Translate this page into:
Effects of quercetin on ultrafine petrol exhaust nanoparticles induced DNA damage, oxidative stress and inflammation in different sections of rat brain
⁎Corresponding authors. marulbiochem@rediffmail.com (Mayakrishnan Vijayakumar), thiyagaramesh@gmail.com (Thiyagarajan Ramesh)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
The major constituent of air pollution is petrol exhaust a complex mixture of particles, gases and chemicals. The aim of the current research was to evaluate whether ultrafine petrol exhaust nanoparticles (PENPs), the particle component of exhaust from petrol engines can induce neurotoxicity in rats. We administered rats with repeated doses of PENPs (90 μg/rat and 180 μg/rat for 6 days (every second day) intratracheally (i.t.). This was followed by the evaluation of several neurotoxicity parameters in various sections of rat brain. PENP exposure caused surge in levels of inflammatory mediators such as reactive oxygen species (ROS) and neurodegenerative disorder indicators like amyloid beta 42 (Aβ42) levels in rat brain. Each section of the brain responded differently upon PENP exposure. Prior treatment with quercetin (60 mg/kg b.wt) inhibited elevation in the aforementioned parameters. Hence, PENP exposure was closely linked to neurotoxicity and the neuroprotective capacity of quercetin was also proved.
Keywords
Petrol exhaust particles
Alzheimer’s disease
Brain
Neuroinflammation
Phytochemicals
Air pollution
1 Introduction
Particulate matter (PM) of airborne origin is the most important constituent of air pollution in urban areas and is found associated with increased incidence of diseases such as cardiovascular and respiratory disorders. Emissions from vehicle exhaust are the major contributors to PM air pollution. According to the size, PM can be categorized into ultrafine particles in size range lesser than 0.2 µm, fine particles in size range lesser than 2.5 µm (PM2.5) and coarse particles of size that ranges between 2.5 µm and 10.0 µm in diameter. Worldwide PM air pollution has been reported to have caused 3.3 million deaths annually (Ronkko and Timonen, 2019).
Crucial constituents of PM2.5 comprise petrol exhaust nanoparticles (PENPs) and the diesel exhaust nanoparticles (DENPs). Both vehicle fuels, petrol and diesel in automobile engines, undergo combustion and produce combustion-derived nanoparticles, and diesel is found to emit more particles/unit of fuel compared to petrol and is widely studied out of the two in regard to adverse health issues. Previous studies also revealed that diesel exhaust particles (DEPs) are more toxic compared to the petrol exhaust particles (PEPs) in regard to the crucial fact that the diesel vehicles are the main contributors to airborne PM (72%) whereas petrol powered vehicles contribute around 10% PM. The ultrafine size and the accompanying distinctive properties of such nano-sized particles have noticeably increased their threat to the environment and human health (Durga et al., 2013). These nano-sized particles were found to be suspended in the air for longer durations and because of their enlarged surface areas were found to travel longer distances and hence humans become exposed to such ultrafine particles through the inhalation route (Haghani et al., 2020).
Once inhaled, studies have reported that the DENPs make way into the blood circulation and get accumulated at different tissues. Inhalation toxicity studies in mice showed that the markers of neurotoxicity and neuroinflammation were elevated after significant exposure to DENPs (Cole et al., 2016) and PM. Such significant contributors of air pollution were found not only associated with cardiovascular and pulmonary diseases but also with disease conditions of the CNS, including Parkinson’s and Alzheimer’s diseases.
Free radicals are generated due to oxidative stress cause damage to the body’s macromolecules with the CNS, especially the brain being significantly prone to oxidative stress due to its very high energy demand, low amounts of anti-oxidants followed by high levels of lipids and proteins (Ramesh et al., 2015a, 2015b). Accumulating data with rat models reveal that air pollutants affect the CNS and cause AD pathology (Cacciottolo et al., 2020). AD is the prevalent cause of dementia globally affecting the lives of millions. It is characterized by loss of memory, deficit language skills, and loss in recognition, movement, and decision-making ability. Pathologically, AD is identified by neurofibrillary tangles and amyloid plaques. DENPs were also found to be associated with cerebrovascular damage and risk of stroke (Greve et al., 2020). Recent research also revealed that DENP exposure was able to induce hippocampus inflammation and associated oxidative stress that may play a vital role in causing Parkinson’s disease. Diesel exhaust were also found to trigger microglial neuroinflammation both in vivo and in vitro (Colasanti et al., 2018).
Quercetin a polyphenol (3,3′,4′,5,7-pentahydroxy flavones) is a widely consumed dietary flavonoid found abundantly in variety of fruits, nuts and vegetables. Quercetin is reported to have protective effects on several human diseases due to its anti-oxidant, anti-inflammatory, anti-hepatotoxic, anti-neoplastic, anti-hypertensive, anti-viral and anti-platelet properties (Anand David et al., 2016). Quercetin was found to inhibit the lipoxygenase and cyclooxygenase pathways inside cells and such pathways mainly serve as the major target for quercetin’s anti-inflammatory properties (Faruk et al., 2019; Doucet et al., 2019). Previous studies illustrated that quercetin had the capacity to cross blood brain barriers and discussed the potential role of anti-oxidant pathways such as Nrf2-ARE pathway and paraoxonase 2 (PON2) pathway, by which quercetin was able to respond to oxidative stress-induced neurotoxicity. Quercetin has also shown to ameliorate pathology caused by Alzheimer’s disease and associated cognitive deficits in Alzheimer’s disease mouse models (Sabogal-Guáqueta et al., 2015). The ability of quercetin to scavenge free radicals and bind to metal ions are also important reasons to choose quercetin for the present study.
Presently, the underlying mechanisms involving regulation of urban air pollution and its association with AD are not well understood. Also, regularly for years only DENPs have been widely analysed for its deleterious effects. Similarly, it is equally essential to research about the mechanism of toxicity linked to PENPs. Our previous in vitro studies on A549 and macrophage cell lines exposed to different concentrations of PENPs proved the pulmonary toxicity of PENPs for the first time. To our knowledge, till date there are no evidence linking PENP exposure and neurotoxicity. Hence, to begin addressing such issues in the present study, we exposed Wistar rats to PENPs at different concentrations and the association of PENPs with oxidative stress, neuroinflammation and AD-like pathology was explored along with the possible neuroprotective capacity of quercetin against oxidative stress, inflammation, DNA damage and AD-like pathology induced by PENPs for the very first time. These data highlight the importance of the lethal effects of airborne PM and the related neurotoxicity effects.
2 Materials and methods
2.1 Purchase of reagents
All the required chemicals were procured from Sigma–Aldrich, India. Reagent kits required for biochemical analyses were obtained from standard suppliers as mentioned in the protocol.
2.2 Particle collection
Light duty multicylinder engines from ALLMECH Pvt Ltd functional at a speed of 1500 rpm on the standard petrol fuel were used to collect PENPs on a Millipore filter paper of size less than 0.5 µm. PENPs were characterized as mentioned in our earlier research work. The collected combustion-derived nanoparticles were mixed with sterile saline (NaCl 0.9%) along with Tween 80 (0.01%). Tween 80, a non-ionic surfactant, was added to impede particle coagulation and increase particle dispersity in solution. Sonication and vortex was done before each experiment in order to prevent particle aggregation. In our earlier studies we have reported the composition and size of PENPs that were used in the present research. Characterization techniques such as high-resolution transmission electron microscopy (HR-TEM) and high-resolution scanning electron microscopy with energy dispersive X-ray analysis (HR-SEM with EDAX) demonstrated the existence of nanoparticles with the key element as carbon.
2.3 Treatment of animals
In the current study, wistar rats (Male, 5 weeks old) of weight range 130–150 g, were used after a 7-day acclimatization period. All measures were taken to minimalize the total number of animals used in the present research. The procured animals were caged in polypropylene cages and kept in a room with 23 °C temperature, 35–40% humidity and alternating 12 h light and 12 h dark cycles. They were given water and standard pellet food. The animal research was performed according to National Institutes of Health guidelines and the guide for use and care of lab animals, and it was also approved by our ethical committee at our Institute (550/02/a/CPCSEA).
Animals were allocated randomly to seven groups (n = 8 in per group): (1) control (C), (2) vehicle control (VC), (3) medium dose PENP treatment (90 μg/rat) (P1), (4) high dose PENP treatment (180 μg/rat) (P2), (5) P1 + quercetin treatment (Q), (6) P2 + quercetin treatment and (7) quercetin treatment alone (Q). The dose of PENPs administered to rats in the present study was chosen from previous studies which have proved to correct arrhythmia in rats (Yokota et al 2005). Following this, the rats were anesthetized by intraperitoneal injection of sodium pentobarbital at a dose of 60 mg/kg body weight. A 24-gauge cannula was introduced into the trachea via the mouth. Either the PENP suspensions (P1/P2) or normal saline only was injected intratracheally using a sterile syringe. Administration was regularly done on alternate days 1, 3, 5 and 7. Prior treatment with quercetin (60 mg/kg body weight) was performed in all groups. Quercetin was dissolved using the solvent olive oil (Sangai and Verma, 2012). The quercetin dose chosen here was based on the doses of quercetin routinely employed in commercial product on mg/kg scaling basis. Studies represented that quercetin is well tolerated at doses 60 mg/kg b.wt orally without evident toxicity (Sangai and Verma, 2012). Forty-eight hours after the last exposure to PENPs, various neurotoxicity end points (Fig. 1) were measured from different sections of rat brain.
End-points after repeated intra-tracheal (i.t.) instillation of saline (control) or PENP (P1/P2) with or without quercetin pretreatment (Q) given 1 h earlier orally in rats. BALF: bronchoalveolar lavage fluid.
2.4 Brain homogenate preparation
The following five sections of the brain such as temporal and frontal lobes, cerebellum, midbrain and the olfactory bulb were dissected from each animal and coated on to a cold aluminum block. The different brain sections were then homogenized using lysis buffer which is composed of protease and phosphatase inhibitors. Following this centrifugation was performed for 5 min at 14,000g, 4 °C. For further experiments, the supernatant was used.
2.5 Determination of levels of Aβ42 and pro-inflammatory cytokines
The five brain sections were analysed for the levels of neurodegenerative disorder marker proteins Aβ42 and pro-inflammatory cytokines (IL-1α, COX 2, TNFα and IL-6) using the ELISA kit procured from Invitrogen.
2.6 Determination of ROS levels
The ROS determination was performed according to the methodology of Hartz et al. (2008). The molecular probe CM-H2DCFDA used in the experiment was purchased from Invitrogen, Inc. To all brain sections, the probe was added at a concentration of 10 µm and incubated for 30–45 min. The color developed was read at 485 nm using a microplate reader.
2.7 Quantification of DNA damage
The procedure involves the quantification of apurinic/apyrimidinic (AP) sites in brain homogenate. AP sites are the most important DNA lesions that are formed during exposure to toxins. They are the best indicators for cell ageing and damage. DNA damage was analyzed in different brain sections using the Invitrogen kit-based method following the given protocol.
2.8 Determination of nitrite ( ), nitrate ( ) and hydrogen peroxide (H2O2) levels in various regions of rat brain
The excised brain tissue was mixed in saline solution and kept for an hour at 4 °C using methanol. The quantity of free radicals such as H2O2, and in various brain regions was estimated according to previous protocols (Mehta et al., 2009).
2.9 Histopathological study
The brain tissue was carefully excised and fixed in 10% formalin for 24 h. After fixation, the excised tissues were embedded in paraffin wax (60–62 °C). The embedded tissues were then made into a paraffin block. Using a microtome, the block was sliced into uniform sections of 4–5 μm thickness and stained with hematoxylin and eosin dye by routine procedures. First, the slides were washed with xyline and fixed in isopropyl alcohol for 10 min. Hematoxylin was added for few minutes and again washed with water. Slides were dipped once in 1% acid alcohol and washed with ammonia water (distilled water 50 mL; liquid ammonia, three drops). Finally, eosin was added and left for 1 min and washed with water. The stained sections were examined for pathological changes at desired magnification under the microscope.
2.10 Statistical analysis
The results of the present study were represented as mean ± SD (each group n = 8). The experiments were executed in triplicates. The statistical method employed in the present study was one-way ANOVA and Duncan’s test to analyze the significance between the various groups. Results were considered to be significant if the ‘p’ value was found to be 0.05 or lesser.
3 Results
3.1 PENP exposure elevated TNFα cytokine levels in all brain regions
As depicted in Fig. 2, the concentration of TNFα was significantly elevated by 115.21% (P2), 29.03% (P2) and 24.44% (P2) in comparison to control animals in the olfactory bulb, frontal and temporal lobes only at the highest concentration of PENP exposed (P2). In contrast to this, the midbrain was sensitive even at lower concentrations of PENP exposed. TNFα levels were seen increased by 52.09% (P1) and 130.53% (P2) in the midbrain. However, TNFα levels of the Q + P1 group for midbrain and TNFα levels of the Q + P2 treated group for other brain regions were seen decreased significantly by 31.39%, 56.97%, 40.61%, 15.16% and 4.31%, respectively, when compared to P1 and P2 groups.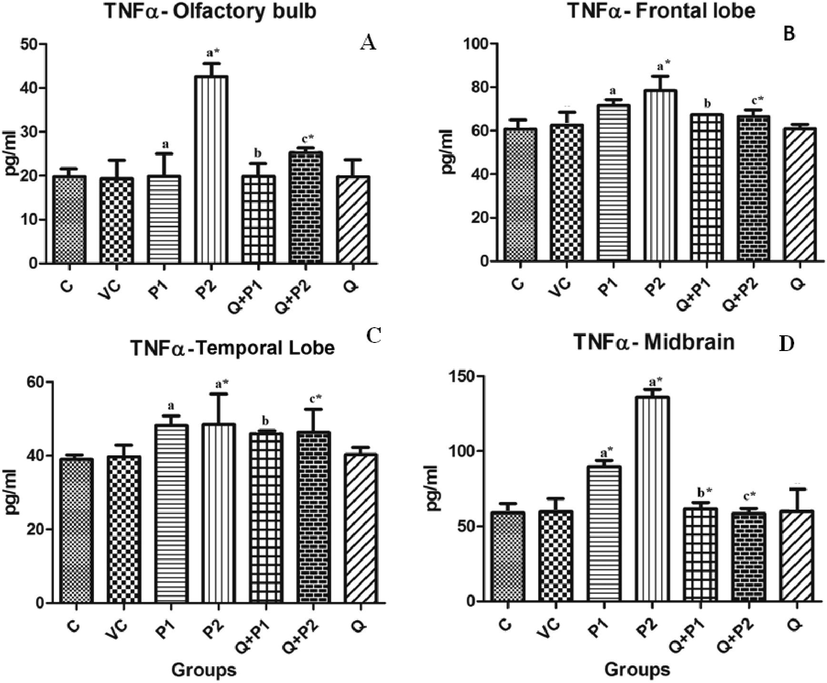
Levels of TNFα upon exposure to PENPs in (A) olfactory bulb, (B) frontal lobe, (C) temporal lobe and (D) midbrain. The cerebellum did not show any significant changes. Data are mean ± SD (n = 8 in each group). Statistical analysis by one-way analysis of variance (ANOVA) followed by Duncan’s tests. *Significant difference (p < 0.05) from control animals.
3.2 PENP elevates other pro-inflammatory cytokines in temporal lobe, midbrain and frontal lobe regions
In order to further demonstrate the effect of air pollution particles on different regions of rat brain, the levels of other pro-inflammatory cytokines such as IL-1α, IL-6 and COX 2 were evaluated. As depicted in Fig. 3A and 3B, in comparison to the control group, the IL-6 and IL-1α levels were significantly increased by 58.43% (P1), 97.79% (P2), and 84.97% (P2) in the temporal region. No evident changes were observed in the levels of IL-1α and IL-6 in the other regions of the brain. In variation to the above, the COX 2 levels were significantly elevated by 100% (P2), 320% (P2) and 455% (P2) in midbrain, frontal and temporal lobes, respectively (Fig. 4A–C). However, upon quercetin treatment, IL-6 levels of Q + P1 and Q + P2 groups, IL-1α levels of Q + P2 group, and COX 2 levels of Q + P2 treated group were decreased significantly (p < 0.05) by 34.83%, 24.43%, 45.23%, 44.15%, 57.14% and 64.86%, respectively.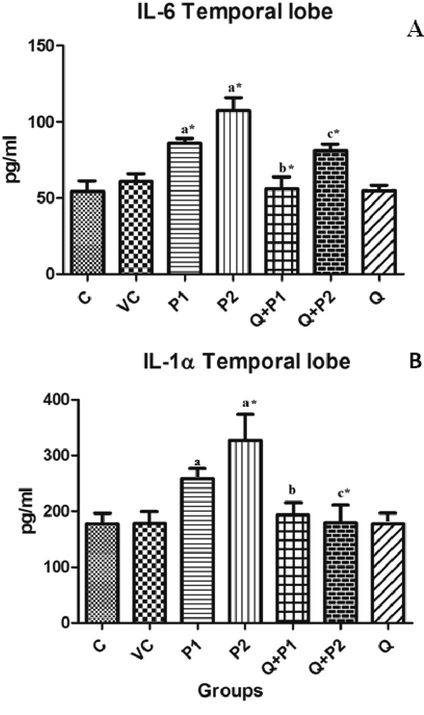
Levels of (A) Cytokine IL-6 in temporal lobe and (B) Cytokine IL-1α levels in temporal lobe upon exposure to PENPs. There were no significant changes in the levels of cytokines IL-6 and IL-1α exhibited by other regions of the brain. Data are mean ± SD (n = 8 in each group). Statistical analysis by one-way analysis of variance (ANOVA) followed by Duncan’s tests. *Significant difference (p < 0.05) from control animals.

Levels of COX 2 in (A) frontal lobe, (B) midbrain and (C) temporal lobe after exposure to PENPs. The olfactory bulb and cerebellum did not show any significant changes. Data are mean ± SD (n = 8 in each group). Statistical analysis by one-way analysis of variance (ANOVA) followed by Duncan’s tests. *Significant difference (p < 0.05) from control animals.
3.3 PENP exposure increases Aβ42 levels in temporal lobe, frontal lobe, cerebellum and olfactory bulb regions of rat brain
Results demonstrated that on exposure to PENPs, the Aβ42 levels were elevated in the temporal lobe, frontal lobe, cerebellum and olfactory bulb in comparison to control group (Fig. 5A-D). Treatment with quercetin ameliorated the surge and decreased the levels compared with the P1 and P2 groups. Exposure of animals to quercetin alone did not significantly affect the levels of Aβ42. There were no significant changes in Alzheimer’s pathology in the midbrain region.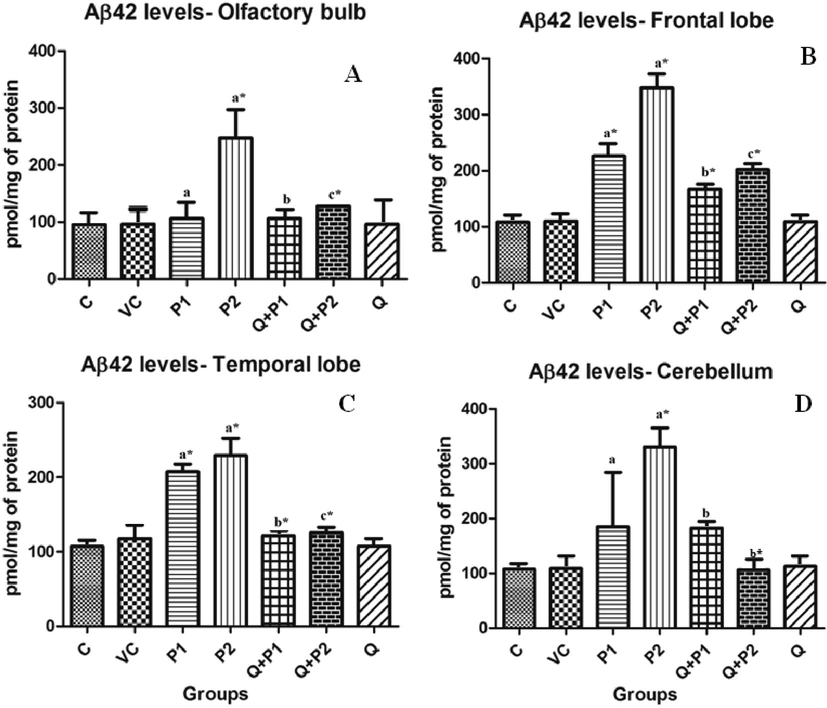
Levels of Aβ 42 in (A) olfactory bulb, (B) frontal lobe, (C) temporal lobe and (D) cerebellum upon exposure to PENPs. There were no significant changes exhibited by the mid-brain. Data are mean ± SD (n = 8 in each group). Statistical analysis by one-way analysis of variance (ANOVA) followed by Duncan’s tests. *Significant difference (p < 0.05) from control animals.
3.4 PENP exposure caused DNA damage in temporal and frontal lobe regions of rat brain
Increased levels of AP sites in rat brain demonstrated DNA damage. Results showed that the AP sites were significantly elevated by 100.38% (P2) and 82.60% (P2) in the temporal and frontal lobe regions of rat brain exposed to PENPs. Pre-treatment of rats with quercetin significantly (p < 0.05) reversed this elevation by 34.21% and 45.83%, respectively (Fig. 6A and 6B).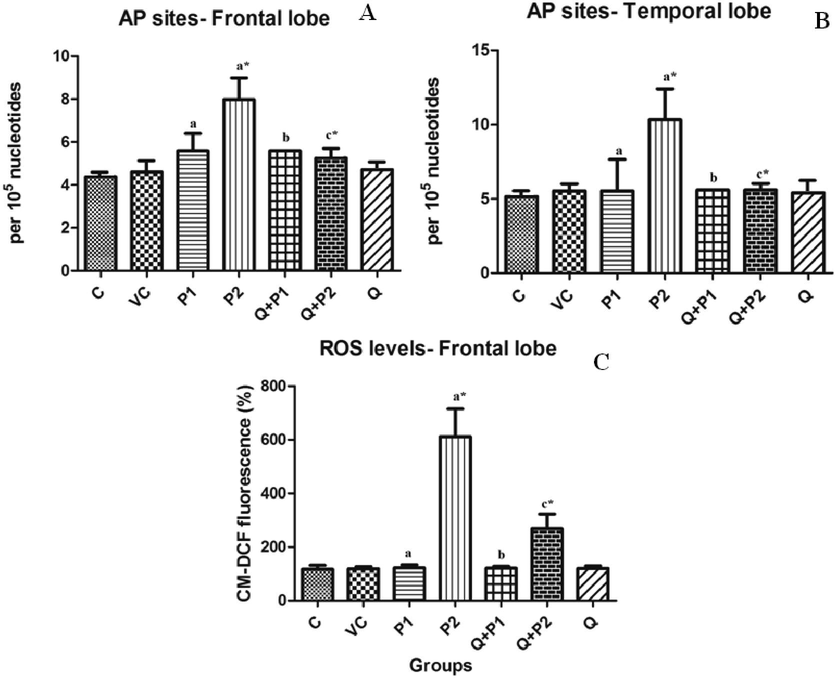
Levels of DNA damage (AP sites) in the (A) frontal lobe, (B) temporal lobe and (C) levels of ROS in Frontal lobe after exposure to PENPs. Data are mean ± SD (n = 8 in each group). Statistical analysis by one-way analysis of variance (ANOVA) followed by Duncan’s tests. *Significant difference (p < 0.05) from control animals.
3.5 PENP exposure causes oxidative stress
ROS levels are an important indicator for oxidative stress. The experiment revealed significant increase in ROS levels on exposure to highest concentration of PENPs in the frontal lobe region of rat brain in comparison to the control group (Fig. 6C). P2 was thus elevated 4.24 times compared to the control animals. Pre-treatment with quercetin reverted the effect and decreased the elevation by 0.56 times compared to the P2 group.
3.6 Effect of PENPs on H2O2, and levels in rat brain
The formation of intracellular radicals such as
,
and H2O2 in different regions of rat brain serves as an important indicator for oxidative stress. Levels of
were seen increased by 1.11 (P2) times (Fig. 7D). In contrast to this, the levels of
were significantly increased in the frontal region, midbrain and temporal lobe by 0.28 (P1), 0.46 (P2), 0.09 (P1), 0.42 (P2) and 0.44 (P2) times when compared to the control group (Fig. 8A–8C). H2O2 levels were found to be elevated in the frontal region, midbrain and cerebellum. P2 was increased in all the above three regions by 0.25, 0.72 and 0.30 times (Fig. 7A–7C). However, upon quercetin treatment, H2O2 levels of Q + P2 group,
levels of Q + P2 group and
levels of Q + P1 and Q + P2 treated groups were decreased significantly (p < 0.05) by 0.17, 30.94, 23.25, 27.95, 36.82, 4.59, 19.96, 25.66 and 37.51 times, respectively.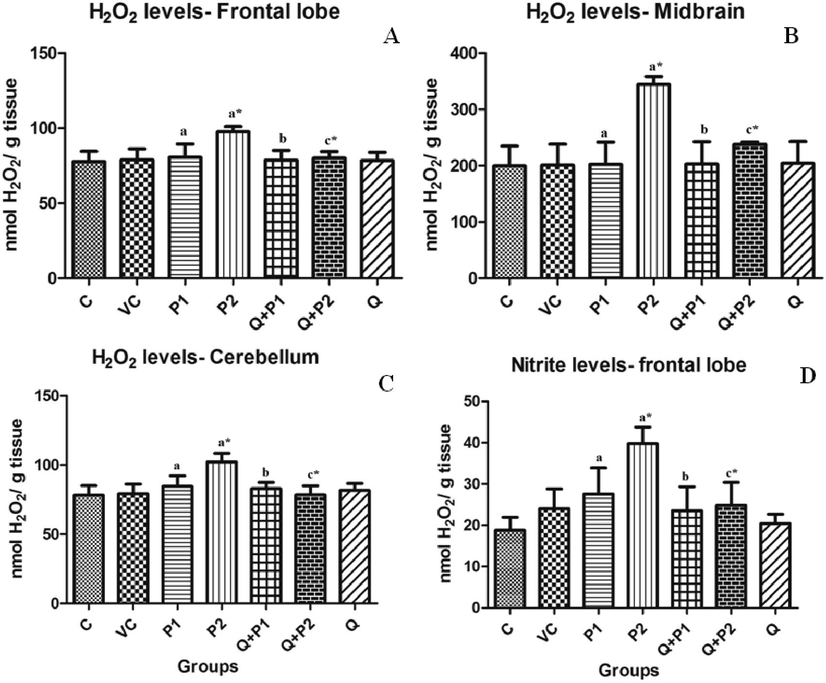
Levels of H2O2 in the (A) frontal lobe, (B) midbrain, (C) cerebellum and (D)
levels in the frontal lobe upon exposure to PENPs. Data are mean ± SD (n = 8 in each group). Statistical analysis by one-way analysis of variance (ANOVA) followed by Duncan’s tests. *Significant difference (p < 0.05) from control animals.
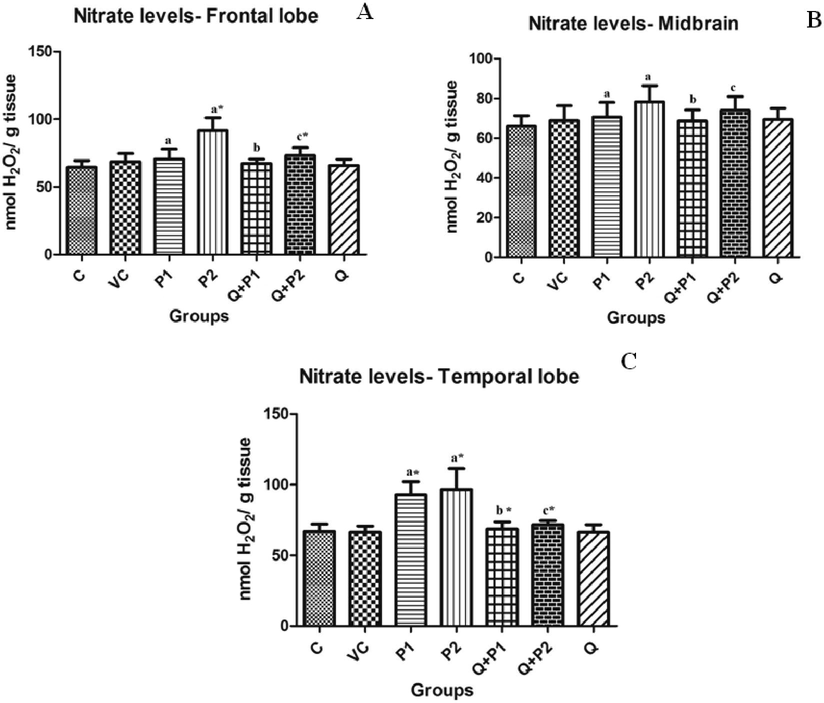
Levels of
in (A) frontal lobe, (B) midbrain and (C) temporal lobe upon exposure to PENPs. There were no significant changes in olfactory bulb and cerebellum. Data are mean ± SD (n = 8 in each group). Statistical analysis by one-way analysis of variance (ANOVA) followed by Duncan’s tests. *Significant difference (p < 0.05) from control animals.
3.7 Histopathological studies
The histology of brain sections is shown in Fig. 9. The control, vehicle control and Q alone treated groups illustrated normal histology of brain. The brain sections of the rats exposed to medium and high doses of PENPs showed reactive gliosis, inflammation, dilated blood vessels and presence of neurofibrillary tangles. Groups pre-treated with quercetin (Q + P1 and Q + P2) showed mild gliosis and mild inflammation similar to that of the control treated groups.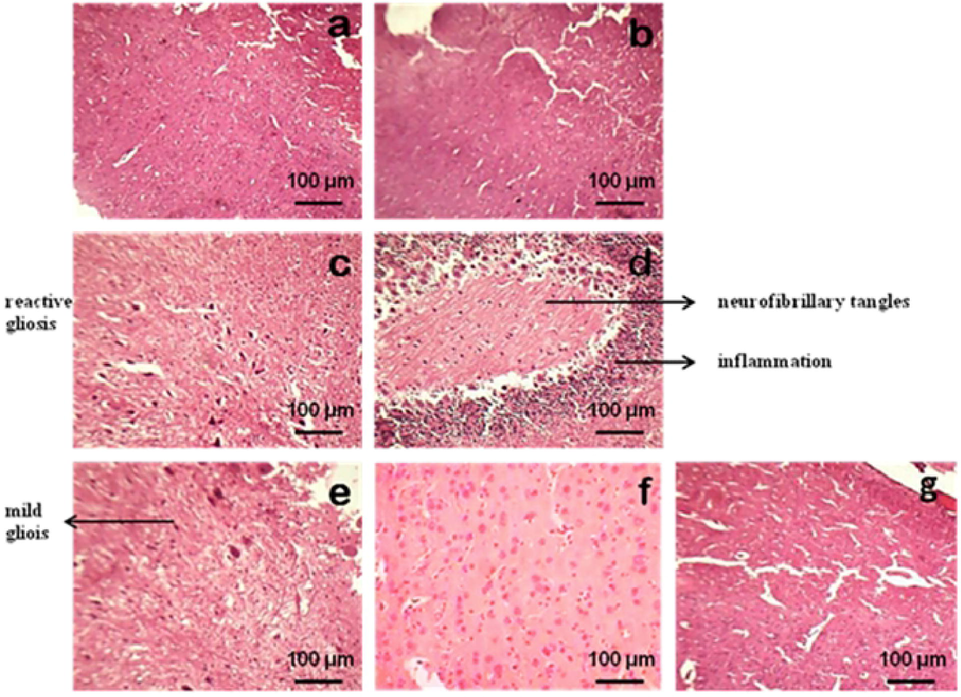
Brain sections of rats exposed to PENPs (A, normal control; B, vehicle control; C, medium dose PENPs treatment (P1) (90 μg/rat); D, high dose PENPs treatment (180 μg/rat); E, P1 + Q (60 mg/kg body weight of Q) treatment; F, P2 + Q (60 mg/kg body weight of Q) treatment; G, Q treatment alone). H & E stain, 100× magnification.
4 Discussion
In the current study, we investigated the association between PENP exposure and neurotoxicity. Lower levels of PENPs compared to levels present in highways or busy roads were employed in the experiment. The present study approach was more related to day-to-day human exposure than single exposure and the dosage employed here is quite relevant to the amount of PM that humans are exposed to everyday (Xing et al., 2016). On the whole, the results obtained from our studies illustrated that exposure to PENPs induced significant neurotoxicity in treated Wistar rats. This was apparent from (a) increase in levels of pro-inflammatory cytokines, (b) increased DNA damage, (c) increase in ROS levels, (d) increase in Aβ42 levels and (e) increase in the activities of H2O2, and . However, pre-treatment with quercetin partially prevented the neurotoxic effects.
Literature report indicates that the nervous system reacts to and identifies different metals, PM, ozone and DENPs with a comparable pathway of inflammation. Inflammation is provoked by the formation of inflammatory mediators like cytokines, chemokines, enzymes (matrix metalloproteinase, cyclooxygenase and nitric oxide synthase) and adhesion molecules that control the entry of the peripheral leukocytes into the brain region and cause damage. In the present study, measuring the increase in activities of pro-inflammatory cytokines in different regions of rat brain suggested their role in neuroinflammation. Also, it was interesting to note that different sections of the rat brain responded differently to the levels of pro-inflammatory cytokines. TNFα, a potent pro-inflammatory cytokine, was reported to increase in neurotoxicity and neurodegenerative disorders. In the current experiment, the midbrain region was more sensitive to PENPs exposure releasing higher quantities of TNFα, especially the substantia nigra region of the midbrain compared to the other regions such as the olfactory bulb, frontal and temporal lobes. The reason could be that the midbrain is rich in microglia (the innate immunological cells of the brain) compared to other parts of the brain. The rich microglia is thus responsible for the maximum release of cytokines such as TNFα. TNFα levels were elevated in all other regions except the cerebellum. The cerebellum has been reported to contain lesser number of microglia and it is usually not involved in Parkinson’s or Alzheimer’s diseases (Liu et al., 2013). Similarly, TNFα levels were increased in the brain sections of experimental animals exposed to gold and titanium dioxide nanoparticles (TiO2) (Khan et al., 2019). A prior study in diesel exhaust particles (DENPs) illustrated that the midbrain showed the maximum release of cytokines, chemokines, microglial markers, nitrite and nitrate levels compared to other brain regions. Similar results were seen in this study on exposure to PENPs, thus indicating midbrain sensitivity to air pollution. The present study also explains the role of different sections of the rat brain towards neuroinflammation.
We subsequently considered investigating the additional response of the brain sections towards PENP exposure by assessing other pro-inflammatory mediators like IL-1α and IL-6 that have been demonstrated to cause neuronal damage in rats and hence reported to result in neurodegenerative diseases (Rothaug et al., 2016). Previous study findings by Kwon et al. (2012) showed that TiO2 nanoparticles entered the brain, elevated IL-6 levels and caused lethal lesions in the brain. IL-1α levels were elevated in the blood brain barrier following silver nanoparticle exposure (Ferdous and Nemmar, 2020). Similarly, studies by Brinchmann et al. (2018) demonstrated that DENPs elevated IL-1α levels in rat brain. Studies by Oberdörster et al. (2009) also demonstrated that the olfactory route serves as a crucial entry point into the CNS for nanoparticles. The extra-pulmonary translocation of nanoparticles and their entry into the brain via the blood brain barrier after intratracheal instillation in animals was demonstrated in previous studies. Thus, nanoparticles, due to the small size, are able to avoid usual phagocytic defenses inside the pulmonary system and gain entry into the systemic circulation and consequently to various extra-pulmonary sites (Miller et al., 2017).
Alzheimer’s disease is a neurodegenerative disease distinguished by the presence of amyloid peptides and neurofibrillary tangles. It is best described by loss of memory and cognitive ability. Proteolysis of integral membrane proteins like amyloid precursor proteins formed 40–42 amino acid length peptides known as beta-amyloid peptides (Aβ40 and Aβ42). Aβ42 has been reported to be the neurotoxic fragment of APP that causes neuronal dysfunction (Calderón-Garcidueñas et al., 2019). Studies reported that increase in APP and Aβ42 levels leads to ischemia, neurotoxic damage and Alzheimer’s disease (Benoit and Maier, 2021).
Experiments involving exposure to silver, copper, iron, silica and silver nanoparticles in animal models demonstrated that these nanoparticles induced AD pathology and other neurotoxic changes in the brain such as neuroinflammation, redox stress, disruption in the BBB function, impairment in neuronal connections and axonal demyelination were evident (Carro et al., 2019). An earliest study finding suggested the significant role of air pollution in neurodegenerative disorders, supported by human epidemiological studies, animal studies, and in vitro research. The research work by Hullmann et al. (2017) provided the evidence for elevated Aβ42 protein levels and accelerated plaque formation post-inhalation exposure to DENPs in the 5XFAD mouse model.
TNFα and IL-1 levels have been reported to increase the level of other inflammatory genes like COX 2. COX 2 is an active inflammatory agent and acts as a preliminary rate limiting enzyme in the production of prostaglandins, prostacyclins and thrombaxane from arachidonic acid (Alexanian and Sorokin, 2017). Literature reported that COX 2-mediated formation of prostaglandins causes ROS accumulation that in turn leads to DNA damage; also, an association between COX 2 mRNA levels and AP sites were seen as an outcome of COX 2-mediated production of prostaglandins. Previous research demonstrated that exposure to air pollution particles caused elevation in COX 2 levels in the brain of experimental models and further induced AD-like pathology in humans via the prostaglandin pathway (Bhatt et al., 2015).
Oxidative stress, free radicals and ROS damage the body macromolecules, with the CNS being more prone to oxidative damage due to its increased energy demands, high cellular content of lipids and proteins and decreased levels of anti-oxidants (Ramesh et al., 2015a, 2015b; Sharifi-Rad et al., 2020). Oxidative stress is a dangerous pathophysiological mechanism reported in various pathologies like cancer, diabetes, cardiovascular diseases, neurological disorders and rheumatoid arthritis. Many studies illustrated oxidative stress to be a vital factor involved in the mode of action of environmental pollutants. Balance between anti-oxidant defences and pro-oxidant exogenous and endogenous factors like free radicals and reactive oxygen species (ROS) can be employed to evaluate the toxic effects on exposure to environmental pollutants. The levels of free radicals or ROS like H2O2, and serve as a key indicator for oxidative stress. Factors like high metabolic activity, high concentrations of iron, low levels of polyunsaturated fatty acids (PUFA) and catecholamines make the CNS more susceptible to the unfavorable effects of free radicals (Singh et al., 2019). Previous studies reported free radical mediated damage to membrane lipids, membrane receptors or membrane enzymes resulting in changes in structure, iron influx and membrane fluidity in the brain. The enzymatic and non-enzymatic oxidants of the brain are not homogenously distributed (Mohammadi et al., 2019). Due to variations in ROS susceptibility to cause brain damage, in the present study, ROS like H2O2, and were accumulated at varying degrees in various brain regions on PENP exposure. Hence, this indicated PENP-induced oxidative stress in different brain regions.
H2O2 is a vital ROS widely evaluated due to its higher stability than or OH− It is also the most dangerous ROS because of its ability to cross membranes (Valverde et al., 2018). The present study clearly demonstrates that PENP exposure caused elevation in levels of H2O2. The elevated formation and ROS release along with decreased anti-oxidant status of the neurological system are the major grounds that lead to oxidative damage and stress in nerve cells. Research illustrated that H2O2 was a significant inducer of cell damage and progression of cancer. Studies also showed that increased H2O2 formation causes cellular and molecular damage hence leading to mutations in tumor suppressor genes and anti-oxidant enzymes along with lipid peroxidation (Aggarwal et al., 2019). Increase in free radical or ROS concentrations in the present study may be an important event leading to PENP toxicity.
Mammalian tissues and cells synthesize NO from l-arginine in the presence of nitric oxide synthase (NOS). The produced NO readily reacts with , , and H2O2 hence forming peroxynitrite (ONOO−). ONOO− is a strong oxidant that readily oxidizes DNA, enzymes, sulfhydryl, lipids, nitrite and nitrate groups (Ramesh et al., 2015b). It also reacts with tyrosine and forms nitro tyrosine which in turn is capable of disrupting protein function; it can also be easily detected in ageing process, neurodegenerative disorders and inflammatory processes. Ca2+ ions regulate NOS activity. Cell membrane damage by peroxidation increases Ca2+ influx into cells, hence increasing NO levels. Our prior research findings showed that short-term exposure to DENPs increased NOS levels in rat brain. Studies reported that high concentrations of NO inhibit Ca2+/K+ channels and Na+–K+ ATPase in the synaptic vesicles of the brain region (Roy et al., 2016). In vitro studies showed that levels were dose dependently increased in PC-12 cells of neural origin. Wu et al. (2014) demonstrated that exposure to TiO2 nanoparticles increased NO levels in the brain of workers, employed in that environment.
Previous studies have reported histopathological indication of neurodegeneration and neuroinflammation in animals exposed to PM, suggesting the prospective for neurotoxic effects of PM (Nazem et al., 2015). The histopathological changes observed in our study have similarities to the AD pathology. Our findings indicate that synthesised nanoparticles exposure is correlated with brain inflammation and neurofibrillary tangles, characteristic of Alzheimer’s disease. The dose of PENPs administered to rats in the present study was chosen from previous studies which have proved to cause cardiac arrhythmia in rats. The quercetin dose chosen here was based on the doses of quercetin routinely employed in commercial product on mg/kg scaling basis. Studies represented that quercetin is well tolerated at doses 60 mg/kg body weight orally without evident toxicity (Sangai and Verma, 2012). Quercetin is a widely known flavonoid known to demonstrate beneficial effects such as anti-oxidant, anti-allergy, anti-ulcer, anti-cancer, anti-inflammatory, anti-viral and anti-tumor activities. Human and animal studies showed that quercetin is hindered with the production of cytokines, p300 activity, accumulation of beta-amyloid plaques and activities of p450 NF-κB cytokine. The polyphenolic flavonoid, quercetin, has shown to ameliorate pathology caused by Alzheimer’s disease and associated cognitive deficits in Alzheimer’s disease mouse model. Recent research discussed the potential role of anti-oxidant pathways such as Nrf2-ARE pathway and paraoxonase 2 (PON2) pathway, by which quercetin was able to respond to oxidative stress-induced neurotoxicity. Earliest study findings evidenced that significant improvement in the neuro-histoarchitecture and enhanced free radical scavenging activity was observed in quercetin administered animals which proved the neuroprotective effect of quercetin. In the present study, our outcomes indicated that quercetin is an important anti-inflammatory and anti-toxic agent that protects against the deleterious effects induced by PENPs. Our findings thus may have the therapeutic implications for the significant use of quercetin in the prevention of neurological effects of air-pollutant particles.
5 Conclusion
Aβ42 is the key indicator of Alzheimer’s pathology. Presently, AD is characterized as a deadly disease that affects the brain and burdens the life of individuals affected by the disease. Studies by Herbert proposed that by 2050, nearly 13.2 million individuals will be impacted by PM air pollution. The role of air pollutants like PENPs in AD pathogenesis and the impact of quercetin in air pollution-induced toxicity amelioration are explored here for the first time. Hence, this project has exposed a significant health risk of environmental importance. Elevated levels of Aβ42 and ROS illustrate a clear link between chronic air pollutant exposure and Alzheimer’s disease pathology. Furthermore, the suggestions for future work includes exploring the protective effects of quercetin on hepato and nephrotoxicity induced by repeated exposure to petrol exhaust nanoparticles by assessing the levels of creatinine, urea, uric acid, liver aspartate transaminase (ALT), liver alanine transaminase (AST), tissue superoxide dismutase (SOD), glutathione (GSH), malondialdehyde (MDA) and catalase. Also the association between air pollution and Parkinson’s disease and the ability of quercetin to ameliorate the effect needs to be explored.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Role of reactive oxygen species in cancer progression: molecular mechanisms and recent advancements. Biomolecules. 2019;9(11):735.
- [Google Scholar]
- Cyclooxygenase 2: protein–protein interactions and posttranslational modifications. Physiol. Genomics. 2017;49(11):667-681.
- [Google Scholar]
- Overviews of biological importance of quercetin: a bioactive flavonoid. Pharmacogn. Rev.. 2016;10(20):84-89.
- [Google Scholar]
- The nickel-chelator dimethylglyoxime inhibits human amyloid beta peptide in vitro aggregation. Sci. Rep.. 2021;11:6622.
- [Google Scholar]
- A pilot study to assess effects of long-term inhalation of airborne particulate matter on early Alzheimer-like changes in the mouse brain. PLoS One.. 2015;10(5):e0127102
- [Google Scholar]
- Lipophilic components of diesel exhaust particles induce pro-inflammatory responses in human endothelial cells through AhR dependent pathway(s) Part Fibre Toxicol.. 2018;15:21.
- [Google Scholar]
- Traffic-related air pollutants (TRAP-PM) promote neuronal amyloidogenesis through oxidative damage to lipid rafts. Free Radic. Biol. Med.. 2020;147:242-251.
- [Google Scholar]
- Combustion and friction-derived nanoparticles and industrial-sourced nanoparticles: the culprit of Alzheimer and Parkinson’s diseases. Environ. Res.. 2019;176:108574
- [Google Scholar]
- Nanoneurotoxicity and potential nanotheranostics for Alzheimer’s disease. EC Pharmacol. Toxicol.. 2019;7(12):1-7.
- [Google Scholar]
- Diesel exhaust particles induce autophagy and citrullination in normal human bronchial epithelial cells. Cell Death Dis.. 2018;9(11):1073.
- [Google Scholar]
- Sex and genetic differences in the effects of acute diesel exhaust exposure on inflammation and oxidative stress in mouse brain. Toxicology. 2016;374:1-9.
- [Google Scholar]
- Identification of peracetylated quercetin as a selective 12-lipoxygenase pathway inhibitor in human platelets. Mol. Pharmacol.. 2019;95(1):139-150.
- [Google Scholar]
- Impact of quercetin on pulmonary toxicity induced by diesel exhaust nanoparticles in albino rat: histological, immunohistochemical and biochemical study. J. Med. Histol.. 2019;3(1):55-64.
- [Google Scholar]
- Health impact of silver nanoparticles: a review of the biodistribution and toxicity following various routes of exposure. Int. J. Mol. Sci.. 2020;21(7):2375.
- [Google Scholar]
- Diesel exhaust impairs TREM2 to dysregulate neuroinflammation. J. Neuroinflammation. 2020;17(1):351.
- [Google Scholar]
- Toxicity of urban air pollution particulate matter in developing and adult mouse brain: comparison of total and filter-eluted nanoparticles. Environ. Int.. 2020;136:105510
- [Google Scholar]
- Diesel exhaust particles induce oxidative stress, proinflammatory signaling, and P-glycoprotein up-regulation at the bloodbrain barrier. FASEB J.. 2008;22:2723-2733.
- [Google Scholar]
- Diesel engine exhaust accelerates plaque formation in a mouse model of Alzheimer’s disease. Particle Fibre Toxicol.. 2017;14(1):35.
- [Google Scholar]
- Size and time-dependent induction of proinflammatory cytokines expression in brains of mice treated with gold nanoparticles. Saudi J. Biol. Sci.. 2019;26(3):625-631.
- [Google Scholar]
- Nasal and pulmonary toxicity of titanium dioxide nanoparticles in rats. Toxicol. Res.. 2012;28:217-224.
- [Google Scholar]
- Cytotoxicity of titanium dioxide nanoparticles in rat neuroglia cells. Brain Inj.. 2013;27:934-939.
- [Google Scholar]
- Pesticide biochemistry and physiology chlorpyrifos induced alterations in the levels of hydrogen peroxide, nitrate and nitrite in rat brain and liver. Pesticide Biochem. Physiol.. 2009;94:55-59.
- [Google Scholar]
- Inhaled nanoparticles accumulate at sites of vascular disease. ACS Nano. 2017;11(5):4542-4552.
- [Google Scholar]
- Sericin alleviates restraint stress induced depressive- and anxiety-like behaviors via modulation of oxidative stress, neuroinflammation and apoptosis in the prefrontal cortex and hippocampus. Brain Res.. 2019;1715:47-56.
- [Google Scholar]
- Rodent models of neuroinflammation for Alzheimer’s disease. J. Neuroinflammation.. 2015;12:74.
- [Google Scholar]
- Nanoparticles and the brain: cause for concern? J. Nanosci. Nanotechnol.. 2009;9(8):4996-5007.
- [Google Scholar]
- Oxidative stress in the brain of cigarette smoke-induced noxiousness: neuroprotective role of Sesbania grandiflora. Metab. Brain Dis.. 2015;30(2):573-582.
- [Google Scholar]
- Brain oxidative damage restored by Sesbania grandiflora in cigarette smoke-exposed rats. Metab. Brain Dis.. 2015;30(4):959-968.
- [Google Scholar]
- Overview of sources and characteristics of nanoparticles in urban traffic-influenced areas. J. Alzheimer’s Dis.. 2019;72(1):15-28.
- [Google Scholar]
- Rothaug, M., Becker-Pauly, C., Rose-John, S., 2016. The role of interleukin-6 signaling in nervous tissue. Biochim. Biophys. Acta – Mol. Cell Res. 1863 (6, Part A), 1218–1227.
- Role of membrane cholesterol and lipid peroxidation in regulating the Na+/K+-ATPase activity in schizophrenia. Indian J. Psychiatry. 2016;58:317-325.
- [Google Scholar]
- The flavonoid quercetin ameliorates Alzheimer’s disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer’s disease model mice. Neuropharmacology. 2015;93:134-145.
- [Google Scholar]
- Quercetin ameliorates bisphenol a-induced toxicity in mice. Acta Poloniae. Pharm. Drug. Res.. 2012;69:557-563.
- [Google Scholar]
- Lifestyle, oxidative stress, and antioxidants: back and forth in the pathophysiology of chronic diseases. Front. Physiol.. 2020;11:694.
- [Google Scholar]
- Oxidative stress: a key modulator in neurodegenerative diseases. Molecules. 2019;24:1583.
- [Google Scholar]
- Hydrogen peroxide-induced DNA damage and repair through the differentiation of human adipose-derived mesenchymal stem cells. Stem Cells Int.. 2018;2018:1615497.
- [Google Scholar]
- Effect of nanoparticles exposure on fractional exhaled nitric oxide (FENO) in workers exposed to nanomaterials. Int. J. Mol. Sci.. 2014;15:878-894.
- [Google Scholar]
- The impact of PM2.5 on the human respiratory system. J. Thorac. Dis.. 2016;8(1):E69-E74.
- [Google Scholar]
- Acute functional enhancement of circulatory neutrophils after intratracheal instillation with diesel exhaust particles in rats. Inhal. Toxicol.. 2005;17(12):671-679.
- [Google Scholar]







