Translate this page into:
Effects of pomegranate juice on the sexual behavior, fertility and protective activity against aluminum exposure in male mice
⁎Corresponding author. gmabutaweel@jazanu.edu.sa (Gasem Mohammad Abu-Taweel)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
This experiment aimed to determine how pomegranate juice (PJ) responds to oxidative stress induced by aluminum in male mice. A total of thirty-six male mice were separated into six groups. The first group (control group) received tap water. Groups two and three were given 20% and 40% PJ, respectively. Group four received 400 mg Al/kg, while groups five and six were given Al + 20% PJ and Al + 40% PJ, respectively. After the oral administration period, the sexual behavior of the mice was recorded. Blood was collected from the heart to measure testosterone concentration. After the animals were sacrificed, the reproductive organs were weighed, and the epididymis and testis were used for further evaluations. Our findings showed that Al decreased the body and organs weights, sexual behavior, sperm count, normal sperm, total and progressive sperm motility, testosterone level, and epithelial diameters of the seminiferous tubes. In addition, the PJ reduced these effects and minimized the aluminum toxicity in male mice. In conclusion, PJ protects against aluminum exposure in male mice by ameliorating sexual behavior and fertility.
Keywords
Aluminum
Pomegranate juice
Sexual behavior
Fertility
Testosterone
1 Introduction
Aluminum (Al) is the third most widely distributed element (8%) in the earth’s crust after oxygen and silicon (Willhite et al., 2014). Aluminum has chemical and physical properties and is widely used in industry, especially the food and beverage industry (Al-Dhabi et al., 2015; Barathikannan et al., 2016; Cuong et al., 2017). Al is added to numerous commercial foods, used in the purification of drinking water and used in therapeutic treatments (Fernandez-Lorenzo et al., 1999; Scancar & Milacic 2006; Malekizadeh & Schenk 2017; Elango et al., 2017, Elango et al., 2016). The element may enter the bodies of organisms in various ways, including through the respiratory system and skin; however, the digestive system remains the primary source of entry into the human body (Fowsiya et al., 2016; Glorybai et al., 2015; Haritha et al., 2016). Aluminum accumulation in body organs, such as those of the central nervous system, due to direct exposure causes neurotoxicity and some morphological alterations (Virk & Eslick 2015; Wang et al., 2016; Kuznetsova et al., 2017). Moreover, exposure to Al during pregnancy and lactation can affect brain development in mouse offspring (Abu-Taweel et al., 2012). In addition, Al causes male sexual dysfunction, decreased sperm concentration and motility, and increased oxidative stress in the testis (Yousef et al., 2005; Guo et al., 2009; Abu-Taweel et al., 2011; Klein et al., 2014; Martinez et al., 2017; Helan et al., 2016; Ilavenil et al., 2017). Furthermore, Al has detrimental effects on male rat fertility at low and high doses (Mouro et al., 2018).
Many antioxidant substances, such as folic acid, propolis, zinc sulphate, vitamin E, citric acid, taurine, ascorbic acid, ginger and black seed, have shown protective effects in mice, rats and rabbits after treatment with aluminum compounds (Mahdy & Farrag 2009; Yeh et al., 2009; Yousef & Salama 2009; Moselhy et al., 2012; Chen et al., 2014; Rawi et al., 2015; Yassa et al., 2017).
Pomegranate fruit (Punica granatum) has been consumed as a natural medicine, especially in the Middle East, because of its health benefits (Park et al., 2016, 2017; Al-Olayan et al., 2014; Ahmed et al., 2015; Alimoradian et al., 2017). Pomegranate fruit has marked antioxidant activity and high levels of polyphenols compared to other fruits (Wang et al., 2004; Kaur et al., 2006; Zarfeshany et al., 2014).
Pomegranate juice (PJ) prevents the damage during spermatogenesis generated by lead (Leiva et al., 2011; Aksu et al., 2017) and carbon tetrachloride (Turk et al., 2016; Surendra et al., 2016). Reactive oxygen species (ROS) formed by Al compounds might be accountable for decreased semen quality (Yousef et al., 2005). Thus, the compounds in PJ, such as polyphenols, flavonoids, and anthocyanins, are considered strong antioxidants that scavenge ROS (Turk et al., 2008; Haber et al., 2011; Abdel Moneim & El-Khadragy 2013; Al-Olayan et al., 2014; Bouasla et al., 2016; Caliskan et al., 2016; Asgary et al., 2017; Derakhshan et al., 2018; Russo et al., 2018).
This experiment was carried out to explore the antioxidant activities of PJ on the sexual behavior, sperm quality and protective activity against aluminum exposure in male mice.
2 Materials and methods
2.1 Animals
Adult, male, SWR/J mice (n = 36, age = 25 days, body weight = 25–35 g) were housed in plastic cages in a room with automatic daily light cycles (12-h light/dark) and regulated temperature (22 ± 2 °C), with ideal humidity. All mice were fed a standard food and had access to tap water ad libitum.
2.2 Experimental design
After the animals adapted, they were separated into six groups (n = 36): tap water (control group), (Group I); 20% PJ (Group II); 40% PJ (Group III); 400 mg Al /kg (Group IV); 400 mg Al/kg + 20% PJ (Group V);and 400 mg Al/kg + 40% PJ (Group VI). The mice were separated in cages by group. The animals were given the various treatments orally for 35 days. One week after the last dose, blood was collected from the animal heart for testosterone hormone analysis. Then, the mice were sacrificed, and their reproductive organs were weighed. The testes and epididymis were immediately used for histological and spermatozoa evaluations. The experimental design was approved by the Institutional Review Board (IRB) of Imam Abdulrahman Bin Faisal University (IAU) (Dammam, Saudi Arabia).
2.3 Sexual behavior
The sexual behavior activities were evaluated by placing age-matched, estrous virgin females in the same cage with the experimental male mice. The sexual behavior of each male mouse was recorded for three hrs. The sexual behavior parameters (i.e., latency of copulation, frequency of copulation, duration of copulation, approach and following, threatening and biting, naso-nasal and naso-genital contacts, rears and wall rears, number of squats, number of wash and digging) were observed according to published protocols (Abu-Taweel, 2019; Park, 2011).
2.4 Sperm count, motility and morphology
The protocol used for the sperm count and motility evaluation was previously published by Oudir et al., (2018). The right epididymis was removed from the attached fat tissues. Then, the caudal epididymis was cut into small pieces in three milliliters of warmed phosphate-buffered saline (PBS) in a 35-mm petri dish and incubated (37 °C) in a water bath shaker for eight minutes. Spermatozoa concentrations were calculated using a hemocytometer. Giemsa stain was used to evaluate the abnormalities in spermatozoa from different fields of semen smears from the mice (approximately 200 sperm). Ten microliters of diluted spermatozoa were placed on a warm slide to evaluate the motility. In general, moving spermatozoa were considered motile, and other spermatozoa were counted as immotile. Spermatozoa moving in a straight line were considered progressively motile. The smears of spermatozoa in all the groups were made on warm slides, dried and stained with Giemsa. A total of 200 spermatozoa from different fields were reported as percentages of normal and abnormal, according to Li et al., (2018).
2.5 Testosterone level analysis
Blood samples were centrifuged to obtain serum and stored at −20 °C to evaluate the testosterone levels. The serum testosterone level was measured using commercial kits with some modifications.
2.6 Testicular histology
The testis were fixed in 10% formaldehyde, dehydrated and embedded in paraffin wax. The testis sections (5 µm) were stained with hematoxylin-eosin (HE) for histological assay evaluations using light microscopy (Olympus, Tokyo, Japan) at magnifications of 400 × and 1000 × . The diameter of the seminiferous tubule (DST) and the heights of the germinal epithelium (HGE) and lumen of seminiferous tubule (LST) were measured according to (Cordeiro et al., 2018).
2.7 Statistical analysis
The data were analyzed for normality using the Kolmogorov–Smirnov test. Then, all the parameters were analyzed (within all groups) by ANOVA post hoc testing followed by Tukey's multiple comparisons test.
3 Results
3.1 Body and reproductive organ weights
The oral administration of Al (400 mg/kg) for 35 days caused significant decreases in the body weights of the male mice compared to those in the other groups, whereas the body weight of the Al + PJ (20%) group was significantly (p < 0.001) higher compared to that of the Al group, especially from day 10 until day 35 (Fig. 1). The testis, seminal vesicle, epididymis and prostate weights significantly (p < 0.001) decreased in the Al group compared to those in the other groups (Figs. 2-4). In addition, the absolute weights of these organs in the Al + PJ-treated mice were significantly higher than those of the Al-treated mice.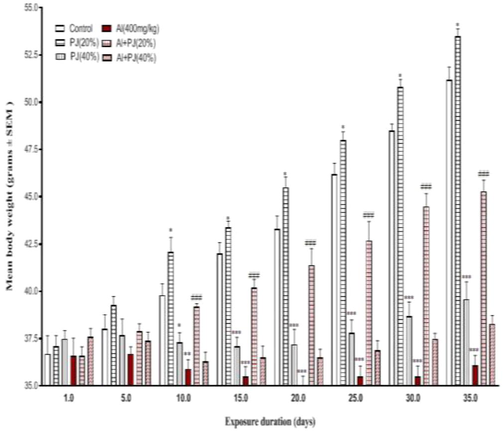
Body weights of male mice treated with aluminum (Al), pomegranate juice (PJ) and Al + PJ. *,** and *** represent significant difference at (p < 0.05) , (p < 0.01) and (p < 0.001) respectively compared to control and PJ groups. ### represent significant difference at (p < 0.001) compared to Al group.
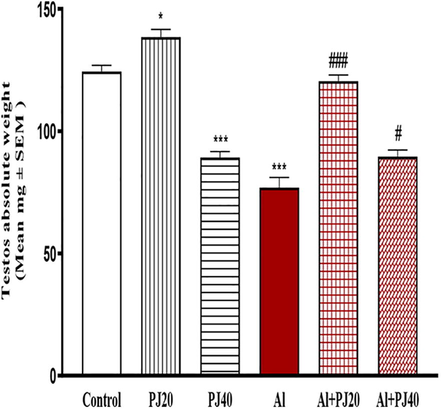
Testes absolute weight of male mice treated with aluminum (Al), pomegranate juice (PJ) and Al + PJ.* and *** represent significant difference at (p < 0.05) and (p < 0.001) compared to control and PJ groups. # and ### represent significant difference at (p < 0.05) and (p < 0.001) compared to Al group.
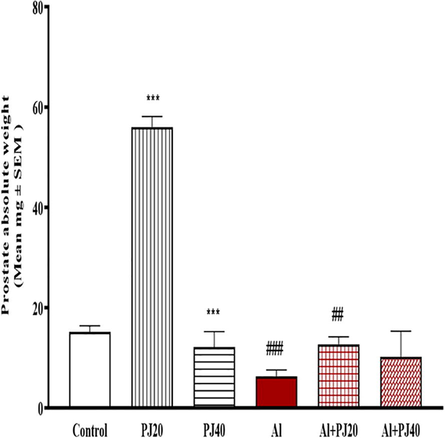
Prostate absolute weight of male mice treated with aluminum (Al), pomegranate juice (PJ) and Al + PJ. *** represent significant difference at (p < 0.001) compared to control and PJ groups. # and ## represent significant difference at (p < 0.05) and (p < 0.01) respectively compared to Al group.
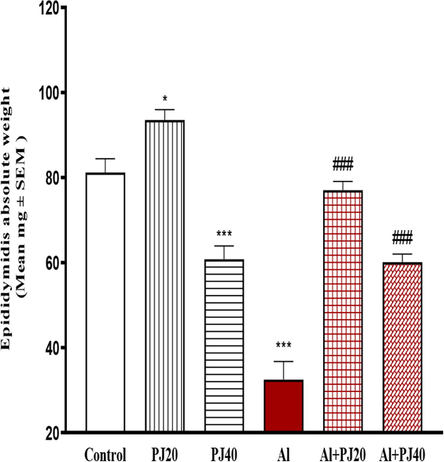
Epididymis absolute weight of male mice treated with aluminum (Al), pomegranate juice (PJ) and Al + PJ. * and *** represent significant difference at (p < 0.05) and (p < 0.001) compared to control and PJ groups.### represent significant difference at (p < 0.001) compared to Al group.
3.2 Sexual behavior
Al exposure led to a significant decrease (p < 0.001) in approach, mount, number of naso-nasal and naso-genital contacts (p < 0.01), following, pelvic thrust and biting (p < 0.001) in treated animals compared to those in the control and pomegranate juice groups (Table 1). As shown in Table 2, wall rears, rears (p < 0.001), and wash and digging (p < 0.01) were decreased, while squats were increased (p < 0.001) compared to those in the control and pomegranate juice groups. Tables 1 and 2 also show the benefits (p < 0.05, p < 0.01 and p < 0.001) of a low dose of pomegranate juice against Al toxicity. *and *** significantly different at (p<0.05, p<0.01 and p<0.001) respectively from the control and PJ groups. #,## and ### significantly different at (p<0.05, p<0.01 and p<0.001) respectively from Al group by ANOVA and Mann-WhitneyU test. ** and *** show significantly different at (p<0.01 and p<0.001) respectively from the control and PJ groups. ## and ### show significantly different at (p<0.05, p<0.01 and p<0.001) respectively from Al group by ANOVA and Mann-Whitney U test.
Median number (with ranges) of acts and postures
Group
Bite
Threat
Pelvic thrust
Mount
N. of Naso-Genital contact
N. of Naso-Nasal contact
Following
Approach
6.00(5.00 – 9.00)
9.00(6.00 – 18.00)
6.00(6.00 –7.00)
9.00(7.00 – 10.00)
36.00(33.00 – 60.00)
63.00(60.00 – 67.00)
30.0028.00 – 32.00)
21.00(21.00 – 28.00)
Control
3.00(2.00 – 4.00)
4.00(3.00 –5.00)
10.00*(9.00 – 11.00)
6.00(4.00 –8.00)
35.00(35.00 – 46.00)
64.00(62.00 – 70.00)
36.00(35.00 – 39.00)
22.00(17.00 – 27.00)
PJ (20%)
5.00(3.00 – 7.00)
7.00(6.00 – 8.00)
1.00***(0.00 – 1.00)
2.00***(1.00 – 3.00)
30.00(28.00 – 32.00)
54.00(50.00 – 55.00)
30.00(18.00 – 31.00)
15.00(14.00 – 20.00)
PJ (40%)
1.00 *(0. 00 –1.00)
2.00*(0.00 – 2.00)
0.00 ***(0.00 – 1.00)
1.00***(0.00 – 2.00)
11.00 **(6.00 – 13.00)
10.00 ***(0.00 – 11.00)
10.00 ***(0.00 – 11.00)
5.00 ***(0.00 – 6.00)
Al (400 mg/kg)
7.00 #(4.00 – 9.00)
5.00(2.00 – 5.00)
8.00 ###(7.00 – 8.00)
8.00 ###(6.00 – 9.00)
21.00 ##(15.00 – 25.00)
44.00 ###(38.00 – 59.00)
22.00 #(18.00 – 25.00)
11.00 ###(9.00 – 13.00)
Al + PJ (20%)
2.00(1.00 –2.00)
6.00(3.00 – 7.00)
3.00 #(3.00 – 5.00)
3.00(3.00 – 4.00)
15.00(10.00 – 17.00)
18.00(15.00 – 20.00)
14.00(13.00 – 17.00)
6.00(3.00 – 8.00)
Al + PJ (40%)
Median number (with ranges) of acts and postures
Group
Digging
Squat
Wash
Rears
Wall rears
7.00(2.00 – 10.00)
1.00(0.00 – 2.00)
4.00(3.00 – 6.00)
16.00(14.00 – 18.00)
22.00(17.00 – 24.00)
Control
7.00(2.00 – 10.00)
1.00(0.00 – 2.00)
4.00(3.00 – 6.00)
27.00 ***(24.00 – 28.00)
35.00 *(30.00 – 39.00)
PJ (20%)
4.00(3.00 – 12.00)
3.00(2.00 – 4.00)
2.00(2.00 – 4.00)
13.00(13.00 – 18.00)
18.00(12.00–25)
PJ (40%)
2.00(1.00 – 3.00)
9.00 ***(8.00 – 12.00)
1. 00 *(0.00 – 1.00)
2.00 ***(0.00 – 3.00)
4.00**(3.00–8.00)
Al (400 mg/kg)
5.00(2.00 – 5.00)
1.00 ###(1.00 – 3.00)
2.00(0.00 – 2.00)
12.00 ###(11.00 – 12.00)
15.00 ###(10.00 – 16.00)
Al + PJ (20%)
3.00(1.00 – 4.00)
4.00 ##(2.00–5.00)
2.00(1.00 – 2.00)
4.00(3.00 – 5.00)
6.00(5.00 – 7.00)
Al + PJ (40%)
3.3 Sperm count, sperm motility and morphology
The sperm count was significantly (p < 0.001) higher in animals administered 20% PJ than that in the other animals and significantly (p < 0.05) lower in the animals treated with Al. In addition, the male mice treated with both Al and PJ (20% or 40%) had significantly higher sperm concentrations (p < 0.001) than that in the mice treated with aluminum (Fig. 5).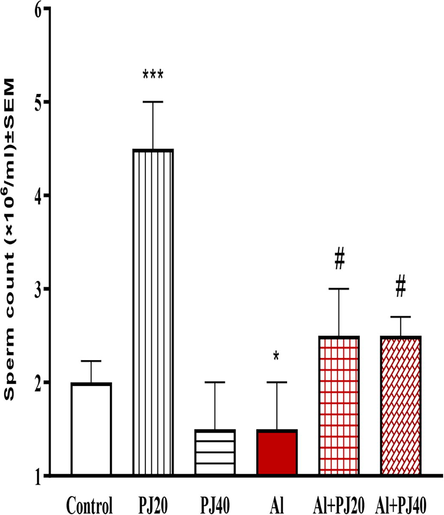
Sperm count of male mice treated with aluminum (Al), pomegranate juice (PJ) and Al + PJ.motile sperm of male mice treated with aluminum (Al), pomegranate juice (PJ) and Al + PJ. *and *** represent significant difference at (p < 0.05) and (p < 0.001) respectively compared to control and PJ groups. # represent significant difference at (p < 0.05) compared to Al group.
The current findings suggested that exposure to Al caused an increase in the immotile sperm percentage, and 20% PJ decreased the negative effects of Al exposure (Fig. 6). Furthermore, PJ was able to reduce the decline in the total and progressive motility of mouse sperm due to exposure to aluminum (Figs. 7 and 8).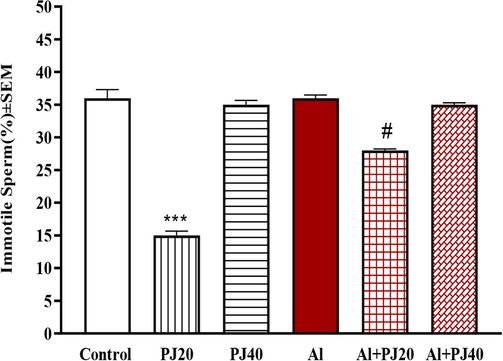
Immotile sperm of male mice treated with aluminum (Al), pomegranate juice (PJ) and Al + PJ. *** represent a significant decrease at (p < 0.001) compared to control and PJ groups. # represent significant difference at (p < 0.01) compared to Al group.
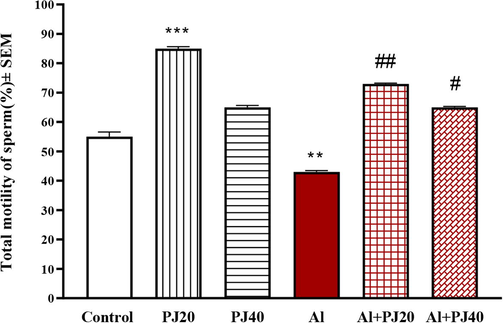
Total motility of sperm of male mice treated with aluminum (Al), pomegranate juice (PJ) and Al + PJ.**and *** represent significant difference at (p < 0.01) and (p < 0.001) respectively compared to control and PJ groups. # and ## represent significant difference at (p < 0.01) and (p < 0.001) respectively compared to Al group.
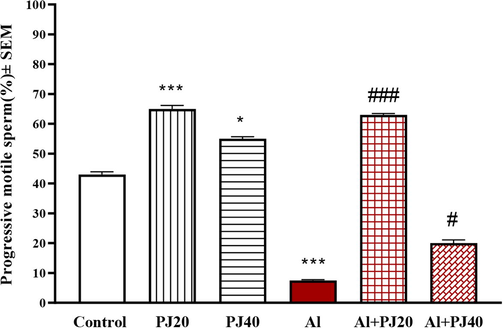
The percentage of progressive motile sperm of male mice treated with aluminum (Al), pomegranate juice (PJ) and Al + PJ. * and *** represent significant difference at (p < 0.05) and (p < 0.001) respectively compared to control and PJ groups. #, ### represent significant difference at (p < 0.05) and (p < 0.001)respectively compared to Al group.
There was no significant difference in the abnormal sperm in the animals that received 400 mg/kg Al compared to the animals that received tap water; however there was a strongly significant (p < 0.0001) increase in the percentage of abnormal sperm in the Al + 40% PJ group compared to that in the other groups (Fig. 9).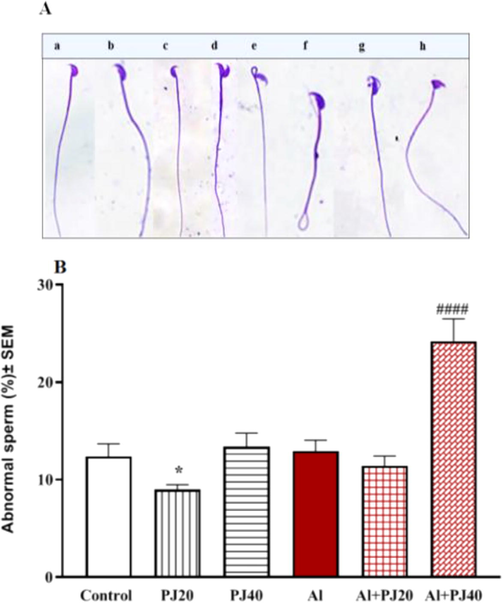
A) Abnormal sperm of male mice treated with aluminum (Al), pomegranate juice (PJ) and Al + PJ. * represent significant difference at (p < 0.05) compared to control, PJ and Al groups, while #### represent significant difference at p < 0.0001) compared to Al and Al + PJ20 groups. B) Mice spermatozoa in different abnormal shapes. a, Normal sperm. b. Without hook. c. Banana shape. d. Double head. e. Mid-piece defect. f. Tail defect. g. Acrosome defect. h. Amorphous shape.
3.4 Testosterone (T) concentration in serum
The testosterone concentration was measured in blood serum at the end of experiment. The exposure to Al (400 mg/kg) led to a significant (p < 0.001) reduction in serum T concentration compared to that in the other groups, and PJ (20% or 40%) ameliorated this depletion in testosterone level (Fig. 10).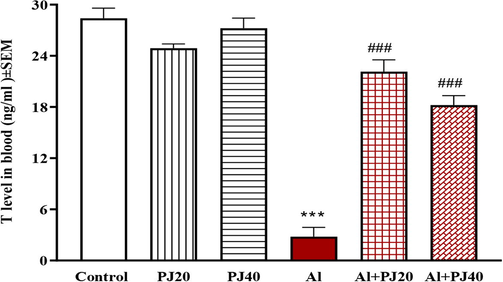
Testosterone level in blood of male mice treated with aluminum (Al), pomegranate juice (PJ) and Al + PJ. *** represent significant difference at (p < 0.001) respectively compared to control and PJ groups. ### represent significant difference at (p < 0.001) compared to Al group.
3.5 Testicular histological evaluations
In the Al + PJ40 group, the seminiferous tubules had a smaller diameter (p < 0.05) than that in the Al group (Fig. 11). In addition, there were not significant differences in the epithelial diameter of the S.T. between the groups (Fig. 12); however, there was a significant increase in the lumen diameter of the S.T. in the aluminum group compared to that in the PJ20 (p < 0.001) and PJ40 (p < 0. 01) groups, and PJ decreased this effect, as shown in the figures (Figs. 13 and 14).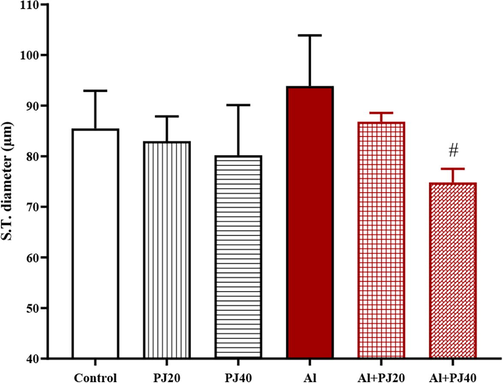
Seminiferous tubes diameter of male mice treated with aluminum (Al), pomegranate (PJ) and Al + PJ.# represent significant difference at (p < 0.05) compared to Al group.
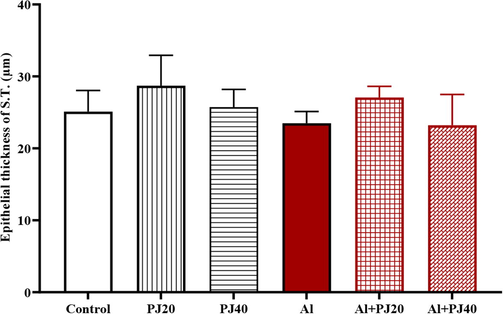
Epithelial thickness of seminiferous tubes of male mice treated with aluminum (Al), pomegranate (PJ) and Al + PJ.
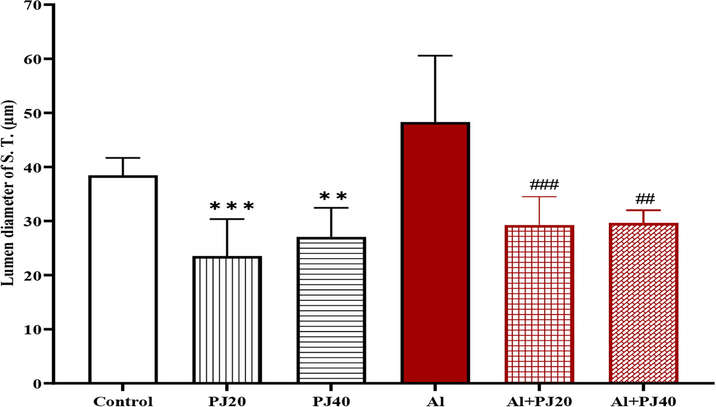
Lumen thickness of seminiferous tubes of male mice treated with aluminum (Al), pomegranate (PJ) and Al + PJ.** and *** represent significant difference at (p < 0.01) and (p < 0.001) compared to control and PJ groups. ## and ### represent significant difference at (p < 0.01) and (p < 0.001) compared to Al group.
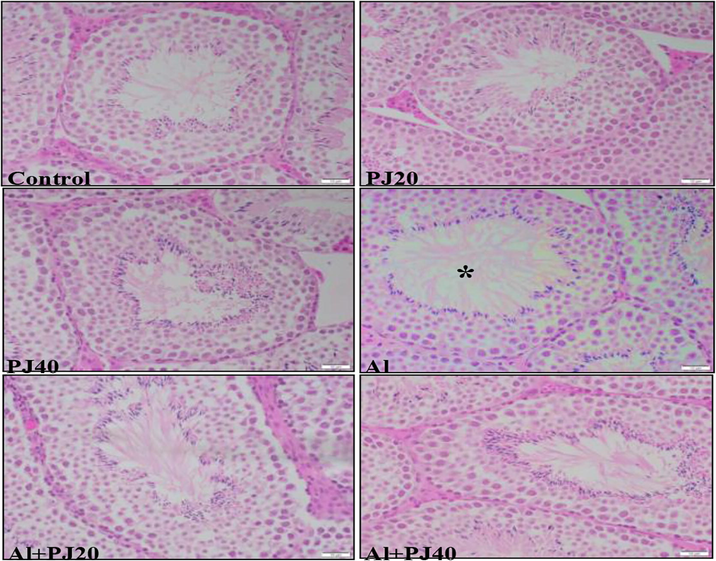
Histological sections of S.T. of mice testis in different groups in this study. * refers to increasing in lumen diameter of S. T. in Al group. Scale bar = 10 µm.
4 Discussion
This study demonstrated the toxicity of aluminum through its effects on the body and reproductive organ weights, sperm count and motility, and testosterone concentration, and this study also demonstrated the effectiveness of PJ in reducing the effects of Al. The decrease in the body and reproductive organ weights in animals treated with Al in this study is consistent with previous reports (Yousef & Salama 2009; Zhu et al., 2014; Miska-Schramm et al., 2017). These studies showed that aluminum-treated animals (i.e., mice, rats and rabbits) exhibited significantly lower body, testes, seminal vesicle and epididymis weights than the control animals. This decrease in body and organ weights may be due to mitochondrial dysfunction and glucose metabolism disruption; thus, mitochondria may be one of the possible targets of the harmful effects of aluminum (Xu et al., 2017). The combination of PJ and aluminum in this study significantly reduced the toxic effects of Al by ameliorating the reduction in the body and organ weights.
Furthermore, the current findings suggest that aluminum can reduce testosterone concentration and motility and increase abnormal spermatozoa. The effects on the testosterone level agree with previous findings in animals exposed to aluminum (Guo et al., 2001, 2005; Yousef et al., 2005; Zhu et al., 2014; Martinez et al., 2017). One study reported that aluminum suppressed T secretion in male rats, thus decreasing sperm count (Sun et al., 2011).
On the other hand, it was reported that Al increased lipid peroxidation (LP) and reduced the antioxidant defense in rat testes (Yousef & Salama 2009). Furthermore, spermatozoa were shown to be oversensitive to oxidative stress because of their limited antioxidant concentrations (Aitken 1995). In the current study, pomegranate juice exhibited a clear antioxidant effect to mitigate the disruption in the testes, especially by maintaining the testosterone concentration and sperm count and quality. Aksu et al., (2017) suggested that the protective effect of pomegranate against Pb toxicity was stronger in the testes compared to that in other organs in male rats.
Our data suggested that the sexual behavior of male mice, such as approach, contact, following, pelvic thrusting and biting, are negatively affected by exposure to aluminum. These deleterious effects may be attributed to the decrease in the concentration of testosterone, which is responsible for sexual behavior. The relationship between the synthesis of T and the sexual behavior of male mice was proven in a previous study (Zang et al., 2016). In addition, PJ improved the impairments in sexual behavior after exposure to Al. It has been well-established that testes function and sexual behavior are controlled by the hypothalamic-pituitary–gonadal axis (HPGA). Sexual behavior may be affected by T levels in Leydig cells, which, in turn, impacts dopamine secretion from the hypothalamus (Sharma et al., 2011). Additionally, the increase in the stress hormone cortisol can decrease gonadotropin-releasing hormone (GnRH) and, therefore, decrease the LH and FSH hormones, which control the T levels and spermatogenesis (Collodel et al., 2008). Oxidative stress can interrupt endocrine function and inhibit T production (Wang et al., 2017). Previous studies reported that PJ benefited antioxidant activity effects and metal detoxification, reducing stress and ameliorating sexual behavior in male rats (Hong et al., 2008; Turk et al., 2008; Dkhil et al., 2013; Aksu et al., 2017; Lydia et al., 2019).
In conclusion, this study demonstrated the toxicity of aluminum by its effects on animal body and organ weights, sperm count, sperm motility and testosterone concentration in male mice; however, PJ exhibits protective activity against these effects by ameliorating sexual behavior and fertility.
Acknowledgement
The authors thank Mr. Mohammed Arafat for technical assistance in this research.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- The potential effects of pomegranate (Punica granatum) juice on carbon tetrachloride-induced nephrotoxicity in rats. J Physiol Biochem. 2013;69:359-370.
- [Google Scholar]
- Curcumin Palliative Effects on Sexual behavior, Fertility and Reproductive Hormones disorders in Mercuric Chloride Intoxicated Mice Offspring. J. of King Saud University - Sci.. 2019;32:1293-1299.
- [Google Scholar]
- Neurobehavioral toxic effects of perinatal oral exposure to aluminum on the developmental motor reflexes, learning, memory and brain neurotransmitters of mice offspring. Pharmacol. Biochem. Behav.. 2012;101:49-56.
- [Google Scholar]
- Aluminum-induced testosterone decrease results in physiological and behavioral changes in male mice. Afr. J. Biotechnol.. 2011;10:201-208.
- [Google Scholar]
- Anti-obesity effects of Taif and Egyptian pomegranates: molecular study. Biosci. Biotechnol. Biochem.. 2015;79:598-609.
- [Google Scholar]
- Free radicals, lipid peroxidation and sperm function. Reprod Fertil Dev. 1995;7:659-668.
- [Google Scholar]
- Effect of pomegranate (Punica granatum L.) juice on kidney, liver, heart and testis histopathological changes, and the tissues lipid peroxidation and antioxidant status in lead acetate-treated rats. Cell Mol Biol (Noisy-le-grand). 2017;63:33-42.
- [Google Scholar]
- Al-Dhabi,N.A, Arasu MV, Rejiniemon TS. 2015. In vitro antibacterial, antifungal, antibiofilm, antioxidant, and anticancer properties of isosteviol isolated from endangered medicinal plant Pittosporum tetraspermum. Evidence-Based Complementary and Alternative Medicine.2015.
- Protective Effects of Pomegranate Juice on Nephrotoxicity Induced by Captopril and Gentamicin in Rats. Iran J Kidney Dis. 2017;11:422-429.
- [Google Scholar]
- Protective effects of pomegranate (Punica granatum) juice on testes against carbon tetrachloride intoxication in rats. BMC Complement Altern Med. 2014;14:164.
- [Google Scholar]
- Pomegranate Consumption and Blood Pressure: A Review. Curr Pharm Des. 2017;23:1042-1050.
- [Google Scholar]
- Barathikannan,K. Venkatadri B, Khusro A, Al-Dhabi NA, Agastian P, Arasu MV, Choi HS, Kim YO. Chemical analysis of Punica granatum fruit peel and its in vitro and in vivo biological properties. BMC Complimnetary and Alternative Medicine. 2016. 16:264.
- Prophylactic effects of pomegranate (Punica granatum) juice on sodium fluoride induced oxidative damage in liver and erythrocytes of rats. Can. J. Physiol. Pharmacol.. 2016;94:709-718.
- [Google Scholar]
- The protective effect of pomegranate juice in paracetamol-induced acute hepatotoxicity in rats. Turk. Pediatri. Ars.. 2016;51:72-78.
- [Google Scholar]
- Study of antagonism of citric acid on aluminum-induced toxicity in mice testis cells. Mol. Cellular Toxicol.. 2014;10:443-450.
- [Google Scholar]
- Effect of emotional stress on sperm quality. Indian J. Med. Res.. 2008;128:254-261.
- [Google Scholar]
- Ivermectin acute administration impaired the spermatogenesis and spermiogenesis of adult rats. Res. Vet. Sci.. 2018;117:178-186.
- [Google Scholar]
- Medically important carotenoids from Momordica charantia and their gene expressions in different organs. Saudi J. Biolog. Sci.. 2017;24:1913-1919.
- [Google Scholar]
- Antioxidant activity and total phenolic content of ethanolic extract of pomegranate peels, juice and seeds. Food Chem Toxicol. 2018;114:108-111.
- [Google Scholar]
- Effect of pomegranate (Punica granatum L.) juice and methanolic peel extract on testis of male rats. Pakistan. J. Zool.. 2013;45
- [Google Scholar]
- Elango, G, Roopan SM, Al-Dhabi NA, Arasu MV, Damodharan KI, Elumalai K. Cocos nucifera coir-mediated green synthesis of Pd NPs and its investigation against larvae and agricultural pest.. Artificial Cells, Nanomedicine, and Biotechnology. 2017.
- Coir mediated instant synthesis of Ni-Pd nanoparticles and its significance over larvicidal, pesticidal and ovicidal activities. J. Mol. Liq.. 2016;223:1249-1255.
- [Google Scholar]
- Aluminum contents of human milk, cow's milk, and infant formulas. J. Pediatr. Gastroenterol. Nutr.. 1999;28:270-275.
- [Google Scholar]
- Photocatalytic degradation of Congo red using Carissa edulis extract capped zinc oxide nanoparticles. J. Photochem. Photobiol., B. 2016;162:395-401.
- [Google Scholar]
- Glorybai, L, Barathi K.K, Arasu MV, Al-Dhabi NA, Agastian P. 2015. Some biological activities of Epaltes divaricata L. - an in vitro study. Annals of Clinical Microbiology and Antimicrobials. 2015, 14:18.
- Serum and testicular testosterone and nitric oxide products in aluminum-treated mice. Environ. Toxicol. Pharmacol.. 2001;10:53-60.
- [Google Scholar]
- Aluminum accumulation induced testicular oxidative stress and altered selenium metabolism in mice. Environ Toxicol Pharmacol. 2009;27:176-181.
- [Google Scholar]
- The influence of aluminum exposure on male reproduction and offspring in mice. Environ. Toxicol. Pharmacol.. 2005;20:135-141.
- [Google Scholar]
- Antioxidant and antiatherogenic effects of pomegranate. Am. J. Health Syst. Pharm.. 2011;68:1302-1305.
- [Google Scholar]
- Arasu MV. Green chemical approach towards the synthesis of SnO2 NPs in argument with photocatalytic degradation of diazo dye and its kinetic studies. J. Photochem. Photobiol., B. 2016;162:441-447.
- [Google Scholar]
- Helan,V. Prince J. J, Naif Abdullah Al-Dhabi, Mariadhas Valan Arasu, A. Ayeshamariam, G. Madhumitha, Selvaraj Mohana Roopan, M. Jayachandran. Neem leaves mediated preparation of NiO nanoparticles and its magnetization, coercivity and antibacterial analysis. Results in Physics. 2016. 6:712–718.
- Pomegranate polyphenols down-regulate expression of androgen-synthesizing genes in human prostate cancer cells overexpressing the androgen receptor. J. Nutr. Biochem.. 2008;19:848-855.
- [Google Scholar]
- Ferulic acid in Lolium multiflorum inhibits adipogenesis in 3T3-L1 cells and reduced high-fat-diet-induced obesity in Swiss albino mice via regulating p38MAPK and p44/42 signal pathways. J. Funct. Foods. 2017;37:293-302.
- [Google Scholar]
- Punica granatum (pomegranate) flower extract possesses potent antioxidant activity and abrogates Fe-NTA induced hepatotoxicity in mice. Food Chem Toxicol. 2006;44:984-993.
- [Google Scholar]
- Aluminum content of human semen: implications for semen quality. Reprod. Toxicol.. 2014;50:43-48.
- [Google Scholar]
- The influence of different aluminium compounds on the hippocampal morphofunctional state and conditioning in mice. Toxicol. Environ. Health Sci.. 2017;9:215-221.
- [Google Scholar]
- Effect of Punica granatum (pomegranate) on sperm production in male rats treated with lead acetate. Toxicol Mech Methods. 2011;21:495-502.
- [Google Scholar]
- Sulphur dioxide and arsenic affect male reproduction via interfering with spermatogenesis in mice. Ecotoxicol Environ Saf. 2018;165:164-173.
- [Google Scholar]
- Lydia, K.K., Charles Irungu Maina, Caleb Oburu Orenge, Collins Kipkorir Kirui, Muriuki, B.G. & Waithaka, P.N., 2019. Effectiveness of Pomegranate (Punica granatum L.) fruit extract on the sexual function in rats. Journal of Microbial & Biochemical Technology 11, 1-6.
- Amelioration of aluminum toxicity with black seed supplement on rats. Toxicol. Environ. Chem.. 2009;91:567-576.
- [Google Scholar]
- High flux water purification using aluminium hydroxide hydrate gels. Sci. Rep.. 2017;7 17437 17437
- [Google Scholar]
- Aluminum exposure for 60days at human dietary levels impairs spermatogenesis and sperm quality in rats. Reprod Toxicol. 2017;73:128-141.
- [Google Scholar]
- The Effect of Aluminum Exposure on Reproductive Ability in the Bank Vole (Myodes glareolus) Biol. Trace Elem. Res.. 2017;177:97-106.
- [Google Scholar]
- Role of ginger against the reproductive toxicity of aluminium chloride in albino male rats. Reprod. Domest. Anim.. 2012;47:335-343.
- [Google Scholar]
- How Bad Is Aluminum Exposure to Reproductive Parameters in Rats? Biol. Trace Elem. Res.. 2018;183:314-324.
- [Google Scholar]
- Male rat exposure to low dose of di(2-ethylhexyl) phthalate during pre-pubertal, pubertal and post-pubertal periods: Impact on sperm count, gonad histology and testosterone secretion. Reprod. Toxicol.. 2018;75:33-39.
- [Google Scholar]
- Accumulation of carotenoids and metabolic profiling in different cultivars of Tagetes flowers. Molecules. 2017;22:313.
- [Google Scholar]
- Metabolic profiling and antioxidant assay of metabolites from three radish cultivars (Raphanus sativus) Molecules. 2016;21:157.
- [Google Scholar]
- Park, J.H., 2011. Assessment of Male Sexual Behavior in Mice. In Gould T. (eds) Mood and Anxiety Related Phenotypes in Mice.: Humana Press.
- Zinc sulphate and vitamin E alleviate reproductive toxicity caused by aluminium sulphate in male albino rats. Toxicol. Ind. Health. 2015;31:221-234.
- [Google Scholar]
- Analysis of phenolic compounds in different parts of pomegranate (Punica granatum) fruit by HPLC-PDA-ESI/MS and evaluation of their antioxidant activity: application to different Italian varieties. Anal. Bioanal. Chem.. 2018;410:3507-3520.
- [Google Scholar]
- Aluminium speciation in environmental samples: a review. Anal. Bioanal. Chem.. 2006;386:999-1012.
- [Google Scholar]
- Spilanthes acmella ethanolic flower extract: LC-MS alkylamide profiling and its effects on sexual behavior in male rats. Phytomedicine. 2011;18:1161-1169.
- [Google Scholar]
- Effects of aluminum exposure on serum sex hormones and androgen receptor expression in male rats. Biol. Trace Elem. Res.. 2011;144:1050-1058.
- [Google Scholar]
- RSM optimized Moringa oleifera peel extract for green synthesis of M. oleifera capped palladium nanoparticles with antibacterial and hemolytic property. J. Photochem. Photobiol., B. 2016;162:550-557.
- [Google Scholar]
- Ameliorating effect of pomegranate juice consumption on carbon tetrachloride-induced sperm damages, lipid peroxidation, and testicular apoptosis. Toxicol. Ind. Health.. 2016;32:126-137.
- [Google Scholar]
- Effects of pomegranate juice consumption on sperm quality, spermatogenic cell density, antioxidant activity and testosterone level in male rats. Clin. Nutr.. 2008;27:289-296.
- [Google Scholar]
- Aluminum Levels in Brain, Serum, and Cerebrospinal Fluid are Higher in Alzheimer's Disease Cases than in Controls: A Series of Meta-Analyses. J. Alzheimers. Dis.. 2015;47:629-638.
- [Google Scholar]
- Bioactive compounds from the seeds of Punica granatum (pomegranate) J. Nat. Prod.. 2004;67:2096-2098.
- [Google Scholar]
- Wang, Y., Chen, F., Ye, L., Zirkin, B. & Chen, H., 2017. Steroidogenesis in Leydig cells: effects of aging and environmental factors. 154, R111.
- Chronic exposure to aluminum and risk of Alzheimer's disease: A meta-analysis. Neurosci. Lett.. 2016;610:200-206.
- [Google Scholar]
- Systematic review of potential health risks posed by pharmaceutical, occupational and consumer exposures to metallic and nanoscale aluminum, aluminum oxides, aluminum hydroxide and its soluble salts. Crit. Rev. Toxicol.. 2014;44(Suppl 4):1-80.
- [Google Scholar]
- Aluminum chloride caused liver dysfunction and mitochondrial energy metabolism disorder in rat. J. Inorg. Biochem.. 2017;174:55-62.
- [Google Scholar]
- Folic acid improve developmental toxicity induced by aluminum sulphates. Environ Toxicol Pharmacol. 2017;50:32-36.
- [Google Scholar]
- Effect of taurine on toxicity of aluminum in rats. Eur. J. Clinic. Nutr. Metab.. 2009;4:e187-e192.
- [Google Scholar]
- Propolis protection from reproductive toxicity caused by aluminium chloride in male rats. Food Chem. Toxicol.. 2009;47:1168-1175.
- [Google Scholar]
- Aluminium-induced deterioration in reproductive performance and seminal plasma biochemistry of male rabbits: protective role of ascorbic acid. Toxicology. 2005;215:97-107.
- [Google Scholar]
- Effects of velvet antler polypeptide on sexual behavior and testosterone synthesis in aging male mice. Asian J. Androl.. 2016;18:613-619.
- [Google Scholar]
- Effects of sub-chronic aluminum chloride on spermatogenesis and testicular enzymatic activity in male rats. Life Sci.. 2014;102:36-40.
- [Google Scholar]







