Translate this page into:
Effects of hydrocarbon contamination on soil microbial community and enzyme activity
*Corresponding author salrumman@kku.edu.sa (Sulaiman A. Alrumman)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 31 October 2014
Peer review under responsibility of King Saud University.
Abstract
Soil enzymatic activities and microbial biomass carbon (Cmic) are considered to be two important soil biological activities influenced by oil contamination occurring in the soil ecosystem. This study focused on changes in the soil microbial community enzymatic activities as a result of the potential inhibitory effects of hydrocarbon contamination. The relationship between hydrocarbons (kerosene and diesel), Cmic and enzymatic activity (dehydrogenase and phosphatase) was evaluated in three amended soil types collected from different areas (Fresh Boyndie, Insch and Brechin) in Aberdeenshire (UK). Results showed that hydrocarbon contamination inhibited enzymatic activities in all the amended soil samples. The extent of inhibition increased significantly with increasing levels of hydrocarbons, and varied with the incubation period. Insch soil had high Cmic values and high numbers of heterotrophic bacteria CFU, but it had the lowest dehydrogenase and phosphatase activities of all three soils. Brechin soils had the highest phosphatase activity. Results also showed that both Insch and Brechin soils had similar numbers of culturable hydrocarbon degrader bacteria across all soil treatments with the exception of kerosene treatments, while Brechin soils had the highest culturable numbers of hydrocarbon degrading fungi across all three soil treatments with the exception of incubated control and kerosene treatments. There were generally strong positive relationships in non-treated samples between bacterial heterotrophs and hydrocarbon degrading bacteria in all three soils. Both incubated Insch and Brechin soil treatments exhibited a strong correlation between fungal heterotrophs and hydrocarbon degraders. However, non-incubated Insch and Brechin soils had a weak relationship between fungal heterotrophs and degrading fungi. Hydrocarbons in soils provide a source of carbon for microbial growth and this helps to explain the high variation in fungal data between soils which may be associated with different microbial communities in each soil.
Keywords
Enzyme activity
Hydrocarbons
Microbial community
Soil contamination
1 Introduction
Petroleum oils are used in large quantities as fuels (Bierkens and Geerts, 2014). Petroleum hydrocarbons are becoming a global problem for the environment. They are highly persistent in the environment, toxic and present significant health risks to human (Hentati et al., 2013). The use of indigenous microorganisms in bioremediation processes can reduce the risks associated with hydrocarbon contaminated soils (Suja et al., 2014).
Soil biological activity, including soil microbial biomass and enzymatic activity, is influenced by a range of physicochemical, environmental parameters and perturbations (Labud et al., 2007). Therefore, soil microbial activity is commonly used to assess disturbed soil.
Consideration of soil Cmic was largely neglected until the mid 1970s. Several methods have been developed to quantify soil Cmic and these methods can be broadly divided into direct or indirect methods (Winding et al., 2005; Kaschuk et al., 2010). Examples of direct methods are microscopy or use of culture media and enumeration of cultures. Indirect methods comprise fumigation extraction (Jenkinson and Powlson, 1976) and substrate induced respiration (Anderson and Domsch, 1978). Indirect methods are more rapid, cost effective and easier to apply than direct methods.
Chloroform (ethanol free) fumigation is the most commonly used indirect method (Winding et al., 2005). Chloroform vapour lyses cells of living soil microorganisms (Jenkinson and Powlson, 1976; Vance et al., 1987; Winding et al., 2005), without affecting the non-living fraction of organic matter. Non-fumigated and fumigated soils are subsequently compared to estimate the size of the freshly lysed biomass. The carbon released by the chloroform is immediately measured either as respired CO2 over a specified period of incubation or by direct extraction of soil with saline solution (i.e. 0.5 M K2SO4). These techniques are known as the chloroform fumigation incubation method (CFI) and the chloroform fumigation direct extraction method (CFE), respectively (Jenkinson and Powlson, 1976; Vance et al., 1987; Winding et al., 2005).
A consideration of the kec-factor (the extractable component of Cmic after fumigation) is required in the fumigation extraction method to convert the flush of microbial biomass carbon mineralised to CO2 over an incubation period. This factor can be estimated, for example, by the addition of a known amount of microbial C to the soil and measuring the amount mineralised after incubation. The use of Kec-factor is controversial, however, common values of kec are 0.45 (Vance et al., 1987), 0.41 (Anderson and Domsch, 1978) and 0.33 depending on intrinsic soil properties such as pH and organic matter content.
If soil quality is to be defined according to the presence and activity of soil microbial populations, then an appropriate technique must be selected. Plate count methods have been used to monitor bioremediation processes (Margesin et al., 2000; Alamri, 2006). It has been reported that the plate count method is a reliable and sensitive technique compared to other methods (e.g., Most Probable Number) used for assessing the potential of biodegradation (Cassidy et al., 2000; Alamri, 2006). Number and species of total microbial population present in soil can be quantified by colony enumeration on selective media. More specific information can be collected by using mineral salt medium (MSM) with a suitable hydrocarbon as a carbon source (Balba et al., 1998).
Soil enzyme activities have been considered as parameters to provide a biological assessment of soil function. Several of the soil enzyme activities have been proposed for evaluating and monitoring remediation of hydrocarbon contaminated soil. In this study, enzyme activity of dehydrogenase and phosphatase will be considered as indicators for assessing recovery of hydrocarbon impacted soils.
Dehydrogenase activity (DHA) has been proposed as a sensitive indicator for evaluating microbial oxidative activity in soils (Turgay et al., 2010; Serrano et al., 2009; Dawson et al., 2007). Quantification of DHA in soil is made by measuring the amount of an artificial electron acceptor reduced by the microbial activity. Compounds such as soluble tetrazolium salts are reduced to red coloured formazans, which can be extracted and measured colorimetrically (Camiña et al., 1998; Shaw and Burns, 2006).
Phosphatase activity (PA) is important to soil P cycling. PA is sensitive to environmental perturbations, thus may be a suitable selection for inclusion in a soil quality index (Amador et al., 1997; Turgay et al., 2010). PA is frequently measured by quantifying the transformation of p-nitrophenyl phosphate. The yellow product of PA (p-nitrophenol) can be quantified colorimetrically (Tabatabai and Bremner, 1969; Shaw and Burns, 2006). Soil quality can be determined according to the presence and activity of soil microbial populations whereas, the enzyme activities have been considered as parameters for evaluating and monitoring remediation of hydrocarbon contaminated soil.
The conceptual model of this study hypothesised different trajectories of response variables to a given time point. This point in time represents soil recovery. Soil recovery itself is a composite of variables and processes, which individually may follow different trajectories: (1) Functions in control soils remain steady. (2) The addition of hydrocarbons causes an increase in a given response variable against control soil, or the addition of hydrocarbons may cause a decrease in a given response variable initially. However, the given response variable coincides with control at the end of incubation. (3) The addition of hydrocarbons causes a decrease in a given response variable against control soil. The main objective therefore of this study is to evaluate the effect of hydrocarbon (diesel and kerosene) contamination on soil microbial activity, in freshly contaminated and incubated soils. Assays that constitute this comprise soil Cmic, culturable counts and soil enzyme activities.
2 Materials and methods
2.1 Determination of soil characteristics
Fresh Boyndie, Insch and Brechin soils were collected in Aberdeenshire (UK) and sieved (2 mm) to remove stones and large root fragments.
The physical–chemical properties of the soils studied were determined by standard methods generally used in chemical-soil laboratories. The following parameters were also determined for each soil: pH, Total N%, Total C% and EC (μS) (Table 1), and hydrocarbon concentrations (Table 2).
Characteristic
Soils
Boyndie
Insch
Brechin
Mean
S.E
Mean
S.E
Mean
S.E
pH (H2O)
7.57
±0.01
6.12
±0.02
6.55
±0.03
pH (CaCl2)
6.89
±0.03
5.32
±0.04
5.56
±0.00
WHC g g−1
0.24
±0.11
0.36
±0.01
0.29
±0.04
OM%
3.71
±0.03
10.59
±0.06
4.96
±0.03
Electro conductivity μS
108.67
±1.76
79.93
±1.39
89.77
±1.80
Total N%
0.12
±0.01
0.38
±0.03
0.15
±0.00
Total C%
2.03
±0.13
4.94
±0.48
2.26
±0.08
Extractable
N–NO3− mg kg−1
9.96
±0.05
2.92
±0.09
1.37
±0.15
N–NH4+ mg kg−1
0.22
±0.03
3.94
±0.24
0.00
±0.00
P–PO43− mg kg−1
33.33
±0.42
3.78
±0.20
13.63
±0.75
Soil texture
Sand%
83.17
±0.00
64.29
±0.00
69.79
±0.73
Silt%
5.46
±0.80
13.22
±0.70
12.08
±0.74
Clay%
11.37
±0.80
22.49
±0.70
18.13
±0.02
Soil texture class
Loamy sand
Sandy loam
Sandy loam
Soil classification (Type (Association))
Iron podzol (Corby)
Brown Earth (Insch)
Brown Earth (Brechin)
Treatment
Soil hydrocarbon (mg kg−1)
Boyndie
S.E
Insch
S.E.
Brechin
S.E.
C
<100a
±000
<100a
±000
<100a
±000
IC
<100a
±000
<100a
±000
<100a
±000
K
2800b
±100
3700b
±100
2900b
±200
IK
<100a
±000
<100a
±000
<100a
±000
D
10070c
±300
11300c
±300
9700c
±300
ID
700d
±000
400d
±100
300d
±100
2.2 Experimental design
Each individual soil sample was then subdivided into three main treatment groups: an unamended control soil (C), kerosene (1% v/w) amended treatment (K), and diesel (1% v/w) amended treatment (D). Kerosene and diesel were added to the soils by nebulisation and the soils thoroughly mixed to ensure homogenous distribution. Each soil treatment was further subdivided into two sub-samples; one of each was then placed in a cold room at 4 °C for two weeks to equilibrate. These sub-samples were defined as “non-incubated treatments”. Hydrocarbon analysis and biological tests, in triplicate, were sequentially performed immediately on each treatment. The second group of sub-samples was defined as “incubated treatments” (incubated control (IC), incubated kerosene amended treatments (IK) and incubated diesel amended treatments (ID)). The amendment and incubation period conditions were as follows. Soil nutrients for the samples were first determined by measuring the available N and P. Based on the initial concentration of hydrocarbon as C 100%, soils were amended by adding NH4NO3 and KH2PO4 as aqueous solutions to both IK and ID to achieve a final C:N:P ratio of 100:10:2 (Margesin and Schinner, 1997). Additionally, the moisture content (MC%) was adjusted to 65% ± 5 for the incubated treatments. Soils were placed in aluminium containers and incubated in a controlled environment (Sanyo-Weiss Gallenkamp LTD, Loughborough, Leicestershire) for ten weeks at 25 °C, 80% humidity and full darkness. Soils were turned weekly and d.d.H2O was added to maintain them at a water holding capacity (WHC) of 65% ± 5. Hydrocarbon analysis and biological tests, in triplicate, were then sequentially performed on each treatment immediately after the incubation period finished.
2.3 Microbial biomass C (Cmic) and plate counts
Cmic was determined using a fumigation-extraction method as described by Vance et al. (1987), and the culturable microorganism number was estimated using the plate count method for soil total heterotrophs and hydrocarbon degraders according to the procedure described by Jørgensen et al. (2000) and Alamri (2006). All results of Cmic were expressed as mg C 100 g−1 oven dry soil. The numbers of colony forming units (CFU) per 100 μl aliquot were counted where possible.
2.4 Soil enzyme assays
Soil enzyme activities have been considered as parameters to provide a biological assessment of soil function and several of the soil enzyme activities have been proposed for evaluating and monitoring remediation of hydrocarbon contaminated soil. In this study, the activity of the following enzymes was determined, dehydrogenase (Trasar-Cepeda et al., 2000 modified by Dawson et al., 2007), and phosphatase (Tabatabai and Bremner, 1969, modified by Dawson et al., 2007), as indicators for assessing the recovery of hydrocarbon impacted soils.
2.5 Hydrocarbon extraction and GC-FID analysis
Hydrocarbon chemical analysis was modified from that described by Francis-Obika (2004) and Alamri (2006). Briefly, in triplicate, approximately 5 g dry weight of each sample was weighed and mixed with 100 μl of Pristane (C19 H40), as an external standard, and 5 g of anhydrous sodium sulphate (Na2SO4, Fischer). The mixture was then ground and homogenised using a mortar and pestle for about 2 min to remove all free water. The ground samples were transferred into 50 ml centrifuge glass tubes, sealed with a screw cap lined with a PTFE septum and then 25 ml of hexane was added to each sample. Samples were sonicated for 30 min and shaken using an end-over-end shaker for 16 h at 60 rpm and then centrifuged for 30 min at 1200 g and 4 °C.
After that, water partitioning was used to clean-up total HC. Twenty ml of each extract was mixed with 100 ml of d.d.H2O in a separation funnel and then manually shaken for 5 min and allowed to stand for 2 min. The d.d.H2O phase was removed and approximately 20 ml of the hexane phase was collected in fresh Wheaton vials. One g of anhydrous sodium sulphate and 1 g of Cu turnings were added. The Wheaton vials were then shaken for 30 min using an end-over-end shaker at 60 rpm. Finally, the suspension was transferred into fresh Wheaton vials. For GC-FID analysis, 4.9 ml of the hexane extract was transferred to a fresh vial and mixed with 100 μl of squalane, as an internal standard. Two millilitre of the extraction was transferred into GC-vials (2 ml glass vials with PTFE seal, Fisher, UK), which were then analysed using a Gas Chromatograph (CE instruments GC 8000) coupled with a Flame Ionisation Detector (GC-FID), Phenomenex ZB1 capillary column (30 m length, 0.32 mm inside diameter, and 0.5 μm ft film thickness) and an Autosampler AS 8000 1 μl injector. The detector temperature was set as 310 °C and injector temperature at 250 °C. The oven temperature was set at 60 °C for 2 min, increased at 5 °C min−1, held at 110 °C for 1 min, increased at 10 °C min−1 to 300 °C, maintained for 15 min, and with a total running time of 47 min. The final hydrocarbon concentration in each treatment was calculated with reference to internal standards and expressed as a % of the initial addition.
2.6 Statistical analysis
Analysis of variance using one-way and two-way ANOVA was carried out and all significance analyses were performed at P ⩽ 0.05 using Minitab for Windows v. 15™. When the data and residuals were not normally distributed or did not have equal variance after transformation, the non-parametric Kruskal–Wallis test was used. In addition, the relationships between data were examined using Pearson’s correlation coefficient. If data were not normally distributed and could not be transformed, Spearman’s rank was applied.
3 Results
3.1 Soil microbial biomass carbon (Cmic)
Results of soil Cmic for all three soils are presented in Fig. 1. Generally, the highest Cmic was associated with the Insch soil samples which differed significantly (P ⩽ 0.05) from the other two soils (Boyndie and Brechin). The values of Cmic in the Boyndie soil were in the range of 17–27 (mg100 g−1 soil dw). There were no significant differences (P > 0.05) between all Boyndie soil treatments either incubated or non-incubated, with the exception of D and ID. Boyndie ID was approximately 30% lower than D. There was no significant (P > 0.05) effect on Boyndie soil treatments caused by the incubation period or hydrocarbon added. However, the interaction between these two variables had a significant (P ⩽ 0.05) effect on soil treatments.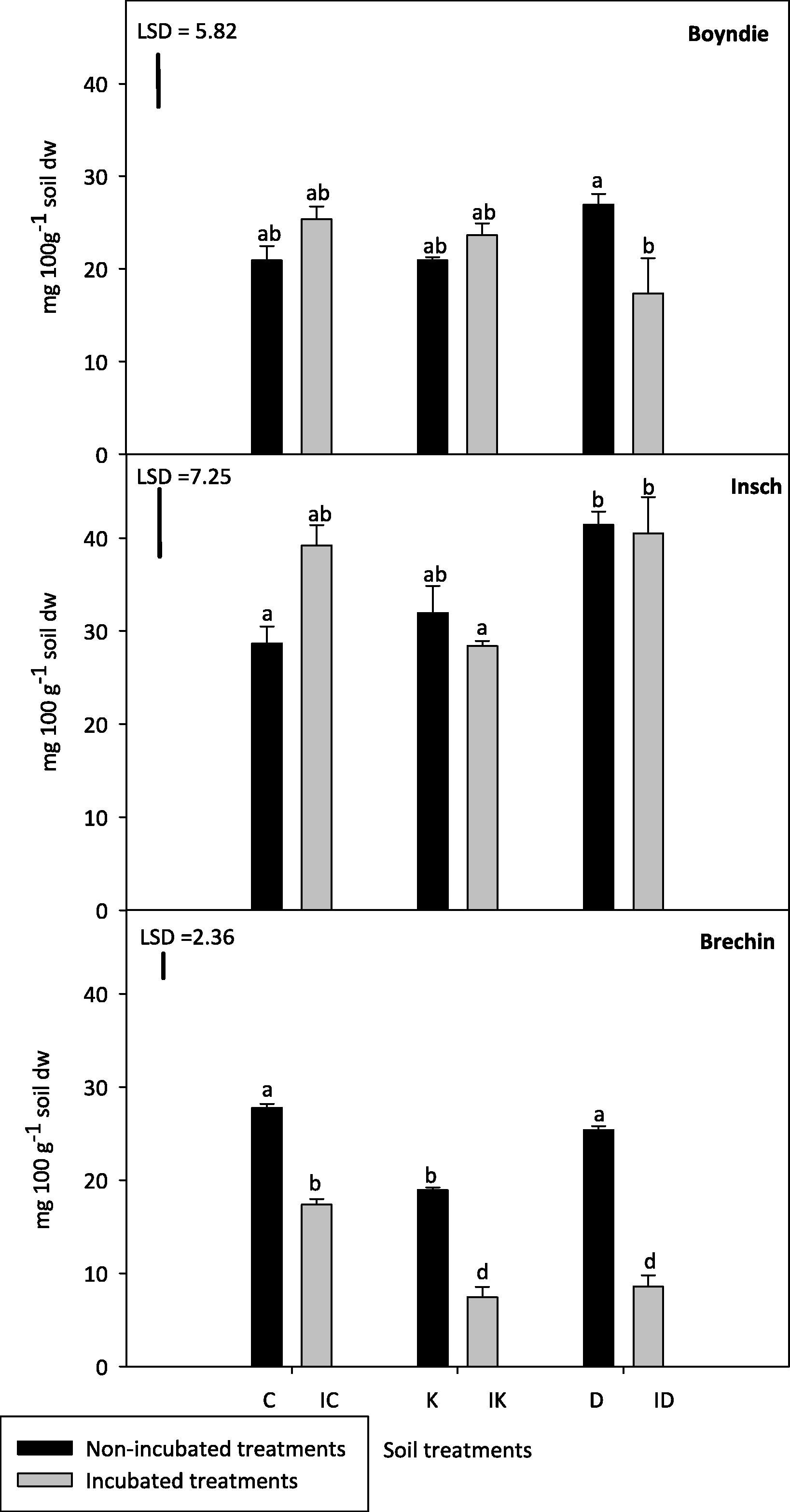
Microbial biomass carbon (Cmic) in the incubated and non-incubated soil treatments. Abbreviations on the x axis: C indicates control, IC indicates incubated control, K indicates kerosene, IK indicates incubated kerosene, D indicates diesel and ID indicates incubated diesel. Different superscript letters show significant differences between treatments for individual soil (One way ANOVA, Tukey test, P ⩽ 0.05). Error bars represent standard error of the mean for n = 3. LSD is the least significant difference at P = 0.05.
In Insch soil treatments (Fig. 1), the mean Cmic in IC was approximately 35% greater than C. There were no significant differences (P > 0.05) between incubated and non-incubated treatments. Both D and ID treatments recorded the highest mean Cmic (approximately 40 mg 100 g−1 soil dw) for each treatment, not only across the Insch soil samples but also compared to the other two soils (Boyndie and Brechin soils). Insch K and its incubated equivalents were 20–30% lower than both D and ID. The interaction between the incubation period and hydrocarbon added had a significant effect (P ⩽ 0.05) on Insch soil treatments. The incubation period alone had no significant effect. However, the addition of hydrocarbon had a significant effect (P ⩽ 0.05) on Insch soil treatments.
Incubated Brechin soil treatments had the lowest Cmic of the three soils (Fig. 1). There was approximately a 50% decrease in the incubated Brechin soil compared to non-incubated Brechin soil. K was significantly (P ⩽ 0.05) lower than D, however, there was no significant difference (P > 0.05) observed between IK and ID. There was no significant difference in Cmic between the non-incubated Brechin soil treatments, C and D had similar Cmic, but K was significantly lower. There was a significant (P ⩽ 0.05) effect on Brechin soil treatments caused by incubation period and hydrocarbon added. Moreover, the interaction between these two variables had a significant (P ⩽ 0.05) effect on soil treatments.
3.2 Plate counts
Table 3 summarises bacterial and fungal culturable heterotrophs and hydrocarbon degrader plate count results for all the three soils. Generally, non-incubated kerosene amended treatments had the highest bacterial numbers. Non-incubated Insch soil treatments had the lowest fungal numbers.
Soil
Treatment
Heterotrophic
Hydrocarbon degrading
Bacteria
SE
Fungi
SE
Bacteria
SE
Fungi
SE
Boyndie
C
5.34E+05a
±6.49E+04
2.75E+03a
±4.16E+02
4.97E+05a
±5.49E+04
9.47E+02a
±2.00E+02
IC
1.12E+06a
±1.60E+05
1.91E+03a
±2.52E+02
4.31E+05a
±0.00E+00
1.32E+03a
±2.33E+02
K
2.13E+06bc
±1.58E+05
2.37E+03a
±2.91E+02
1.81E+06b
±8.69E+04
1.21E+03a
±8.82E+01
IK
2.73E+06b
±2.40E+04
2.15E+03a
±3.76E+02
5.97E+05a
±9.07E+04
6.99E+02a
±2.33E+02
D
1.85E+06c
±1.15E+05
2.10E+03a
±2.60E+02
1.06E+06c
±6.36E+04
1.30E+03a
±1.53E+02
ID
2.19E+06bc
±1.13E+05
1.91E+03a
±1.86E+02
5.43E+05a
±5.90E+04
6.55E+02a
±1.67E+02
LSD
4.57E+05
8.63E+02
1.08E-01
6.02E+02
Insch
C
6.72E+05a
±1.01E+05
1.29E+03ac
±1.33E+02
5.15E+04ad
±3.76E+03
3.73E+02a
±3.33E+01
IC
5.19E+05a
±2.96E+04
9.89E+02a
±2.03E+02
4.41E+04d
±6.43E+03
3.25E+02a
±5.77E+01
K
2.54E+07b
±4.26E+05
1.26E+03ac
±1.15E+02
7.26E+05b
±6.93E+04
3.70E+02a
±8.82E+01
IK
3.97E+06d
±3.46E+05
2.59E+03ac
±8.96E+02
8.58E+04a
±1.34E+04
4.11E+02a
±1.73E+02
D
1.02E+07c
±6.77E+05
1.33E+03ac
±5.77E+01
2.76E+05c
±3.53E+04
3.48E+02a
±5.77E+01
ID
3.62E+06d
±8.82E+04
7.62E+03b
±1.11E+03
8.17E+04a
±1.31E+04
1.94E+03b
±3.79E+02
LSD
8.66E+05
2.33E-01
1.50E-01
4.48E+02
Brechin
C
1.32E+06a
±1.51E+05
3.43E+03abc
±4.18E+02
5.95E+04a
±2.41E+04
2.26E+03a
±1.20E+03
IC
3.98E+05d
±2.19E+04
2.11E+03ab
±4.26E+02
4.42E+04ad
±1.00E+04
8.30E+02a
±3.48E+02
K
1.93E+07b
±1.71E+06
4.68E+03ac
±5.77E+01
2.40E+06b
±4.03E+05
6.74E+03bc
±9.07E+02
IK
1.19E+06a
±1.33E+05
1.56E+03ab
±7.37E+02
6.35E+04ade
±1.45E+04
5.47E+02a
±3.33E+02
D
7.41E+06c
±5.61E+05
4.39E+03ac
±5.77E+01
4.13E+05c
±1.15E+04
6.87E+03bc
±1.45E+03
ID
2.83E+06e
±1.76E+05
7.99E+03ac
±1.72E+03
1.33E+05ae
±3.38E+04
3.61E+03ac
±1.12E+03
LSD
1.12E-01
3.36E-01
2.85E-01
2.58E+03
3.2.1 Heterotrophic bacteria
A comparison of heterotrophic bacteria in the hydrocarbon amended treatments across all three soils showed that Insch soil treatments had approximately 25% more colonies than Brechin soil treatments. Insch soil treatments had approximately 80% more culturable CFU than Boyndie soil treatments (Table 2). Kerosene amended treatments had significantly greater numbers of CFU (P ⩽ 0.05) than the diesel amended treatments (approximately 50%) in both Insch and Brechin soils. There were no significant differences (P > 0.05) measured in bacterial heterotrophic numbers in Boyndie soil between K and D. Both the non-incubated C and the IC equivalent recorded the lowest culturable number of bacterial heterotrophs across all three soils. There were no significant differences (P > 0.05) in bacterial heterotroph counts between the incubated control and non-incubated C soils, with the exception of the Brechin soil, where C was significantly (P ⩽ 0.05) higher than IC. The culturable number of bacterial heterotrophs broadly decreased by approximately 50–80% in IK and ID treatments for both Insch and Brechin soils compared to non-incubated treatments. There was no significant difference (P > 0.05) between non-incubated and incubated Boyndie treatments (Table 2).
3.2.2 Hydrocarbon degrading bacteria
Both Insch and Brechin soils had similar numbers of culturable hydrocarbon degrader bacteria across all soil treatments with the exception of K treatments (Table 3). Brechin K treatment culturable numbers were about 70% greater than Insch K treatment CFU numbers. Generally, Boyndie soil had the highest CFU numbers of hydrocarbon degrading bacteria of all the three soil treatments with the exception of Brechin K treatment which had the highest overall. The lowest culturable numbers were recorded in the Insch soil treatments. Both K and D amended treatments had 50–90% higher CFU degrader numbers than the incubated treatments. For each individual soil, there was no significant difference (P > 0.05) in hydrocarbon degrading bacterium culturable numbers between incubated hydrocarbon amended treatments and the C soil equivalents. Culturable numbers of hydrocarbon degrading bacteria were about 70% greater in both incubated and non-incubated C for Boyndie soils compared to the other two soils (Table 3).
3.2.3 Heterotrophic fungi
Insch soils had 20–50% lower culturable numbers of heterotrophic fungi compared to the other two soils, with the exception of the IK and ID treatments. There were no statistical differences (P > 0.05) between the IK treatments in all three soils. Insch and Brechin ID had the highest culturable numbers of heterotrophic fungi (Table 3). Generally, Brechin soils had the highest culturable numbers of fungal heterotrophs with the exception of the IK treatment which had approximately 50% of the culturable numbers of the non-incubated treatments. Boyndie soils had similar numbers of fungal heterotrophs with no significant differences (P > 0.05) between all treatments for both incubated and non-incubated.
3.2.4 Hydrocarbon degrading fungi
Brechin soils had the highest culturable numbers of hydrocarbon degrading fungi across all three soil treatments with the exception of IC and IK treatments (Table 3). Brechin IC degrading fungi population sizes were approximately 50% of C numbers. No significant differences (P > 0.05) were observed between IK treatments across all the three soil treatments. Similarly, there were no statistical differences (P > 0.05) in the culturable number of hydrocarbon degrading fungi in Boyndie and Insch soils with the exception of ID treatment which was significantly (P ⩽ 0.05) higher (Table 3). The hydrocarbon degrader fungus culturable number decreased by about 50% in Boyndie and Brechin incubated hydrocarbon amended treatments compared to non-incubated treatments. In contrast, the count in Insch ID was approximately 80% higher compared to D treatment.
3.2.5 Relationship between heterotrophs and degraders
The relationships between heterotrophic bacteria and hydrocarbon degrading bacteria are illustrated in Fig. 2. There were generally strong positive relationships in non-treated samples between bacterial heterotrophs and hydrocarbon degrading bacteria in all three soils. However, the correlation declined after incubation in all three soils (Fig. 2).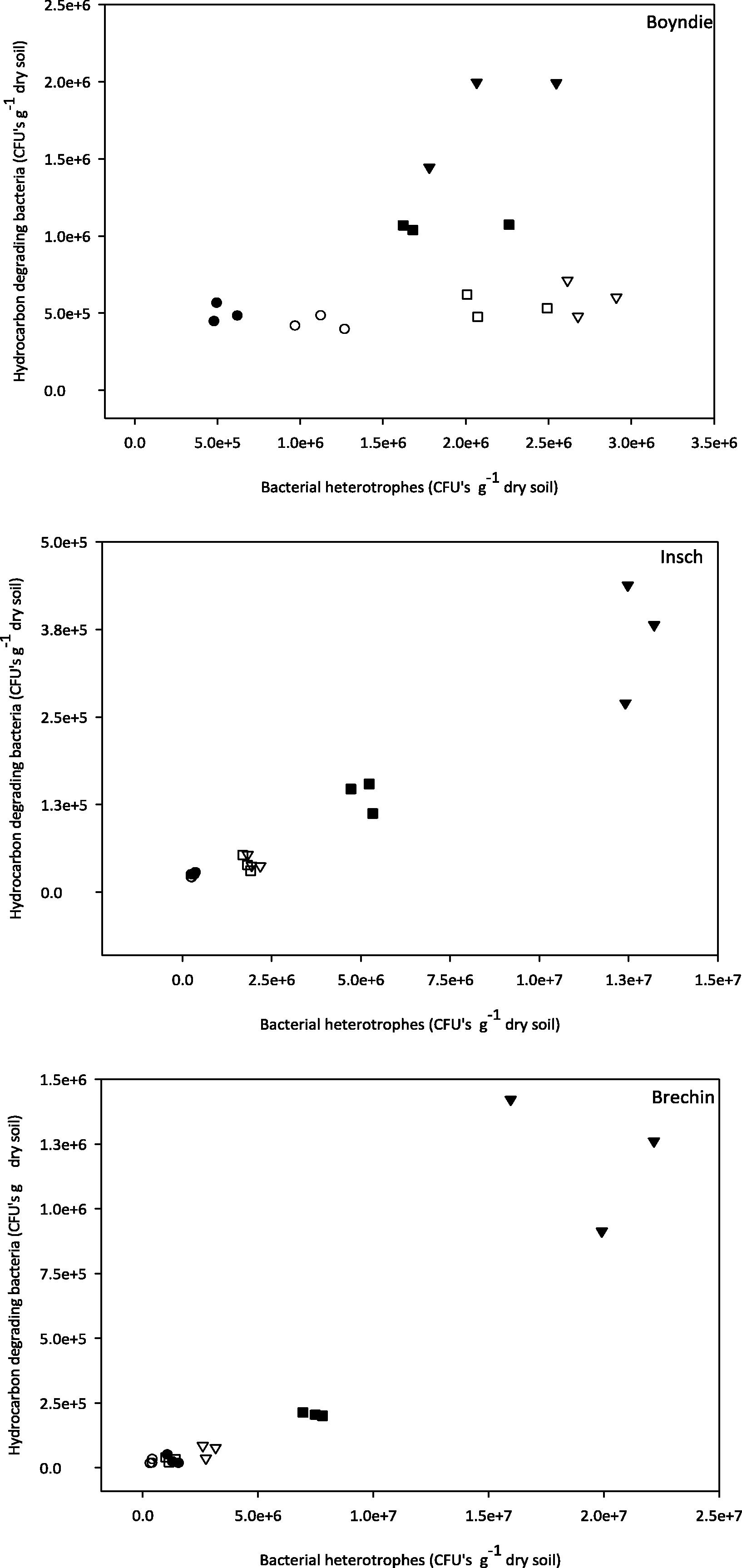
The relationship between measurable counts and enumeration of heterotrophic bacteria and hydrocarbon degrading bacteria in non-incubated and incubated treatments in the three soils. (●) indicates control. (○) indicates incubated control, (▾) indicates kerosene, (▽) indicates incubated kerosene, (■) indicates diesel and (□) indicates incubated diesel.
The relationships between heterotrophic fungi and hydrocarbon degrading fungi are presented in Fig. 3. There was no clear correlation in the Boyndie soil between heterotrophic fungi and hydrocarbon degrading fungi (Fig. 3). Both incubated Insch and Brechin soil treatments exhibited a strong correlation between fungal heterotrophs and hydrocarbon degraders. However, non-incubated Insch and Brechin soils had a weak relationship between fungal heterotrophs and degrading fungi (Fig. 2).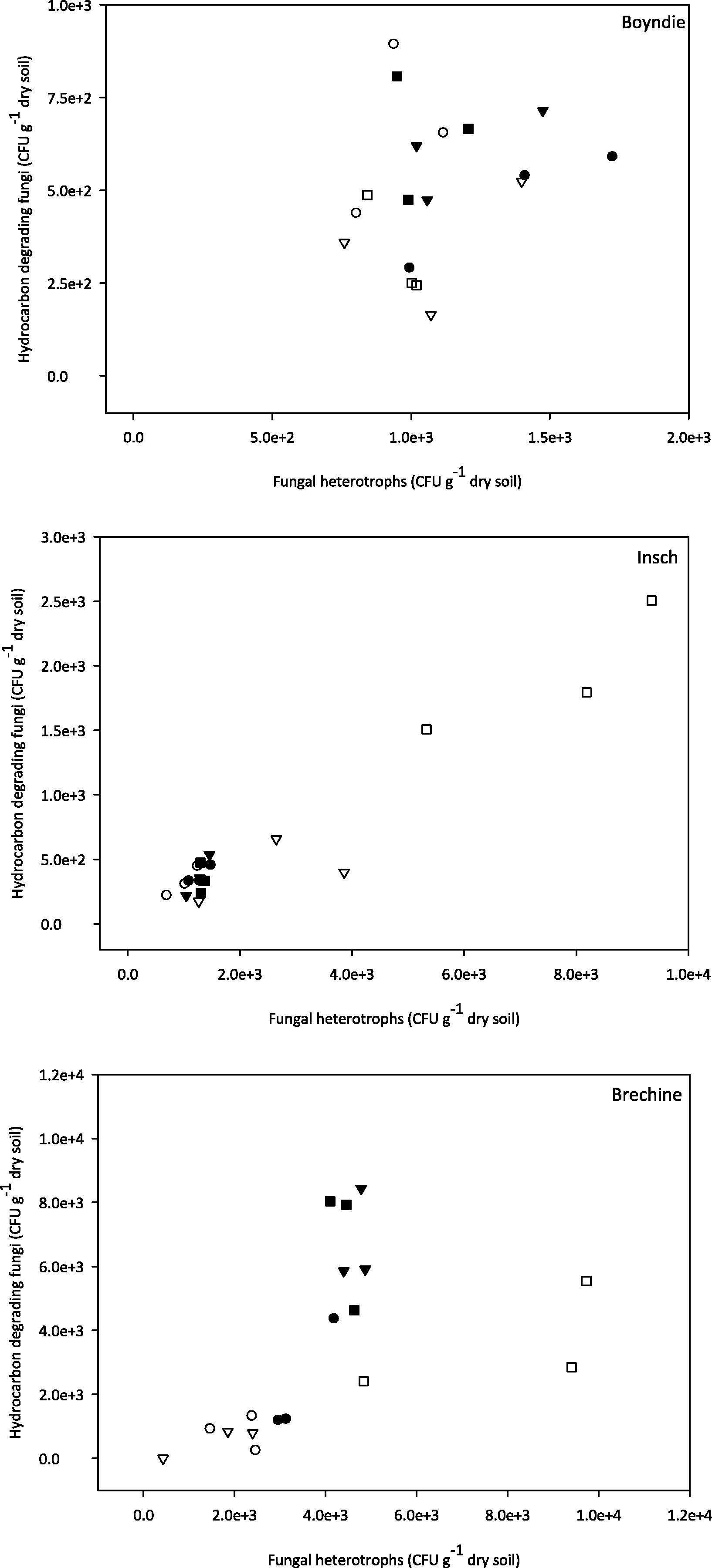
The relationship between measurable counts and enumeration of heterotrophic fungi and hydrocarbon degrading fungi in non-incubated and incubated treatments in the three soils. (●) indicates control. (○) indicates incubated control, (▾) indicates kerosene, (▽) indicates incubated kerosene, (■) indicates diesel and (□) indicates incubated diesel.
3.3 Enzyme activity
3.3.1 Dehydrogenase activity (DHA)
Boyndie and Brechin soils generally had the highest dehydrogenase activity (DHA) and Insch soil had significantly (P ⩽ 0.05) lower DHA than the other two soils as presented in Fig. 4. Incubated Brechin soil had significantly higher DHA than Boyndie and Insch equivalents. There was no significant difference (P > 0.05) measured in DHA between the non-incubated Boyndie and Brechin soils with the exception of that in kerosene amended treatment. Boyndie K DHA was significantly (P ⩽ 0.05) higher than Brechin K treatment. There was no significant difference (P > 0.05) between DHA in the non-incubated Insch and Brechin treatments, with the exception of Insch diesel amended soil, which had lower DHA.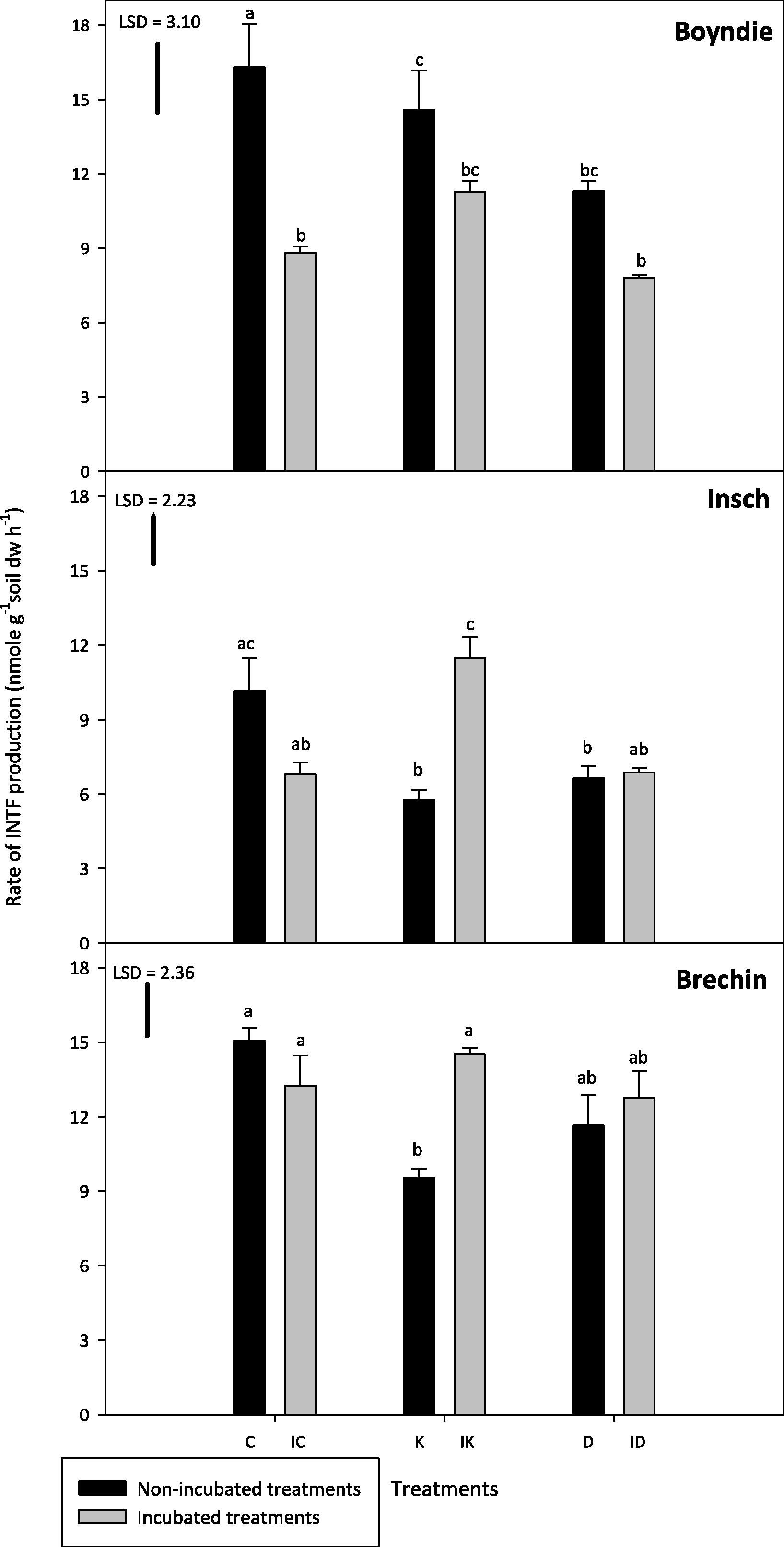
Dehydrogenase activity (DHA) in the incubated and non-incubated soil treatments. Abbreviations on the x axis: C indicates control, IC indicates incubated control, K indicates kerosene, IK indicates incubated kerosene, D indicates diesel and ID indicates incubated diesel. Different letters on the bars show significant differences between treatments (One way ANOVA, Tukey test, P ⩽ 0.05). Error bars represent standard error of the mean for n = 3. LSD is the least significant difference at P = 0.05.
Boyndie soil (freshly contaminated) had higher DHA than the incubated soils by approximately 20–40% (Fig. 4). The DHA assay was able to discriminate between the controls, which was significantly (P ⩽ 0.05) higher than both the K and D treatments. There was no significant difference (P > 0.05) between K and D treatments. There were no significant differences (P > 0.05) between all incubated Boyndie treatments. There were also no differences found between freshly hydrocarbon contaminated Boyndie soils and the incubated equivalent.
Insch soils had the highest DHA compared to the other two soils. For Insch soil, the IK treatment was approximately 50% higher than the other Insch soil treatments, with the exception of the control soil (Fig. 4). There were no significant differences (P > 0.05) observed between either C and the incubated control or D and its incubated equivalent. K and D were significantly (P ⩽ 0.05) lower than C by about 35%. There was no significant difference (P > 0.05) observed between K and D treatments.
Brechin soil had the lowest DHA in K treatments. However, DHA increased significantly in the IK by approximately 30% greater compared to the similar sample before incubation. There were no statistically significant differences (P > 0.05) observed between the other Brechin soil treatments, including D and the incubated equivalent, C and IC (Fig. 4).
3.3.2 Phosphatase activity (PA)
Brechin soils had the highest PA but Insch soils had the lowest PA of all three soils (Fig. 5) There were no significant differences in PA between Boyndie and Brechin hydrocarbon amended (kerosene and diesel) treatments both before and after incubation. Boyndie soils differed significantly (P ⩽ 0.05) from Insch soils, with the exception of ID treatments. Generally, there was a clear decline observed in PA across all incubated treatments for the three soils compared to the non-incubated soil treatments.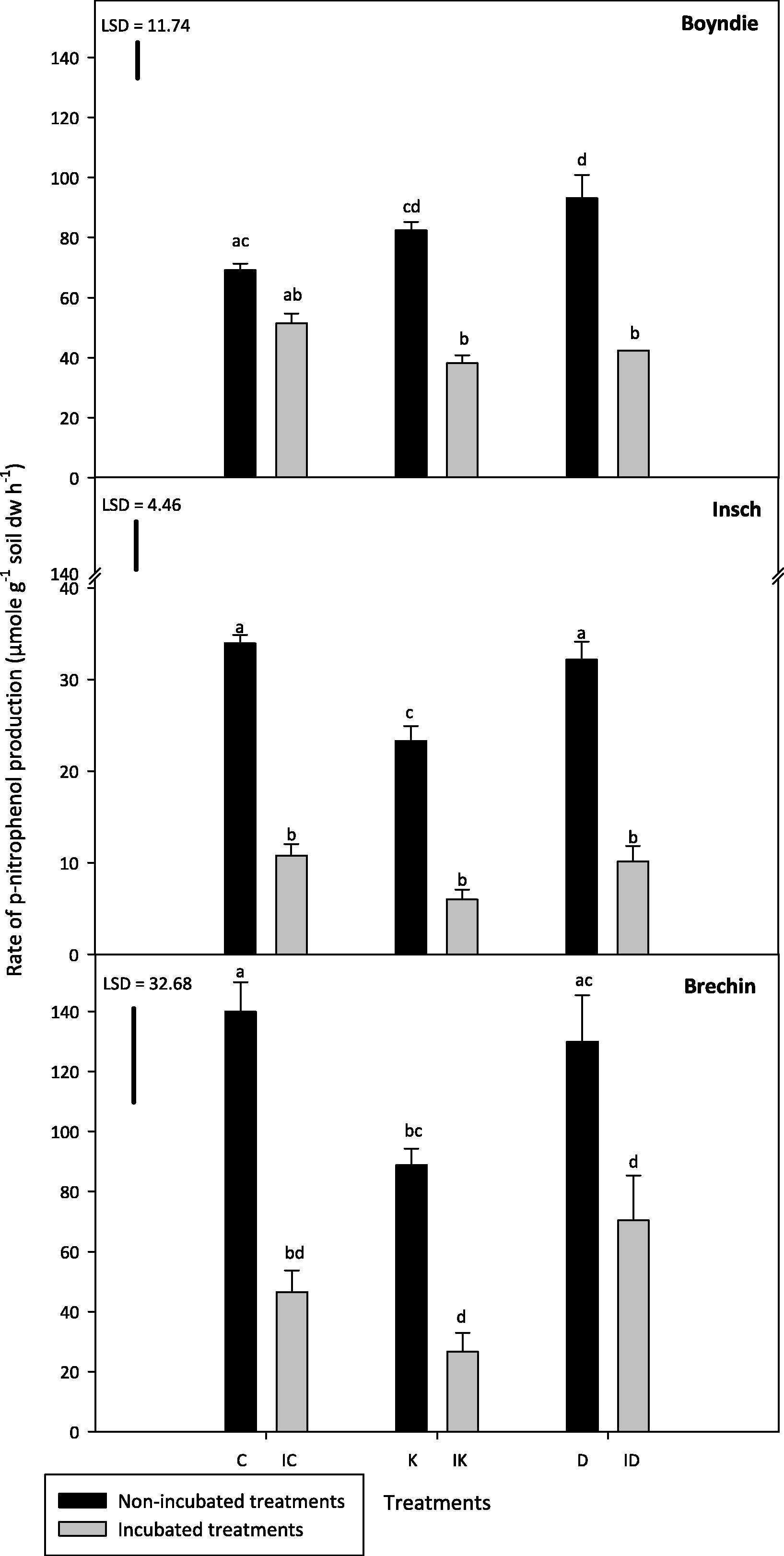
Phosphatase activity (PA) in the incubated and non-incubated soil treatments. Abbreviations on the x axis: C indicates control, IC indicates incubated control, K indicates kerosene, IK indicates incubated kerosene, D indicates diesel and ID indicates incubated diesel. Different letters on the bars show significant differences between treatments (One way ANOVA, Tukey test, P ⩽ 0.05). Error bars represent standard error of the mean for n = 3. LSD is the least significant difference at P = 0.05.
There were no significant differences (P > 0.05) for Boyndie soil PA between C and the incubated equivalent soil. However, both K and D were about 50–60% higher than the incubated equivalents (Fig. 5). D and K had the highest PA. There were no significant differences (P > 0.05) between all of the incubated Boyndie treatments. PA in D differed significantly (P ⩽ 0.05) and was greater than C for Boyndie soil.
Insch K had the lowest PA for non-incubated treatments. K treatment differed significantly (P ⩽ 0.05) from the other two non-incubated Insch soil treatments (C and D). PA in all incubated Insch treatments was approximately 60–70% lower than in the non-incubated treatments. There were no significant differences (P > 0.05) between all of the incubated Insch soils (Fig. 5).
Both Brechin C and D had the highest phosphatase activity, not only for the Brechin soil treatments, but also relative to all the three soils. K treatment was about 35% lower than both non-incubated Brechin soil treatments, however, with no significant difference (P > 0.05) with D treatment. There were no significant differences (P > 0.05) observed in PA between incubated Brechin soil treatments but PA was approximately 50–70% lower than in the non-incubated samples (Fig. 5).
4 Discussion
Evaluating the effect of different hydrocarbons on soil microbial activity and determining the suitability of analytical techniques for discriminating between soil treatments are essential for investigating petroleum-polluted soils.
For soil microbial biomass carbon (Cmic), soil characteristics play an important role in controlling the effect of hydrocarbon contaminants on the diversity and function of soil microbial communities. High organic matter and clay content are often associated with high concentrations of Cmic, a finding confirmed in the present study where Insch soils were found to have higher Cmic values than other soil types that contain lower levels of organic matter and clay. It has previously been reported that organic matter protects soil microorganisms against the effect of hydrocarbons which would lead to lower inhibition of soil Cmic (Labud et al., 2007). Organic matter and clay content can absorb hydrocarbons and decrease bioavailability during their aqueous phase (El-Tarabily, 2002; Eibes et al., 2006).
The addition of diesel and kerosene to soils can act as significant sources of C for microbial growth and activity (Bundy et al., 2002), and thereby contribute to higher Cmic values. Diesel treatments have higher Cmic than kerosene treatments and this may be due to the fact that diesel is a less toxic source of carbon. The effect of hydrocarbon toxicity is influenced by hydrocarbon type (Labud et al., 2007). The higher the concentration of low-boiling and/or unsaturated compounds, aromatics and acids, the more toxic hydrocarbons are to microorganisms in the soil (Xu and Johnson, 1995). Hydrocarbon toxicity occurs due to disruption of plasma membranes. Lighter fractions (e.g. kerosene) produce greater disruption than heavier diesel fractions (Fernandes et al., 2003; Gouda et al., 2008). The physical properties of hydrocarbons play a key role in their behaviour in soils. Kerosene fractions are more volatile than diesel and are lighter in open systems where fractions may be lost to the atmosphere. Therefore, increased toxicity, decreased availability of short chain hydrocarbons (Cermak et al., 2010) and decreased total HC (as a potential C substrate) all contribute to lowering Cmic values for kerosene treated soils. Understanding hydrocarbon mobility and behaviour in soil provides important information for exposure assessments (hazard and risk) and assessments of potential hydrocarbon bioavailability. Therefore, fugacity models (Mackay and Paterson, 1991; Mackay, 2001) have been used to predict the distribution and concentration of hydrocarbon contaminants in different soil phases (air, aqueous, solid) (Coulon et al., 2010b).
In addition to consistency in an assay, there is also a need for consistency in procedures and to optimise preparation stages. Incubation time may lead to unavoidable experimental artefacts. It has been demonstrated that storage conditions and watering regimes both strongly influence microbial activities, but that this is co-mediated by the soil type (Gonzalez-Quiñones et al., 2009; Yong et al., 2007). In this work it was clear that the incubation period for control Brechin soils exerted a stronger negative effect on soil Cmic, than for the other two soil types.
For plate counts, it is known that zymogenous populations proliferate following addition of readily assimilated substrates at the expense of autochthonous groups (Killham, 2006). Hydrocarbons in soil provide a source of C for microbial growth and this explains the high CFU for heterotrophic bacteria in freshly contaminated soils compared to control soils of all three types. The addition of N and P to nutritionally balance the incubated hydrocarbon amended treatments may also enhance the growth of zymogenous populations and increase CFU counts of heterotrophic bacteria compared to incubated controls (Margesin et al., 2000).
The CFU count for heterotrophic bacteria was influenced by soil properties and by hydrocarbon type. High organic matter and clay content, as found in Insch soils, may reduce the bioavailability of hydrocarbons through sorption and increase the enumeration compared to sandier, textured soils (Labud et al., 2007). Soil pH is an important factor that can affect soil microorganism diversity (Killham, 2006). Relatively low pH values (6.0–6.5) of Insch and Brechin soils are associated with higher heterotrophic bacteria CFU counts compared to the higher pH Boyndie soil (pH 7.5).
Heterotrophic bacteria CFU counts were affected by hydrocarbon type. Kerosene impacted soils had a lower Cmic than diesel impacted soils, but soil heterotrophic bacteria counts were higher for the diesel impacted soils. Luria Bertani (LB) is a general growth medium, but is perhaps beneficial for communities that have survived in kerosene impacted soils. The diesel impacted heterotrophic communities simply respond differently to LB. The bio-stimulation effect of LB is different for different treatments. Hence, the kerosene population is stimulated to a greater extent by LB than the diesel impacted population, and this outcome can be seen across all three soils.
The addition of hydrocarbon was the main driver for the increase of CFU for both heterotrophic bacteria and hydrocarbon degrading bacteria. Studies have reported that an increase in the numbers of hydrocarbon utilisers is positively correlated with hydrocarbon concentration (Margesin et al., 2000; Bundy et al., 2002; Alamri, 2006). The increase in numbers of culturable hydrocarbon degrading bacteria demonstrates how rapidly indigenous soil microorganisms are able to adapt to new substrates (Margesin et al., 2000). The findings of the present study indicate that hydrocarbon degrading bacteria CFU was high in treatments of freshly hydrocarbon-contaminated soils. Kerosene had a stronger stimulatory effect compared to diesel, and this may have increased the numbers of kerosene degrading bacteria compared to diesel treatments. Soil microbial communities, including hydrocarbon degrading bacteria, are soil specific (Bundy et al., 2002). The number of CFUs for hydrocarbon degrading bacteria was the highest in the Boyndie soil, possibly because it has a different microbial community compared to the other two soils.
Soil type and pH also had an effect on heterotrophic and degrading fungi. Variation in fungi counts between soils may be associated with different microbial communities in each soil. The Brechin soil had a higher number of heterotrophic and degrading fungi than the other two soils; (1). The pH of Brechin soil is different from the other two soils and/or (2). The microbial community present in the Brechin soil differs from the other two soils.
For enzyme activity, dehydrogenase activity (DHA) is a measure of the oxidation–reduction capability of the active soil microbial biomass (Shaw and Burns, 2006). Soil characteristics such as organic matter and soil texture are known to affect DHA. Although Insch soil had high Cmic values and a high number of heterotrophic bacteria CFU, it had the lowest DHA. Additionally, Insch soil contained high levels of organic matter which may limit available carbon sources with low DHA. A comparison of the incubated control with its equivalent in Boyndie soil shows that the incubation period had a negative effect on DHA. Incubation time may affect the balance of soil nutrients compared to natural soil. It has been reported that measuring levels of DHA does not allow discrimination between soil treatments (Trasar-Cepeda et al., 2000). The high concentration of hydrocarbons decreased the DHA. Kerosene added to soils has a toxic effect on soil microorganisms, as shown in the non-incubated Insch and Brechin soils, and this can lead to lower DHA. In contrast, the bioavailability of kerosene decreased with incubation time and this is associated with an increase in DHA.
Unlike DHA, phosphatase activity (PA) phosphatases are not purely intracellular and are not dependent on cell activity. They are also not ubiquitous and may be absent from parts of the microbial community. Phosphatase activity is also influenced by soil P-status. PA in all three soils was negatively influenced by the incubation period reflecting the added nutrients and increased P availability. Turgay et al. (2010) reported that some enzymes, including phosphatases, are involved in N and P cycling and may not directly influence hydrocarbon presence. The low pH of Insch soil (6.12) may also be a reason for low PA compared to the other two soils as more P will be desorbed from soil colloids at a lower pH (Płaza et al., 2010).
5 Conclusion
The influence of hydrocarbons on enzymatic activity is related to soil physicochemical properties. Insch soil had high Cmic values and a high number of heterotrophic bacteria CFU, but had the lowest dehydrogenase and phosphatase activity of all the three soils. Brechin soils had the highest phosphatase activity. There were generally strong positive relationships between bacterial heterotrophs and hydrocarbon degrading bacteria in non-treated samples of all three soils. Both incubated Insch and Brechin soil treatments showed strong correlations between fungal heterotrophs and hydrocarbon degraders. Conversely, non-incubated Insch and Brechin soils showed weak relationships between fungal heterotrophs and degrading fungi. In conclusion, the results of the current study show that soil characteristics play an important role in controlling the effect of hydrocarbon contaminants on the diversity and function of soil microbial communities. Measuring soil enzymatic activities can provide information about the function and structure of soil microbial communities in hydrocarbon contaminated soils. These measures could be used as rapid and cost-effective means for evaluating and monitoring remediation of hydrocarbon contaminated soil.
Acknowledgment
I would like to gratefully acknowledge the government of Saudi Arabia for the scholarship and financial support.
References
- Alamri, S.A., 2006. Development and application of a microbiologically based tool kit to predict and monitor petroleum hydrocarbon bioremediation (Ph.D. thesis), University of Aberdeen.
- Spatial distribution of soil phosphatase activity within a riparian forest. Soil Sci.. 1997;162:808-825.
- [Google Scholar]
- A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biol. Biochem.. 1978;10:215-221.
- [Google Scholar]
- Bioremediation of oil-contaminated soil: microbiological methods for feasibility assessment and field evaluation. J. Microbiol. Methods. 1998;32:155-164.
- [Google Scholar]
- Environmental hazard and risk characterisation of petroleum substances: a guided “walking tour” of petroleum hydrocarbons. Environ. Int.. 2014;66:182-193.
- [Google Scholar]
- Microbial communities in different soil types do not converge after diesel contamination. J. Appl. Microbiol.. 2002;92:276-288.
- [Google Scholar]
- Measurement of dehydrogenase activity in acid soils rich in organic matter. Soil Biol. Biochem.. 1998;30:1005-1011.
- [Google Scholar]
- A comparison of enumeration methods for culturable Pseudomonas fluorescens cells marked with green fluorescent protein. J. Microbiol. Methods. 2000;40:135-145.
- [Google Scholar]
- Toxicity of petroleum hydrocarbon distillates to soil organisms. Environ. Toxicol. Chem.. 2010;29:2685-2694.
- [Google Scholar]
- Multimedia fate of petroleum hydrocarbons in the soil: oil matrix of constructed biopiles. Chemosphere. 2010;81:1454-1462.
- [Google Scholar]
- Application of biological indicators to assess recovery of hydrocarbon impacted soils. Soil Biol. Biochem.. 2007;39:164-177.
- [Google Scholar]
- Enzymatic degradation of anthracene, dibenzothiophene and pyrene by manganese peroxidase in media containing acetone. Chemosphere. 2006;64:408-414.
- [Google Scholar]
- Total microbial activity and microbial composition of a mangrove sediment are reduced by oil pollution at a site in the Arabian gulf. Can. J. Microbiol.. 2002;48:176-182.
- [Google Scholar]
- Solvent tolerance in bacteria: role of efflux pumps and cross-resistance with antibiotics. Int. J. Antimicrob. Agents. 2003;22:211-216.
- [Google Scholar]
- Francis-Obika, C.O., 2004. Development and application of techniques to monitor natural attenuation of petroleum hydrocarbons in the environment (Ph.D. thesis), University of Aberdeen.
- Influence of cold storage on soil microbial community level physiological profiles and implications for soil quality monitoring. Soil Biol. Biochem.. 2009;41:1574-1576.
- [Google Scholar]
- Bioremediation of kerosene II: a case study in contaminated clay (laboratory and field: scale microcosms) World J. Microbiol. Biotechnol.. 2008;24:1451-1460.
- [Google Scholar]
- Toxicity assessment for petroleum-contaminated soil using terrestrial invertebrates and plant bioassays. Environ. Monit. Assess.. 2013;185 2989e2998
- [Google Scholar]
- The effects of biocidal treatments on metabolism in soil-I. Fumigation with chloroform. Soil Biol. Biochem.. 1976;8:167-177.
- [Google Scholar]
- Bioremediation of petroleum hydrocarbon-contaminated soil by composting in biopiles. Environ. Pollut.. 2000;107:245-254.
- [Google Scholar]
- Three decades of soil microbial biomass studies in Brazilian ecosystems: Lessons learned about soil quality and indications for improving sustainability. Soil Biol. Biochem.. 2010;42:1-13.
- [Google Scholar]
- Soil Ecology (fourth ed.). Cambridge, UK: Cambridge University Press; 2006. p. :242.
- Effect of hydrocarbon pollution on the microbial properties of a sandy and a clay soil. Chemosphere. 2007;66:1863-1871.
- [Google Scholar]
- Mackay, D., 2001. Multimedia Environmental Models: the Fugacity Approach, second ed., Lewis Publishers, MI.
- Evaluating the multimedia fate of organic chemicals: a level III fugacity model. Environ. Sci. Technol.. 1991;25:427-436.
- [Google Scholar]
- Efficiency of indigenous and inoculated cold-adapted soil microorganisms for biodegradation of diesel oil in alpine soils. Appl. Environ. Microbiol.. 1997;63:2660-2664.
- [Google Scholar]
- Monitoring of bioremediation by soil biological activities. Chemosphere. 2000;40:339-346.
- [Google Scholar]
- Ecotoxicological and microbiological characterization of soils from heavy-metal and hydrocarbon-contaminated sites. Environ. Monit. Assess.. 2010;163:477-488.
- [Google Scholar]
- Evaluation of soil biological activity after a diesel fuel spill. Sci. Total Environ.. 2009;407:4056-4061.
- [Google Scholar]
- Enzyme activity profiles and soil quality. In: Bloem J., Hopkins D.W., Benedetti A., eds. Microbiological Methods for Assessing Soil Quality. Cambridge, MA: CABI Publishing; 2006.
- [Google Scholar]
- Effects of local microbial bioaugmentation and biostimulation on the bioremediation of total petroleum hydrocarbons (TPH) in crude oil contaminated soil based on laboratory and field observations. Int. Biodeter. Biodegrad.. 2014;90:115-122.
- [Google Scholar]
- Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem.. 1969;1:301-307.
- [Google Scholar]
- Limitations of soil enzymes as indicators of soil pollution. Soil Biol. Biochem.. 2000;32:1867-1875.
- [Google Scholar]
- Effect of humic deposit (leonardite) on degradation of semi-volatile and heavy hydrocarbon and soil quality in crude-oil contaminated soil. Environ. Monit. Assess.. 2010;170:45-58.
- [Google Scholar]
- An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem.. 1987;19:703-707.
- [Google Scholar]
- The use of microorganisms in ecological soil classification and assessment concepts. Ecotoxicol. Environ. Saf.. 2005;62:230-248.
- [Google Scholar]
- Root growth, microbial activity and phosphatase activity in oil-contaminated, remediated and uncontaminated soils planted to barley and field pea. Plant Soil. 1995;173:3-10.
- [Google Scholar]
- Cold storage and pre-treatment incubation effects on soil microbial properties. Soil Sci. Soc. Am. J.. 2007;71:1299-1305.
- [Google Scholar]







