Translate this page into:
Effects of nitric oxide and silicon application on growth and productivity of pepper under salinity stress
⁎Corresponding author. ssoylemez@harran.edu.tr (Selçuk Söylemez)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Background
Salinity is one of the most prevalent abiotic stresses which adversely affect plant growth, yield, and fruit quality by restricting water uptake and disrupting metabolic activities. Nitric oxide (NO) can be used to alleviate the adverse effects of salinity stress on plant growth and yield; however, limited knowledge is available on the frequency of NO application. There are limited studies on determining frequency of NO application to alleviate adverse effects of salinity stress on plant growth.
Methods
This study was conducted to determine the frequency of NO application and associated impacts on pepper growth under salinity stress. The plants were grown in perlite medium and irrigated with a nutrient solution having 0 and 100 mM NaCl salinity. Salinity stress was imposed one month after planting, and simultaneously 2 mM silicon (Si) and 100 µM NO were applied at 0-, 7-, 14- and 28-days interval after imposing salinity.
Results
Yield and plant growth were reduced by salinity stress, whereas proline and MDA (malondialdehyde) in the leaves were increased. The Si application increased stem diameter and dry weight, whereas decreased plant height and root length. Furthermore, Si application decreased stomatal conductivity under salinity-free conditions, while increased it under salinity stress. Application of NO at 7- and 14-days interval increased most of the studied traits, whereas decreased plant height, leaf dry weight, stomatal conductivity, and proline content.
Conclusions
While nitric oxide applied at 7-day intervals decreased marketable yield, application at 14-day intervals increased it by ∼16.5%. Based on the results, it is thought that weekly application of Si and NO application at 14-days interval could increase marketable yield of pepper under saline environments.
Keywords
Growth traits
Membrane permeability
Stomatal conductance
Productivity
Marketable yield
1 Introduction
Salinity is one of the most devastating abiotic stresses that limit plant growth and yield, particularly in arid and semi-arid climates. Like all crop plants, pepper’ growth is adversely affected by various abiotic stresses. Various stresses (biotic or abiotic) alter metabolic functions of plants, and reduce their growth, development, and yield. Soil salinity increases osmotic pressure in the rhizosphere layer; thus, plants experience physiological drought and resultantly their growth is inhibited (Farooq et al., 2018a). In addition, Na and Cl ions taken up in excess from saline soils cause ion imbalance, prove toxic and cause nutritional imbalances due to their antagonistic effect (Munns and Termaat, 1986; Lauchli, 1986; Farooq et al., 2018b). Soil salinity is an important problem in arid and semi-arid climatic regions, and it is constantly increasing around the world (Kantar and Elkoca, 1998). The ability of plants to continue their normal growth development can be ensured by eliminating the stress factors that exert adverse effects on growth (Farooq et al., 2017; Onen et al., 2017). Breeding salt-tolerant plants, and low-salt water source along with drainage to remove the salts from root zone are the possible solutions of salinity; however, both are time-consuming and expensive. Doğru and Canavar (2020) reported that various phytohormones are used to reduce the negative effects of salinity stress on plant growth. In the recent years, studies have been focused on the use of external applications of various compounds (hormones, amino acids, minerals, etc.) to alleviate the negative impacts of salinity stress in plants. Nitric oxide (NO) and silicon (Si) are among these compounds which have been used to improve salinity tolerance of plants (Ijaz et al., 2021).
The positive and negative effects of NO on plants have been reported in several studies during recent years (Bolwell, 1999; Beligni and Lamattina, 2001; Wendehenne et al., 2001; Lamattina et al., 2003; Neill et al., 2003). The NO is synthesized in plants through biochemical and molecular mechanisms (Del Rio et al., 2004). Nitric oxide is accepted as an important signaling molecule since it initiates various physiological reactions in plants. It is known to have important contribution in plant development from seed to fruit ripening. However, NO is produced in different organs, depending on plant species, severity of salinity and temperature stresses, and nutrient deficiency. It has been proven that NO is a very active molecule, which biologically protects plants from the damage caused by oxidative stress. The NO can exert both beneficial and harmful effects on plant cells depending on its amount of application. Nitric oxide is involved in mitochondrial and chloroplast functions in mature cells, cell wall lignification (Ferrer and Ros-Barceló, 1999), iron deposition and ion regulation in cells (Garcia-Mata et al., 2003). It plays an important role in the regulation of plant metabolism and transformation of plant leaves from green to yellow and from yellow to purple (senescence), promotion of cell death (Pedroso et al., 2000), and opening and closing of stomata (Garcia-Mata and Lamattina, 2007; Guo et al., 2003; Sakihama et al., 2003; Bright et al., 2006). Nabi et al. (2019) reported that external application of NO-donors ameliorated negative effects of stress on plants and increased antioxidant activity. Likewise, Hajihashemi et al. (2020) reported that pre-treatment of Chenopodium quinoa (quinoa) seeds with sodium nitroprusside (SNP), H2O2 and CaCl2 lowered the negative effect of salt stress on seed germination.
Silicon, on the other hand, is a versatile and useful element whose importance in plants has not been sufficiently understood (Takahashi et al., 1990; Singh et al., 2005). There has been worldwide interest in Si in recent years as it positively affects the development processes of plants. Sistani et al. (1997) and Ma (2004) observed that Si protects plants against abiotic and biotic stresses and increases their development under extreme climatic conditions (such as high temperature, drought). Tomatoes and cucumbers accumulate <1% Si in their tissues. Tomatoes and cucumbers continue their healthy growth under normal growing conditions; however, utilize Si to grow normally on the onset of any stress. The vital activities of the plants may be disrupted if Si is deficient in the environment under stressful environments. Silicon ameliorates the stress effects on photosynthesis by protecting photosynthetic machinery and its functions (Rastogi et al., 2021). Silicon increases plant's resistance to salt and drought stress by supporting photosynthesis, regulating osmotic adjustment capacity, reducing transpiration, increasing antioxidant capacity and eliminating toxic ions (Chen et al., 2018). The Si is in the dormant structure in the plants and transported to upper leaves by transpiration. Silicon transported to the upper leaves by transpiration accumulates under the cuticle, making the leaves stand upright, increasing photosynthetic activity and improving transpiration. The Si accumulation in leaves and stem helps to strengthen the cell wall and provides a hardening effect. Silicon also reduces transpiration; thus, enables plants to gain resistance against low and high temperatures, drought stress, radiation, and UV stress. Fauteux et al. (2005) reported that Si reduces the incidence and degree of disease infestation in plants. However, the protective effect of Si on plants has not been fully understood (Currie and Perry, 2007). Ahmad et al. (2021) and Liu et al. (2020) reported that Cd and As applications negatively affected plant growth in Brassica juncea and maize, respectively, and this negative effect decreased with the combined application of NO and Si.
Orosco-Alcalá et al. (2021) reported that pepper plants are sensitive to salinity and biomass production decreases by 14% for each unit increase in EC. Similarly, the researchers stated that pepper plants can die if subjected to high salinity for a long time, also high salinity increases the blossom end rot and reduces the fruit quality.
Climate scientists predict that global warming and climate change would decrease clean water resources, resulting in increased salinity in coastal areas. Therefore, use of salt water will become necessary in crop production to ensure food security of increasing global population. Therefore, current study investigated the possible role of NO and Si in alleviating the adverse effects of salinity stress on growth and development of pepper. The impact of NO and Si applications on plant growth, yield and some biochemical parameters was evaluated. It was hypothesized that NO and Si application would reduce the negative impacts of salinity stress on plant growth, yield, and biochemical parameters.
2 Materials and methods
2.1 Experiment details
The experiment was carried out in an unheated greenhouse with polycarbonate cover (heated only on frosty days) and a soilless production system during 2019. The ‘Mert F1’ pepper variety was used as plant material in the study. The variety is early and suitable for greenhouse and moderately tolerant to salinity. Perlite was used as growing medium in the experiment. Perlite was filled in 8-liter plastic pots and 1 pepper seedling was transplanted in each pot. Pots were placed in the greenhouse 120 cm apart from each other. The plants were planted on 23.09.2019 and the experiment was terminated on 08.02.2020.
All plants were treated equally till 30 days after planting and watered with the same nutrient solution to ensure a healthy adaptation. At the end of adaptation period, only nutrient solution was applied to the control pots, whereas nutrient solution + 100 mM NaCl solution was applied to the pots subjected to salinity stress. Salinity was increased by 25 mM daily and reached 100 mM at the end of the 4th day to prevent sudden shock to the plants. The Si and NO were also applied to the roots with 100 µM SNP (sodium nitroprusside) for 0, 7, 14 and 28 days and 2 mM Si nutrient solution was applied once a week. Diseases and pests were controlled by recommended plant protection measures throughout the experiment. All pots received the equal amount of irrigation. The irrigation amount was determined according to the drainage volume. To prevent excessive salt accumulation in soilless agriculture, 20–25% of the given nutrient solution must be drained. For this purpose, 3 pots were separated for each treatment (salt: 0 mM, Si: 0 mM and NO: 0 days) and water was given until 20–25% of it drained, and the amount of irrigation water was determined (Soylemez et al., 2020). The amount of irrigation water determined was measured in a graduated measuring cylinder and applied to other plants.
The nutrient solution recommended by Cokuysal et al. (2016) was used in the experiment. The nutrient solution consisted of 15.5 mM NO3, 1.25 mM NH4, 6.5 mM K, 1.5 mM Mg, 4.75 mM Ca, 30 µM BO3, 0.75 µM Cu, 5 µM Zn, 0.5 µM Mo, 10 µM Mn, and 15 µM Fe. The EC of the nutrient solution was adjusted to 2.25 dS m−1, while pH was adjusted to 5.8–6.5 using nitric acid.
2.2 Measurements
Plant height was measured from media surface and the growth tip with a measuring tape. Stem diameter was measured from 1 to 2 cm above the growth media level using a digital vernier caliper. Leaf area of the harvested at the end of the experiment were determined using the “Imagej” computer program (Easlon and Bloom, 2014).
Leaf, stem, and root were placed separately in paper bags, dried in an oven at 65 °C until constant weight, dry weights were determined on a precision scale. Total biomass was determined by adding root, stem, and leaf dry biomass.
A portable chlorophyll meter (Minolta SPAD-502) was used to determine chlorophyll index values of leaves. The chlorophyll index was determined by reading 6 fully developed young leaves per plant. Stomatal conductance was determined in mmol m−2 s−1 using leaf porometer (Decagon Devices. Inc. Pullman, USA) in young leaves that have completed their development at noon (Ghimire et al., 2018).
The 0.5 g of fresh leaves were kept in 20 ml distilled water at 40 °C for 30 min and the EC1 value of the solution was measured. Afterwards, the same samples were kept at 100 °C for 10 min and the EC2 value of the solution was determined after they came to room temperature. Membrane stability (MS) of plants was calculated with the following formula of Premchandra et al. (1990).
Proline contents were determined according to Bates et al. (1973). To prepare an acid-ninhydrin mixture, 1.25 g of ninhydrin was dissolved in 30 ml of glacial acetic acid and 20 ml of 6 M phosphoric acid. Leaves weighing 0.5 g were homogenized in 10 ml of 3% sulfosalicylic acid and passed through Whatman No. 2 filter paper after homogenization. The homogenized samples were transferred into a 2 ml sieve tube to which 2 ml glacial acetic acid, 2 ml acid ninhydrin, and 4 ml Toluene was added, and left at 100 °C water bath for 1 h, and the reaction was terminated in ice. The upper phase was taken, and the absorbance was measured the toluene control at a wavelength of 515 nm in the spectrophotometer. A pre-prepared L-Proline solution was used as a standard.
Marketable fruits were weighed at green stage and marketable yield per plant was calculated. Mean fruit weight was calculated by dividing the marketable fruit weight by the number of marketable fruits.
2.3 Statistical analysis
The experiment was carried out according to factorial design with 4 replications. The NaCl applications (0 and 100 mM) was main factor, whereas Si (0 and 2 mM) application was sub-factor and NO (0-, 7-, 14- and 28-day intervals) application was regarded as sub-sub factor. Hence, the experiment consisted of (16) treatments (2 × 2 × 4 = 16), each treatment was replicated four times and each replicate was represented by a single pot. The total number of experimental units were 64. Statistical analysis of the recorded data was carried out using analysis of variance in TARIST 4.0 statistical package. The LSD (least significant difference) post-hoc test was applied to compare treatments’ means.
3 Results and discussion
3.1 Plat growth parameters
Salinity stress and Si applications caused significant reductions in plant height. The plant height was decreased as the application interval of NO. The effect of salinity stress × Si × NO interaction on plant height was non-significant. Salinity stress significantly decreased plant height. The application of NO at 7-day intervals increased plant height of the plants subjected to salinity stress with 0 mM Si application. The increased interval of NO application decreased plant height (Fig. 1). The Si application in salinity-free environment shortened plant height, while its application under salinity stress increased plant height. In salinity stress × Si × NO interaction, the tallest plants (114.40 cm) were obtained from 0 mM salinity, 0 mM Si and NO application at 7-day intervals, while the shortest plants (51.10 cm) were recorded for 100 mM salinity, 0 mM Si and NO application at 28 days interval (Fig. 1).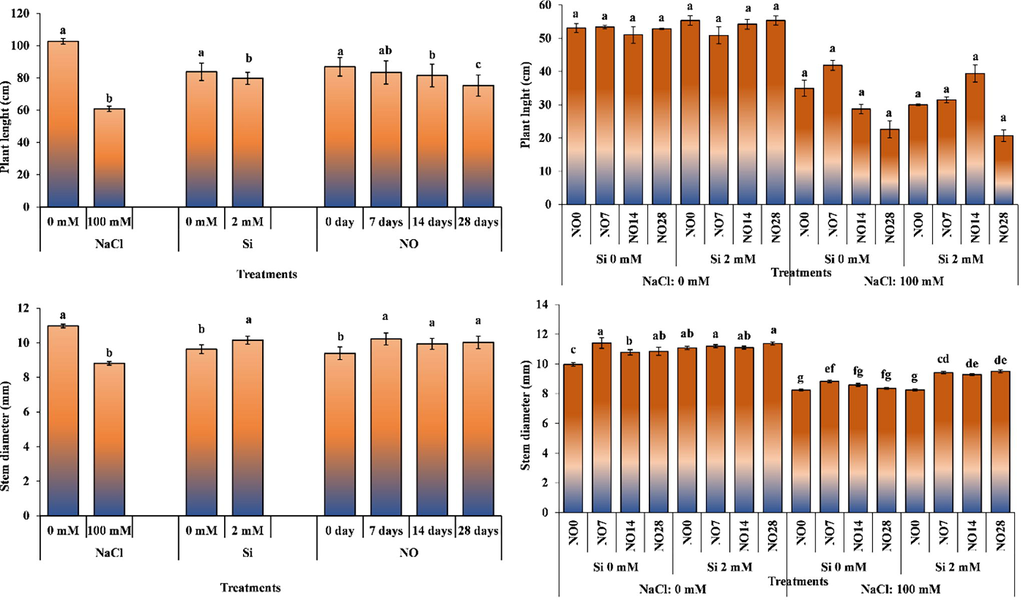
The impact of individual and interactive effects of Si and NO applications on plant height and stem diameter of pepper grown under salinity stress. The means having different letters are statistically different from each other (p < 0.05) (n = 4). LSD for plant height: NaCl = 3.817, Si = 3.817, NO = 5.428, NaCl × Si × NO = non-significant, LSD for stem diameter: NaCl = 0.199, Si = 0.199, NO = 0.281, NaCl × Si × NO = 0.5612.
The decrease in plant height is a typical response of the plants to salinity stress. The short height of the plants exposed to salinity stress and Si can be explained with decreased chlorophyll index. Heidari (2012) reported that a decrease in chlorophyll content may adversely affect photosynthesis. The decreased chlorophyll contents might have suppressed plant height by reducing the products of photosynthesis. In addition, plants grown under salinity stress cannot benefit from the available water sufficiently due to high osmotic stress. Plants that cannot benefit from the available water close their stomata; thus, photosynthesis is adversely affected as CO2 entry to the plant is prevented, resultantly plant growth and development is suppressed. Furthermore, toxic levels of Na and Cl ions accumulate in plants grown in saline conditions resulting in inhibited growth. Similar to the current study, different earlier studies on tomato (Soylemez and Pakyurek, 2017), pepper (Yurtseven et al., 1996) and watermelon (Sharf-Eldin et al., 2018) have reported a decrease in plant height under as a result of salinity stress. Cengiz (2017) reported that NO did not increase plant height of pepper. The results of the current study on plant height are similar to Cengiz (2017).
Salinity stress, Si and NO significantly altered stem diameter (Fig. 1). While salinity stress decreased stem diameter, Si and NO applications increased it. The interactive effects of salinity stress × Si × NO on stem diameter were significant (Fig. 1). The stem diameter of the plants grown in saline conditions was significantly reduced compared to those grown in salinity-free environment. Generally, Si applications under saline and non-saline conditions increased stem thickness of the plants. The stem diameters of the NO-treated plants were thicker than the plants receiving no NO application. The highest stem diameter (11.41 mm) was recorded for the plants grown under 0 mM salinity, 0 mM Si, and NO application every 7 days. The lowest stem diameter (8.24 and 8.25 mm) was recorded for the plants grown under 100 mM salinity, 0 mM Si and no NO application and 100 mM salinity and 2 mM Si application.
Yakit and Tuna (2006) reported decrease in stem diameter of maize plants in response to salinity. Kiran et al. (2019) observed that stem diameter of eggplant plant grown under salinity and drought stress decreased compared to the control plants. It has been reported that Si application increased stem diameter of lamb's lettuce (Valerianella locusta L.) under salinity stress compared to no Si application (Oztekin and Tutal, 2021). It is evident that salinity stress prevented plant growth and reduced stem diameter. The findings of our study are in agreement with the results of several previous studies.
Analysis of variance revealed that the effect of salinity stress and Si application on root length was significant, while NO remained non-significant in this regard (Fig. 2). Salinity and Si applications decreased root length. Application of NO every 14 days increased root length; however, the effect was non-significant.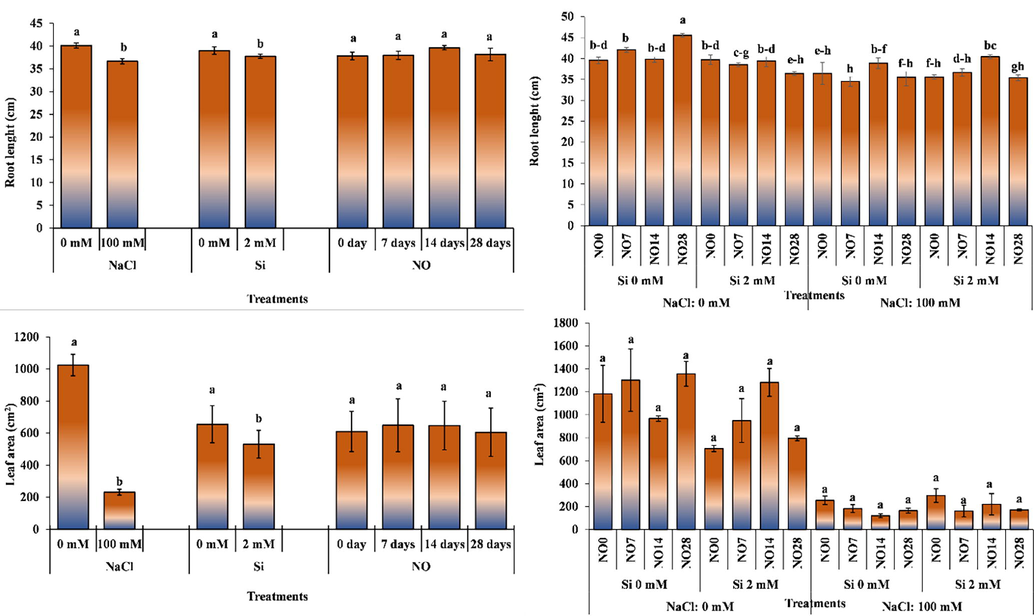
The impact of individual and interactive effects of Si and NO applications on root length and leaf area of pepper grown under salinity stress. The means having different letters are statistically different from each other (p < 0.05) (n = 4). LSD for root length: NaCl = 1.575, Si = 1.172, NO = non-significant, NaCl × Si × NO = 3.372, LSD for leaf area: NaCl = 159.499, Si = 118.731, NO = non-significant, NaCl × Si × NO = non-significant.
The interactive effects of salinity stress × Si × NO on root length was significant (Fig. 2). The plants grown under salinity-free conditions, no Si application and NO application at 28 days interval resulted in deeper penetration of roots, while the NO application at 0- and 14-days interval under no salinity 0- and 2-mM Si application resulted in longer roots. The root length of the plants subjected to salinity stress was generally increased with Si applications. The NO application at 14 days interval had significant effect on the plants grown under salinity stress and 0- and 2-mM Si application. The highest root length (45.50 cm) was recorded for the plants grown under 0 mM salinity, 0 mM Si and NO application at 28 days interval, while the lowest value (34.53 cm) was noted for the plants grown under 100 mM salinity, 0 mM Si and NO application at 7 days interval.
It has been observed that the root depths of the plants exposed to salt stress were less. Baran et al. (1996) on pepper, Akinci et al. (2004) on eggplant, Sekmen et al. (2005) in tomato plant and Aktas and Kilic (2013) in soybean reported a decrease in root length under saline environments like our study.
Salinity stress and Si applications had significant effect on leaf area, while NO applications remained non-significant (Fig. 2). Salinity stress and Si applications reduced the leaf area by 77.38% and 19.02%, respectively. The application of NO every 7 days increased leaf area by 6.38% compared to the control.
The interactive effect of salinity × Si × NO was non-significant for leaf area (Fig. 2). The plants NO application at 7-day intervals to the plants grown under 0 Mm salinity and Si application observed an increase in leaf area compared to the control group, while a decrease was recorded when NO was applied at 14 and 28-day intervals. Likewise, Si applications leaf area of the plants grown under salinity-free environments; however, NO application in addition to Si increased leaf area. Leaf area generally decreased with the imposition of salinity stress. The highest leaf area (1302.77 cm2) was noted for the plants grown under 0 mM salinity and Si and NO application at 7-day intervals, while the lowest leaf area (101.29 cm2) was recorded for the plants grown under 100 mM salinity, 0 mM Si and NO application at 28 days interval.
Plants grown in saline environments experience physiological drought due to high osmotic pressure in the soil. In other words, although there is water in the environment, the plant cannot benefit from it due to the high osmotic pressure. Plants reduce their leaf area to adapt the saline environments. Kaya and Dasgan (2013) reported that total leaf area of beans decreased with increasing salinity. Similarly, Aksoy (2019) reported decreased leaf area of tomato, Bora (2015) for pepper and Sharf-Eldin et al. (2018) for watermelon. The findings of our study and the results of these researchers are in line with each other.
3.2 Biomass production
Salinity stress and NO applications had significant effect on leaf dry weight, while Si application had non-significant effect (Fig. 3). While salinity reduced dry weight of leaves by 73.22%, there was no significant difference between Si applications. Nitric oxide applications, on the other hand, decreased leaf dry weight.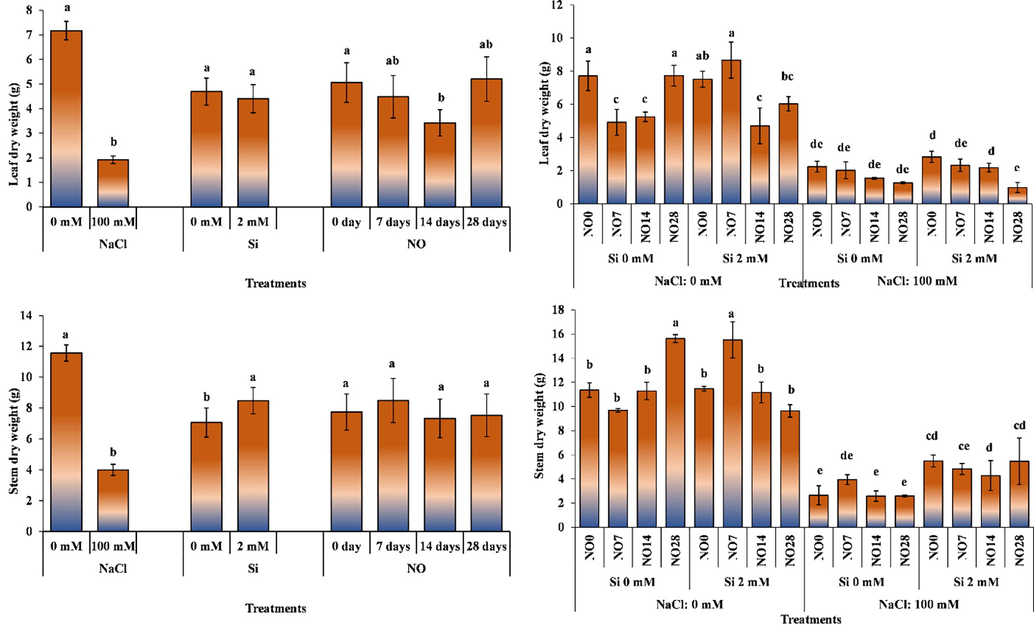
The impact of individual and interactive effects of Si and NO applications on leaf and stem dry weight of pepper grown under salinity stress. The means having different letters are statistically different from each other (p < 0.05) (n = 4). LSD for leaf dry weight: NaCl = 1.262, Si = non-significant, NO = 1.196, NaCl × Si × NO = 1.691, LSD for stem dry weight: NaCl = 1.532, Si = 1.141, NO = non-significant, NaCl × Si × NO = 1.930.
Regarding salinity × Si × NO interaction (Fig. 3), application of NO at 7- and 14-day intervals decreased leaf dry weights, while NO application every 28 days increased dry weight of the leaves. Si application in salt-free environment decreased leaf dry weight, while its application in saline environment increased leaf dry weight. Increased interval of NO application under saline conditions decreased leaf dry weight. While the highest leaf dry weight (8.67 g plant−1) was recorded for the plants grown under 0 mM salinity, 2 mM Si, and NO application every 7 days, the lowest (0.98 g plant−1) was recorded under 100 mM salinity, 2 mM Si, and NO application at 28 days interval.
The results of the current study indicated that Si application alleviated the negative effects of salt stress. Other researchers working on different plants also reported decrease in leaf dry weight under salinity stress (Yurtseven et al., 1996; Bora, 2015). Cengiz (2017) applied three different doses of NO donor SNP to pepper plants and reported that 1 to 100 µM applications reduced leaf weight compared to the control, while 0.01 µM SNP application was in the same statistical class as the control. The results of the researchers and the findings of current study show similarities.
Salinity stress and Si application significantly altered shoot dry weight, while NO supplication has non-significant impact in this regard (Fig. 3). Shoot dry weight of the plants grown in saline conditions decreased compared to control plants. Silicon application significantly increased shoot dry weight.
The interactive effect of salinity stress, Si and NO application was significant for shoot dry weight. The plants grown under 0 mM salinity, 2 mM Si and NO application at 7-days interval recorded a significant increase in shoot dry weight (Fig. 3). Silicon application under salinity-free environment generally decreased shoot dry weight, while its application under saline environment alleviated the adverse effects of salinity stress and increased shoot dry weight. The increasing interval of NO application under saline environment resulted in a linear reduction of shoot dry weight.
Several earlier studies have determined that salinity stress results in the necrosis of older leaves and decreased fresh and dry weights of the plants (Dasgan et al., 2002; Demir, 2009; Akay Rastgeldi et al., 2014). On the other hand, Khan et al. (2019) stated that Si application can reduce negative effects of salinity by regulating the antioxidant defence system; thus, reducing lipid peroxidation and ultimately protecting membrane integrity and decreasing plasma membrane permeability. Similarly, high Na+ concentration causes excessive production of ROS in plants, negatively affects plant growth and metabolism, but Si application reduces Na+ accumulation (Khan et al., 2019).
Salinity stress and NO application had significant effect on root dry weight, whereas Si application remined non-significant (Fig. 4). Root dry weight of the plants grown in saline conditions decreased by 69.77% compared to the control. Silicon applications increased root dry weight in a non-significant manner, while NO application every 7 days significantly increased root dry weight. Extending NO application interval decreased root dry weight.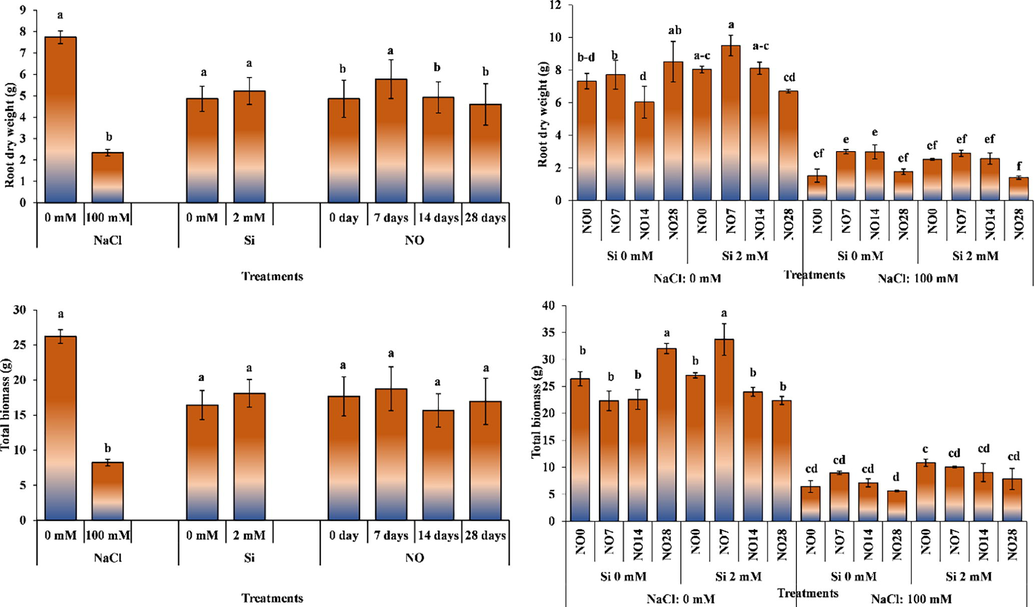
The impact of individual and interactive effects of Si and NO applications on root dry weight and total biomass production of pepper grown under salinity stress. The means having different letters are statistically different from each other (p < 0.05) (n = 4). LSD for root dry weight: NaCl = 0.740, Si = non-significant, NO = 0.778, NaCl × Si × NO = 1.558, LSD for total biomass production: NaCl = 1.816, Si = non-significant, NO = non-significant, NaCl × Si × NO = 5.102.
The interactive effect of salinity stress, Si and NO application was significant for root dry weight. Nitric oxide and Si application generally increased root dry weight. The plants receiving no salinity and Si produced the highest root dry weight with NO application every 28 days, while NO application at 7-day intervals with 2 mM Si under salinity-free environment resulted in the highest root dry weight. While Si application in saline environment increase root dry weight with NO at 0-day interval, it decreased it in other NO applications. Root dry weight decreased as NO application interval increased in saline environment.
The studies conducted on melon, pepper and tomato reported a significant decrease in dry weight of plants with increasing salinity (Chartzoulakis and Klapaki, 2000; Debouba et al., 2006; Soylemez et al., 2017). Silicon applied to maize grown under salt stress increased root dry weight (Rohanipoor et al., 2013). The findings of the above researchers support the results of current study.
Salinity stress had significant effect on total plant biomass, whereas Si and NO application remained non-significant (Fig. 4). Salinity stress significantly decreased total dry biomass of the plant, Si application increased it; however, the increase was non-significant. Application of NO at 7-day interval increased plant dry biomass in non-significant manner.
The interactive effect of salinity stress, Si and NO application was significant for total plant dry weight (Fig. 4). The highest and the lowest total plant dry biomass was recorded for the plants grown under 0- and 100-mM salinity, respectively. Silicon increased the total dry biomass of plants under non-saline environments. In the interactions, the highest total dry weight (33.70 g plant−1) was recorded for the plants grown under 0 mM salinity, 2 mM Si and NO application a 7 days interval. The lowest total plant dry biomass (5.62 g plant−1) was recorded for the plants grown under 100 mM salinity, 0 mM Si and NO application at 28 days interval.
Decreased dry biomass of plants under salt stress have been attributed to the disruption of osmotic balance of NaCl ions taken in excess to the plant body and the decrease in metabolic activity (Giuffrida et al., 2009). The studies on tomatoes reported that salt stress reduce plant dry weight (Aksoy, 2019). Furthermore, it has been reported that salt stress reduced total plant dry biomass of tomato and lamb's lettuce (Valerianella locusta L.) grown in soilless culture, while Si application alleviated the negative effects salt stress by increasing plant dry weight (Oztekin et al., 2017; Oztekin and Tutal, 2021).
Celik and Eraslan (2015) applied NO to corn plant grown under salt stress and reported that salt stress and NO applications reduced the dry weight of the plant. On the other hand, Kausar et al. (2013) stated that NO applied to wheat plant under salt stress has a positive effect on plant growth and yield. The findings obtained in our study and the results of the earlier studies are in line with each other.
3.3 Physiological and biochemical traits
The individual effects of salinity stress, Si and NO application significantly altered chlorophyll index. Salinity stress and Si applications decreased chlorophyll index, whereas NO applications increased it compared to control plants. Increasing interval of NO application increased chlorophyll index.
The interactive effect of salinity stress, Si and NO application was non-significant for chlorophyll index (Fig. 5). Salt stress decreased chlorophyll index. Silicon application in salt-free environment decreased chlorophyll index, while increased it under saline environment. Application of NO at 7 and 14-day intervals in salt-free conditions increased chlorophyll index, while combined application of Si and NO under salt stress decreased chlorophyll index compared to control.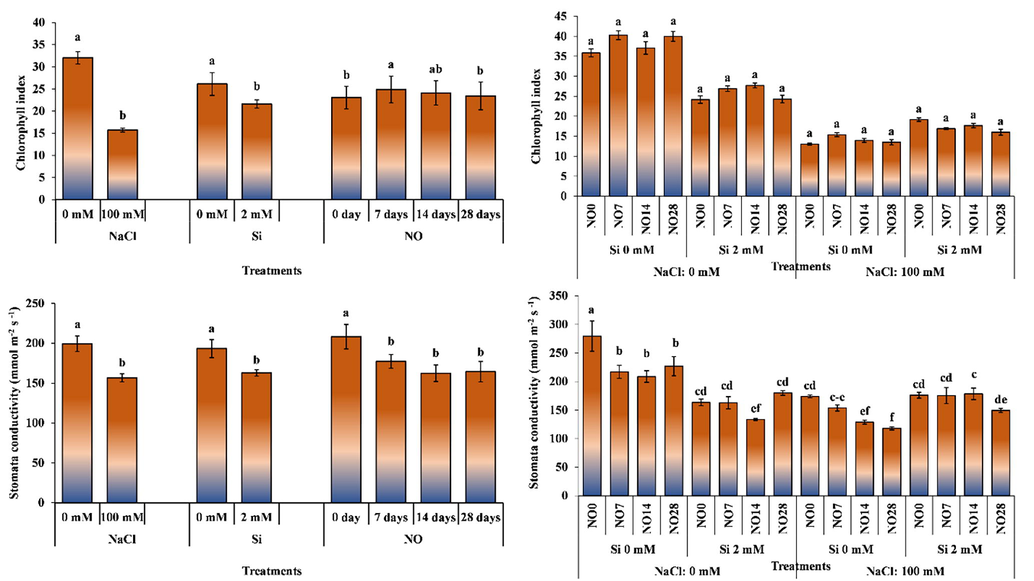
The impact of individual and interactive effects of Si and NO applications on chlorophyll index and stomatal conductance of pepper grown under salinity stress. The means having different letters are statistically different from each other (p < 0.05) (n = 4). LSD for chlorophyll index: NaCl = 1.133, Si = 1.133, NO = 1.192, NaCl × Si × NO = non-significant, LSD for stomatal conductance: NaCl = 11.564, Si = 11.564, NO = 18.20, NaCl × Si × NO = 27.08.
The highest chlorophyll index (40.27) was recorded for the plants grown under 0 mM salinity and Si application, and NO application 7-day intervals, while the lowest value (13.03) was obtained for the plants grown under 100 mM salinity, 0 mM Si and NO application at 0-day interval. It has been reported that chlorophyll and photosynthetic pigments are damaged and the amount of chlorophyll decreases under salt stress (Kusvuran et al., 2008; Izci, 2009; Demirel et al., 2012). Increasing salt concentration due to irrigation water salinity causes a decrease in the amount of chlorophyll (Kaya et al., 2007; Kusvuran et al., 2008). Celik and Eraslan (2015) reported that SNP applied to maize plant under salt stress increased chlorophyll. Similarly, Hajihashemi et al. (2021) reported that SNP application eliminated several salt-induced adverse effects on tissue structure and increased photosynthetic pigments, carbohydrate and protein content, and antioxidant activity, and ROS accumulation and lipid peroxidation under salt stress. Rohanipoor et al. (2013) reported that salt stress decreased the amount of chlorophyll in maize grown under salt stress; however, Si application increased it.
Salinity stress, Si and NO application had significant effect on stomatal conductance (Fig. 5). Salinity stress and application of Si and NO decreased stomatal conductance. The interactive effect of salinity, Si and NO application was also significant for stomatal conductance (Fig. 5). The highest stomatal conductance (319.50 mmol m−2 s−1) was noted for the plants grown under non-saline environments, 0 mM Si application and NO application at 0 days interval (control). The stomatal conductance decreased under salt stress. Silicon applied in salt-free environment decreased stomatal conductance, while increased it under salt stress. Nitric oxide applications generally decreased stomatal conductance. Stomatal conductance decreased with increasing interval of NO application.
Plants experience physiological drought under salinity stress due to high osmotic pressure of soil water and close their stomata to reduce plant water loss. Stomata closure prevent the entry of CO2 into plant structure, which negatively affect photosynthesis, development, and productivity of plants. Deveci and Tuğrul (2017) investigated the effect of salt stress on leaf physiological properties in spinach and reported that stomata were closed in response to salinity. Avcu et al. (2013) stated that Si applied to plants reduces stomatal conductance. Celik and Eraslan (2015) indicated that stomatal resistance of maize plants increased with NO application under salt stress. The results reported by the researchers are similar to the findings of current study.
Membrane permeability was significantly affected by salinity, while Si and NO had non-significant effect in this regard. Membrane permeability of plants grown under salinity-free environments was 44.45%, whereas it increased to 54.88% in the plants grown under salt stress. Silicon and NO applications had no effect on membrane permeability; however, NO application at 7-day intervals slightly increased membrane permeability.
The interactive effect of salinity, Si and NO applications significantly altered membrane permeability (Fig. 6) Salt stress increased membrane permeability. Silicon applied in a salt-free environment decreased membrane permeability; however, did not cause any change in membrane permeability under salt stress. The highest value of membrane permeability (57%) was recorded for the plants grown under 100 mM salinity, 2 mM Si and NO application at 7-day intervals, while the lowest value (37%) was noted under 0 mM salinity, 2 mM Si and NO application at 28 days interval.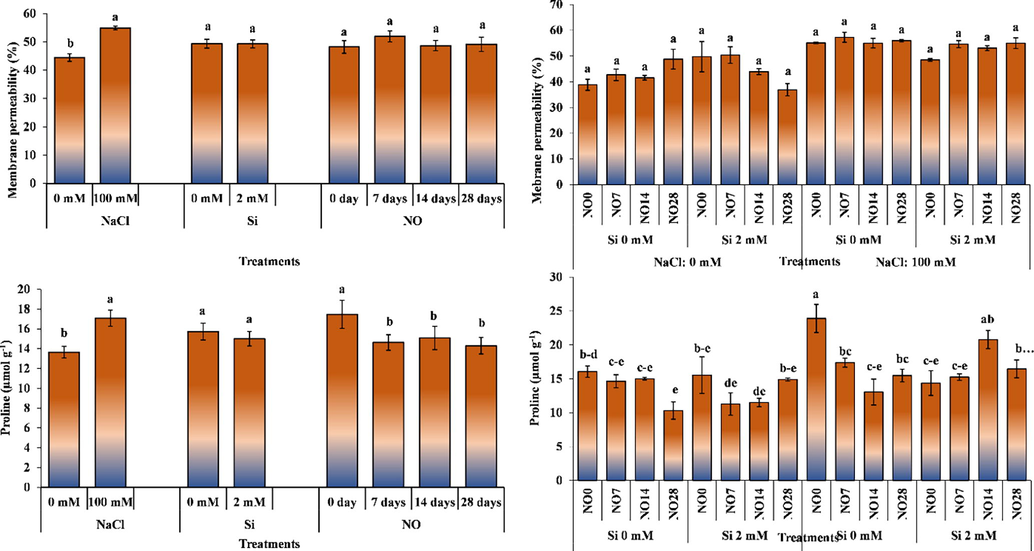
The impact of individual and interactive effects of Si and NO applications on membrane permeability and proline contents of pepper grown under salinity stress. The means having different letters are statistically different from each other (p < 0.05) (n = 4). LSD for membrane permeability: NaCl = 0.003, Si = non-significant, NO = non-significant, NaCl × Si × NO = non-significant, LSD for proline contents: NaCl = 1.879, Si = non-significant, NO = 1.977, NaCl × Si × NO = 5.316.
Kaya and Higgs (2003) noted that salt stress applied to pepper plants disrupted cell structure. Bora (2015) reported that salt stress increased membrane permeability in pepper. Kaya and Dasgan (2013) reported increased membrane permeability in beans, and Alkhatib et al. (2021) in eggplant. The results obtained in current study are in harmony with these earlier studies.
Proline content was significantly affected by salt stress and NO application, while Si had non-significant effect (Fig. 6). Salt stress increased the proline content, while NO application decreased it. Si applications reduced the proline content; however, the reduction was non-significant.
The interactive effect of salt stress, Si and NO application was significant. The NO application under 0 mM salinity and Si decreased the proline content. While NO decreased proline content in plants grown under 100 mM salt and 0 mM Si, it increased it in plants receiving 2 mM Si.
Various researchers working on different plants have reported that salt stress increases leaf proline content (Celik and Eraslan, 2015). Kirecci and Yurekli (2019) observed that NaCl and NO applications in sunflower increased proline content compared to control treatment. The results of our study show similarities with the findings of earlier studies.
3.4 Marketable yield
Salt stress and NO application significantly altered marketable yield; however, Si application had non-significant effect in this regard (Fig. 7). Marketable yields significantly decreased in salt-treated plants. The application of NO every 14 days increased marketable yield, while its application every 28 days was in the same statistical group as of control plants.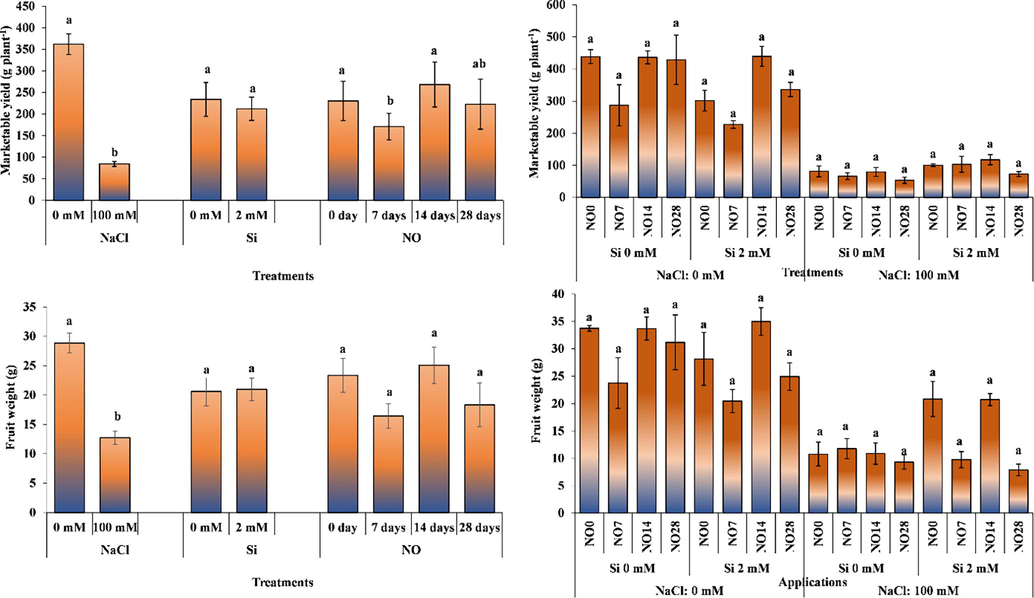
The impact of individual and interactive effects of Si and NO applications on marketable yield and fruit weight of pepper grown under salinity stress. The means having different letters are statistically different from each other (p < 0.05) (n = 4). LSD for marketable yield: NaCl = 58.56, Si = non-significant, NO = 61.60, NaCl × Si × NO = non-significant, LSD for fruit weight: NaCl = 4.471, Si = non-significant, NO = non-significant, NaCl × Si × NO = non-significant.
The interactive effect of salt stress, Si and NO application remained non-significant for on marketable yield (Fig. 7). The Si and NO application increased marketable yield under salinity stress in a non-significant manner. Silicon application decrease yield of the plants grown under non-saline environment, while it increased marketable yield under salt stress. While the highest marketable yield was obtained from 2 mM Si and NO application at 14 days interval under salt stress, the lowest marketable yield was obtained from the plants grown under 0 mM Si and NO application at 28 days interval. The reason of low marketable yield than average is due to the short trial period.
Salt stress not only hindered plant growth but also negatively affected yield. Salt stress disrupts osmotic and ionic balance at the cell level, inhibits photosynthesis, damage photosynthesis cell metabolism; thus, resulting in abnormal plant growth. Doğru and Canavar (2020) reported that salinity reduced plant growth by decreasing photosynthetic pigments that influence photosynthetic activity. Similarly, Reina-Sanchez et al. (2005), Flores et al. (2010), Soylemez and Pakyurek (2017) and Aksoy (2019) reported that salt stress reduces marketable yield. In our study, Si and NO applied under saline conditions alleviated the effect of salt stress by increasing stomatal conductance and increased marketable yield by positively affecting photosynthesis.
Silicon and NO did not affect mean fruit weight; however, salt stress significantly altered it. Salt stress decreased average fruit weight. Silicon applications increased mean fruit weigh, but it was non-significant. Application of NO at 14-day interval increased mean fruit weight compared to control plants, while application of NO at 7-day and 28-day intervals decreased it.
Three-way interaction of salt stress, Si and NO application has non-significant effect o on mean fruit weight (Fig. 7). Plants grown under 0 mM salinity produced heavier fruits than the plants subjected to 100 mM salinity. Silicon application decreased mean fruit weight in under non-saline environments, it increased in fruit weight in the plants subjected to salt stress. The effect of NO applications on average fruit weight fluctuated, and generally, application every 14 days gave better results. The highest average fruit weight (33.73 g) was recorded for the plants grown under 0 mM salinity, 2 mM Si and NO application at 14 days interval. The lowest (7.88 g) fruit weight was recorded for the plants grown under 100 mM salinity, 2 mM Si, NO application at 28 days interval.
Soylemez and Pakyurek (2017) reported that salt stress reduced average fruit weight of tomato. Again, in another study on tomato, salt stress reduced fruit weight by 73.5% (Ali and Ismail, 2014). Salt stress in eggplant plant have a reducing effect on fruit weight (Talhouni et al., 2017). The results obtained by the researchers and the results of the current study are similar.
4 Conclusion
The results revealed that salinity stress significantly decreased plant growth parameters, yield characteristics, chlorophyll index and stomatal conductance. Although the beneficial effects of Si and NO varied according to different studied parameters to alleviate the adverse effects of salinity, their individual effects exerted positive impacts in general. However, simultaneous use of Si and NO had negative effect on the studied traits under salt stress. Silicon application weekly and NO application at 14-days interval could increase marketable yield under saline conditions. It is recommended that future works should be concentrated on determining the impact of Si and NO on growth and yield parameters by applying these during different growth phases to find a suitable and optimum combination. Furthermore, different doses of NO should also be tested to find the optimum dose for increasing growth and yield of pepper under salinity stress.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Synergistic effects of nitric oxide and silicon on promoting plant growth, oxidative stress tolerance and reduction of arsenic uptake in Brassica juncea. Chemosphere. 2021;262:128384
- [CrossRef] [Google Scholar]
- Response of eggplant varieties (Solanum melongena) to salinity in germination and seedling stages. N. Z. J. Crop Hortic. Sci.. 2004;32(2):193-200.
- [CrossRef] [Google Scholar]
- Photosynthetic and ultrastructural properties of eggplant (Solanum melongena) under salinity stress. Horticulturae. 2021;7(7):181.
- [CrossRef] [Google Scholar]
- Akay Rastgeldi, Z.H., Pakyürek A.Y., Söylemez S., 2014. Effect of different salt concentration on some plant growth parameters and content of Na, K, Ca and Mg in pepper. 10. Vegetable Agriculture Symposium, 2-4 September 2014. Namık Kemal University, Agricultural Faculty, Tekirdağ/Turkey, p: 226-232 (in Turkish).
- Aksoy, E., 2019. The effects of humic acid on plant development, yield, some quality and biochemical properties on tomato grown in salty conditions. Ms. Thesis Harran University, Graduate School of Natural and Applied Sciences, Department of Horticulture, Sanlıurfa-Turkey, p:102 (in Turkish). https://tez.yok.gov.tr/UlusalTezMerkezi/tezDetay.jsp?id=UK06K7CFBEh8Zcy_nC13Fw&no=tqNO7bl2bYjP8oRmu9f90g.
- Effect of salt treatment on seed germination and sprout of quality in vegetable soybean sprout growing (Glycine max L.) Yuzuncu Yıl Univ. J. Agric. Sci.. 2013;23(3):236-241. in Turkish
- [Google Scholar]
- Tomato fruit quality as influenced by salinity and nitric oxide. Turk. J. Bot.. 2014;38:122-129.
- [CrossRef] [Google Scholar]
- Effects of selenium and silicon on salt-stressed tomato. Tarım Bilimleri Arastırma Dergisi. 2013;6(1):183-188. in Turkish
- [Google Scholar]
- Changes of the root parameters of pepper plant Capsicum annuum L. grown at different media. J. Agric. Sci.. 1996;2(02):1-4. in Turkish
- [CrossRef] [Google Scholar]
- Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205-207.
- [CrossRef] [Google Scholar]
- Nitric Oxide in plants: The history is just beginning. Plant Cell. Environ. 2001;24:267-278.
- [CrossRef] [Google Scholar]
- Role of reactive oxygen species and NO in plant defence responses. Curr. Opin. Plant Biol.. 1999;2:287-294.
- [CrossRef] [Google Scholar]
- Bora, M., 2015. Determination of physiological, morphological and chemical changes occurred by the application of various salt concentration in different vegetation periods in pepper. Ms. Thesis, Namık Kemal University, Graduate School of Natural and Applied Sciences, Tekirdağ-Turkey, p:92. https://tez.yok.gov.tr/UlusalTezMerkezi/TezGoster?key=sY7m19PfcL6F1NUw-cr80OKPrUKDLoRP_56IMdO4dSCbr0jHwrZJQ7VlJuNQENHe.
- ABA induced NO generation and stomatal closure in arabidopsis are dependent on H2O2 synthesis. Plant J.. 2006;45:113-122.
- [CrossRef] [Google Scholar]
- Cengiz, A., 2017. Determination of the relationship between drought stress and nitric oxide in pepper plant. Ms. Thesis, Yuzuncu Yıl university, Graduate School of Natural and Applied Sciences,Van-Turkey, p:54. https://tez.yok.gov.tr/UlusalTezMerkezi/TezGoster?key=RrI-Krk3A-RkF4YfHofukwpBTskIJ94B3M9gGBx7H5Q7_RqIVLobvirlyevjMBIK.
- Response of two greenhouse pepper hybrids to NaCl salinity during different growth stages. Sci. Horticult.. 2000;86:247-260.
- [CrossRef] [Google Scholar]
- How does silicon mediate plant water uptake and loss under water deficiency? Front. Plant Sci.. 2018;9:281.
- [CrossRef] [Google Scholar]
- Silica in plants: biological, biochemical and chemical studies. Ann. Bot.. 2007;100:1383-1389.
- [CrossRef] [Google Scholar]
- Effects of exogenous nitric oxide on mineral nutrition and some physiological parameters of maize grown under salinity stress. Suleyman Demirel Universitesi Ziraat Fakultesi Dergisi. 2015;10(1):55-64.
- [Google Scholar]
- Cokuysal, B., Anac D., Eryuce, N., Colak Esetlili, B., Ozkan, C.F., Tepecik, M., 2016. Topraksız tarım ve bitki besleme teknikleri. Editor: Dilek ANAC. Nobel Yayıncılık, publication No:0391, Ankara.
- Determination of screening techniques to salinity tolerance in tomatoes and investigation of genotype responses. Plant Sci.. 2002;163:695-703.
- [CrossRef] [Google Scholar]
- NaCl stress effects on enzymes involved in nitrogen assimilation pathway in tomato “Lycopersicon esculentum” seedling. J. Plant Physiol.. 2006;163:1247-1258.
- [CrossRef] [Google Scholar]
- Nitric oxide and nitric oxide synthase activity in plants. Phytochemistry. 2004;65(7):783-792.
- [CrossRef] [Google Scholar]
- Demir, S., 2009. Investigation on salt tolerance of the local melon populations (Koçhisar kavunu) grown in Salt Lake region. Ms. Thesis, Ankara University, Graduate School of Natural and Applied Sciences,, Ankara-Turkey, p:96 (in Turkish). https://tez.yok.gov.tr/UlusalTezMerkezi/TezGoster?key=NtBAevXNhYaNqJFoAcdBdjl5IUgsVA7diLZKQr6U1oN6TXNpUGxmTOxfcoXDoV3Q.
- Estimation of growth curve parameters for pepper (Capsicum annuum cv. kapija) under deficit irrigation conditions. J. Agric. Faculty Ege Univ.. 2012;49(1):37-43.
- [Google Scholar]
- Deveci, M., Tuğrul, B., 2017. The effect of salt stress on spinach leaf physiological characteristics. Akademik Ziraat Dergisi, 6:89-98 (in Turkish). https://dergipark.org.tr/en/download/article-file/378324
- Physiological and biochemical components of salt tolerance in plants. Academic Platform – J. Eng. Sci.. 2020;8(1):155-174.
- [CrossRef] [Google Scholar]
- Easy leaf area: Automated digital image analysis for rapid and accurate measurement of leaf area. Appl. Plant Sci.. 2014;2(7):1400033.
- [CrossRef] [Google Scholar]
- Silicon and plant disease resistance against pathogenic fungi. FEMS Microbiol. Lett.. 2005;249:1-6.
- [CrossRef] [Google Scholar]
- Impact of abiotic stresses on grain composition and quality in food legumes. J. Agric. Food Chem.. 2018;66:8887-8897.
- [CrossRef] [Google Scholar]
- Plant Nutrients and Abiotic Stress Tolerance. Singapore: Springer Singapore; 2018. p. :391-413.
- Range expansion potential of two co-occurring invasive vines to marginal habitats in Turkey. Acta Oeocol.. 2017;84:23-33.
- [CrossRef] [Google Scholar]
- Differential effects of nitric oxide on peroxidase and H2O2 production by the xylem of zinnia elegans. Plant Cell Environ. J.. 1999;22:891-897.
- [CrossRef] [Google Scholar]
- The efectiveness of grafting to improve tomato fruit quality. Sci. Hortic.. 2010;125:211-217.
- [CrossRef] [Google Scholar]
- Nitric oxide regulates K+ and Cl channels in guard cells through a subset of abscisic acid-evoked signaling pathways. Proc. Natl. Acad. Sci.. 2003;100:11116-111121.
- [CrossRef] [Google Scholar]
- Abscisic acid (ABA) inhibits light-induced stomatal opening through calcium- and nitric oxide-mediated signaling pathways. Nitric Oxide. 2007;17:143-151.
- [CrossRef] [Google Scholar]
- Transpiration and stomatal conductance in a young secondary tropical montane forest: Contrasts between native trees and invasive understorey shrubs. Tree Physiol.. 2018;38(7):1053-1070.
- [Google Scholar]
- How sodium chloride concentration in the nutrient solution influences the mineral composition of tomato leaves and fruits. HortScience. 2009;44(3):707-711.
- [CrossRef] [Google Scholar]
- Identification of a plant nitric oxide synthase gene involved in hormonal signaling. Science. 2003;302:100-103.
- [CrossRef] [Google Scholar]
- Ijaz, M., Sattar, A., Sher, A., Ul-Allah, S., Mansha, M.Z., Khan, K.A., Shahzad, M.A., Al-Sadi, A.M., Arif, M., Aljuaid, B.S., El-Shehawi, A.M., Farooq, S., 2021. Sulfur application combined with planomicrobium sp. Strain MSSA-10 and farmyard manure biochar helps in the management of charcoal rot disease in sunflower (Helianthus annuus L.). Sustain. https://doi.org/10.3390/su13158535.
- Cross-talk between nitric oxide, hydrogen peroxide and calcium in salt-stressed Chenopodium quinoa Willd. At seed germination stage. Plant Physiol. Biochem.. 2020;154:657-664.
- [CrossRef] [Google Scholar]
- Effect of sodium nitroprusside on physiological and anatomical features of salt-stressed Raphanus sativus. Plant Physiol. Biochem.. 2021;169:160-170.
- [CrossRef] [Google Scholar]
- Effects of salinity stress on growth, chlorophyll content and osmotic components of two basil (Ocimum basilicum L.) genotypes. Afr. J. Biotechnol.. 2012;11(2):379-384.
- [CrossRef] [Google Scholar]
- In vitro effect of different salt concentration on photosynthetic pigments from cotton (G. hirsutum L.) Alinteri Zirai Bilimler Dergisi. 2009;17:7-13. in Turkish
- [Google Scholar]
- Salt tolerance in crop plants. Ataturk Univ. J. Agric. Facult.. 1998;29(1):163-174. in Turkish
- [Google Scholar]
- Protective role of foliar applied nitric oxide in Triticum Aestivum under saline stress. Turk. J. Bot.. 2013;37:1155-1165.
- [Google Scholar]
- Screening of the bean genotypes for their tolerans to salinity and drought stresses at the early plant growth phase. C.U Fen ve Muhendislik Bilimleri Dergisi. 2013;29(2):39-48. in Turkish
- [Google Scholar]
- Supplementary KNO3 improves salt tolerance in bell pepper plants. J. Plant Nutr.. 2003;26(7):1367-1382.
- [CrossRef] [Google Scholar]
- Improved salt tolerence of melon by the addition of proline and potassium nitrate. Environ. Exp. Bot.. 2007;60:397-403.
- [CrossRef] [Google Scholar]
- Silicon and salinity: Crosstalk in crop-mediated stress tolerance mechanisms. Front. Plant Sci.. 2019;10:1429.
- [CrossRef] [Google Scholar]
- Change in physiological and biochemical parameters under drought stress in salt-tolerant and salt-susceptible eggplant genotypes. Turk. J. Agric. For.. 2019;43(6):593-602.
- [CrossRef] [Google Scholar]
- The effects of salt stress, nitric oxide and hormone applications on antioxidant defense in sunflower plant leaves. KSU J. Agric Nat. 2019;22(3):360-369. in Turkish
- [CrossRef] [Google Scholar]
- Kusvuran, S., Dasgan, H.Y., Abak, K., 2008. Farklı bamya genotiplerinin kuraklık stresine tepkileri. VII. Vegetable Agriculture Symposium, 26-29 Agust, Yalova-Turkey (in Turkish).
- Nitric oxide: The versatility of an extensive signal molecule. Annu. Rev. Plant Biol.. 2003;54(1):109-136.
- [Google Scholar]
- Responses and adaptations of crops to salinity. Acta Horticult.. 1986;190:243-246.
- [CrossRef] [Google Scholar]
- Combined application of silicon and nitric oxide jointly alleviated cadmium accumulation and toxicity in maize. J. Hazard. Mater.. 2020;395:122679
- [CrossRef] [Google Scholar]
- Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Sci. Plant Nutr.. 2004;50(1):11-18.
- [CrossRef] [Google Scholar]
- Nitric oxide regulates plant responses to drought, salinity, and heavy metal stress. Environ. Exp. Bot.. 2019;161:120-133.
- [CrossRef] [Google Scholar]
- Higher tolerance to abiotic stresses and soil types may accelerate common ragweed (Ambrosia artemisiifolia) invasion. Weed Sci.. 2017;65:115-127.
- [CrossRef] [Google Scholar]
- Grafting improves salinity tolerance of bell pepper plants during greenhouse production. Hortic. Environ. Biotechnol.. 2021;62(6):831-844.
- [CrossRef] [Google Scholar]
- Oztekin, G.B., Tuzel, Y., Tuzel, I.H., 2017. Effects of silicon to salinity stress on soilless tomato grown in greenhouse. Akademik Ziraat Dergisi, 6:243-256 (in Turkish). https://dergipark.org.tr/en/download/article-file/378599.
- Effects of silicon addition to nutrient solution aganist salinity stress on Lam’s lettuce (Valerianella locusta (L.) Laterr) production. Erciyes Univ. J. Inst. Sci. Technol.. 2021;37(1):36-46. in Turkish
- [Google Scholar]
- Nitric oxide induces cell death in taxus cells. Plant Sci.. 2000;157:173-180.
- [CrossRef] [Google Scholar]
- Cell membrane stability, an indicator of drought tolerance, as affected by applied nitrogen in soybean. J. Agric. Sci.. 1990;115:63.
- [Google Scholar]
- Does silicon really matter for the photosynthetic machinery in plants? Plant Physiol. Biochem.. 2021;169:40-48.
- [CrossRef] [Google Scholar]
- Plant water uptake and water use efficiency of greenhouse tomato cultivars irrigated with saline water. Agric. Water Manag.. 2005;78:54-66.
- [CrossRef] [Google Scholar]
- Effect of silicon on some physiological properties of maize (Zea mays) under salt stress. J. Biol. Environ. Sci.. 2013;7(20):71-79.
- [Google Scholar]
- Involvement of nitric oxide in the mechanism for stomatal opening in Vicia faba leaves. Biol Plant. 2003;46:117-119.
- [Google Scholar]
- Effect of the plant activator on some physiological characteristics and total protein content of tomato plants under salt stress. J. Agric. Faculty Ege Univ.. 2005;42(1):85-95. in Turkish
- [Google Scholar]
- Enhancement of salt tolerance in watermelon using grafting. Arab Univ. J. Agric. Sci.. 2018;26(1):327-335.
- [CrossRef] [Google Scholar]
- Effect of rice hull ash silicon on rice seedling growth. J. Plant Nutr.. 1997;20(1):195-201.
- [CrossRef] [Google Scholar]
- Responses of rootstocks to nutrient induced high EC levels on yield and fruit quality of grafted tomato cultivars in greenhouse conditions. Appl. Ecol. Environ. Res.. 2017;15(3):759-770.
- [Google Scholar]
- Promotive effects of epibrassinolide on plant growth, fruit yield, antioxidant, and mineral nutrition of saline stressed tomato plants. Pak. J. Bot.. 2017;49(5):1655-1661.
- [Google Scholar]
- The effect of waterpad polymer application on yield and some quality properties of cucumber under restricted irrigation conditions. Turk. J. Agric. Nat. Sci.. 2020;7(4):1031-1042. in Turkish
- [CrossRef] [Google Scholar]
- Takahashi, E., Ma, J.F., Miyake, Y., 1990. The possibility of silicon as an essential element for higher plants. Comments on agricultural and food chemistry 2:99-122. https://worldveg.tind.io/record/19607/files/d013366.pdf
- Talhouni, M., Sonmez, K., Ellialtioğlu, S., Kusvuran, S. 2017. Analysis of some plant and fruit characteristics of grafted eggplants grown under salinity stress. Akademik Ziraat Dergisi, 6:71-80 (in Turkish). https://dergipark.org.tr/en/download/article-file/378318
- Nitric oxide: comparative synthesis and signalling in animal and plant cells. Trends Plant Science. 2001;6:177-183.
- [CrossRef] [Google Scholar]
- The effects of Ca, K and Mg on the stress parameters of the maize (Zea mays L.) plant under salinity stress. Akdeniz University Journal of the Faculty of. Agriculture. 2006;19(1):59-67. in Turkish
- [Google Scholar]
- The effect of irrigation water salinity on some yield parameters of pepper (Capsicum annuum) during different growing periods. J. Agric. Sci.. 1996;2(2):5-10. in Turkish
- [CrossRef] [Google Scholar]







