conditions for simultaneous production of microalgal lipids and carotenoids. Front. Microbiol.. 2016;7 Article 54
- [Google Scholar]
- Optimization of nitrogen, phosphorus and salt for lipid accumulation of microalgae: towards the viability of microalgae biodiesel. Nat. Sci.. 2016;8:557-573.
- [Google Scholar]
- Effect of salinity, pH, light intensity on growth and lipid production of microalgae for bioenergy application. J. Biol. Sci.. 2015;15:260-267.
- [Google Scholar]
- The synthesis of nucleic acid in cultures of Escherchia coli, strains band B / R. J. Bacterial.. 1949;58:317.
- [Google Scholar]
- Effect of dissolved nutrients on the distribution of algal flora in selected lakes of U.A.R. II. Lake Nasser. Women’s Coll. Ann. Rev.. 1970;6:15-24.
- [Google Scholar]
- Effect of salinity on the growth rate and nutrient stoichiometry of two Baltic Sea filamentous cyanobacterial species. Estonian J. Ecol.. 2014;63(2):55-70.
- [Google Scholar]
- Reno, U., Regaldo, L., Gagneten, A.M., 2020. Circular economy and agro-industrial wastewater: potential of microalgae in bioremediation processes. In book: Valorisation of Agro-industrial Residues – Volume I: Biological Approaches. 10.1007/978-3-030-39137-9-5.
- The use of lead citrate at high pH as an electron opaque stain in electron microscopy. J. Cell Biol.. 1963;17:208.
- [Google Scholar]
- Sucrose determination as a mean of estimations of the “Draw Bak Tax” on exported halawa tehinia. Bull. Fac. Sci. Cairo Univ.. 1964;39:209.
- [Google Scholar]
- A method for the determination of deoxyribonucleic acid, ribonucleic acid and phosphorproteins in animal tissues. J. Biol. Chem.. 1945;161:83.
- [Google Scholar]
- Shaaban, A.M., Haroun, B.M., Ibraheem, I.B.M., 2004. Assessment of the impact of Microcystis aeruginosa and Chlorella vulgaris in the uptake of some pollutants from culture media. 3rd Int. Conf. Biol. Sci. (ICBS) Fac. Sci. Tanta Univ. 28 – 29 April 2004. 3, 433-450.
- Exploitation of algal-bacterial consortia in combined biohydrogen generation and wastewater treatment. Front. Energy Res. 2019
- [CrossRef] [Google Scholar]
- Statistical Methods (6th ed.). Iowa State Univ: Press. Ames. Iowa, USA; 1967. p. :275.
- A practical handbook of seawater analysis. Bull. Fish. Res. Board Canada. 1968;167:1-311.
- [Google Scholar]
- Manometric Techniques: A Manual Describing Methods Applicable to the Study of Tissue Metabolism. Published by Minneapolis. Germany): Burgess Publishing Company. Antiquariat Dr. Götzhaber (Luckenbach; 1969.
- Algal flora of saline soils of Coimbatore (Tami,1 Nadu) Acta Botanica-Indica. 1989;17(2):241-244.
- [Google Scholar]
- Role of light and photosynthesis on the acclimation process of the cyanobacterium spirulina platensis to salinity stress. J. Appl. Phycol.. 1996;8(2):119-124.
- [Google Scholar]
- Effects of nitrogen and phosphorus on the growth of Levanderina fissa: How it blooms in Pearl River Estuary. J. Ocean Univ. China. 2017;16:114-120.
- [Google Scholar]
- Wilkinson, M., 1981. Survival strategies of attached algae in estuarine. In survival and feeding strategies of esturine organisms. Eds. W.V. Jones and W.J. Wolfe. Plenum Publishing Co. London.
- Protein hydrolysis. A description of the method used at the department of animal Physiology in Copenhagen. Acta Agri. Scondinavia. 1966;16(115)
- [Google Scholar]
- Protein hydrolysis. A description of the method used at the department of animal Physiology in Copenhagen. Acta Agri. Scondinavia. 1966;16:115.
- [Google Scholar]
- Removal of Cu2+ and Ni2+ by free and immobilized microalgae. Biomed. Environ. Sci.. 1992;5:99-108.
- [Google Scholar]
- Effect of NaCl salinity on the growth, metabolites, and antioxidant system of Microcystis aeruginosa. J. Freshwater Ecol.. 2013;28(4):477-487.
- [Google Scholar]







