Translate this page into:
Effects of diminazene aceturate on flip-flop plasma pharmacokinetics of piroxicam in dogs
⁎Corresponding author. pharn_saga2006@yahoo.com (Saganuwan Alhaji Saganuwan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
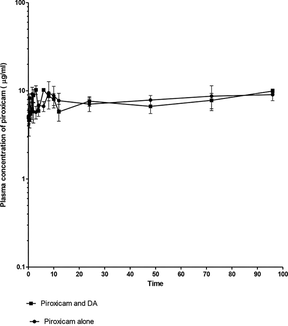
Figure 3. Mean plasma concentration–time curve of 3.5 mg/kg intramuscular piroxicam, and 3.5 mg/kg of piroxicam/diminazene aceturate (3.5 mg/kg) in dogs respectively (n = 20).
Abstract
Objectives
Pain, fever and inflammation associated with protozoan infections caused by Trypanosomes, Babesia, Entamoeba, Leishmania and Pneumocystis have necessitated the search for polypharmacy,that could be used for treatment of protozoan infections in dogs.
Methods
Randomized cross-over controlled trial was adopted for kinetic study of piroxicam (3.5 mg/kg) and piroxicam (3.5 mg/kg) administered with diminazene aceturate (3.5 mg/kg) in Nigerian indigenous dogs. Ten dogs comprised 5 males and females, each of about 8 ± 2 months and weighed 10 ± 0.5 kg were administered piroxicam, after one month the dogs were administered piroxicam and diminazene aceturate at different thigh muscles. Single dose was administered to avoid toxicity. Blood samples were collected at 0, 0.08, 0.25, 0.5, 1.0, 1.5, 2.0, 3.0, 4.0, 6.0, 8.0, 10, 12, 24, 48, 72 and 96 h for plasma analysis of piroxicam.
Results
Findings have shown that piroxicam was significantly (p < 0.05) slowly absorbed (5.823 ± 1.46 h−1) and eliminated (0.935 ± 0.75 h−1) as compared to piroxicam/diminazene aceturate group (7.405 ± 1.75 h−1) and (0.137 ± 0.10 h−1), respectively. Concentration maximum (Cmax = 12.659 ± 0.85 µg/ml), peak time (Tmax = 13.675 ± 9.21 h), absorption half-life (T1/2α = 0.016 ± 0.04 h), elimination rate constant (β = 0.137 ± 0.10 h−1), area under curve zero to infinity (AUC0-∞ = 778.885 ± 66.99 mg/L/h), area under moment curve (AUMC = 9820.140 ± 5.33 mg/h2/L), fraction absorbed zero to 96 h (Fab0-96h = 7.505 ± 0.00%) were significantly lower in piroxicam/diminazene aceturate (p < 0.05) as compared to Cmax(18.560 ± 2.97 µg/ml), Tmax (45.000 ± 11.49 h), T1/2α(0.250 ± 0.08 h), β (0.935 ± 0.75 h−1), AUC0-∞ (814.472 ± 86.43 mg/L/h), AUMC (36274.840 ± 9010.44 mg/h2/L), and Fab0-96h (7.848 ± 0.00%) in the piroxicam treated group, respectively. The elimination half-life was significantly lower (p < 0.05) in piroxicam (33.634 ± 9.34 h) as compared with piroxicam/diminazene aceturate (34.850 ± 11.94 h) treated group.
Conclusion
Hence piroxicam displays flip-flop phenomenon of absorption, and could be coadministered with diminazene aceturate at single or twice dose for treatment of trypanosomosis, amoebiasis, leishmaniasis, pneumocytis and babesiosis in dogs.
Keywords
Piroxicam
Pharmacokinetics
Flip-flop
Absorption
Diminazene aceturate
Dog
1 Introduction
Diminazene aceturate is an aromatic diamidine which consists, two amidinophenyl moieties linked by a triazene. It is the most widely used for treatment of trypanosomosis and babesiosis in domestic livestock. It is injectable with narrow safety margin, and appropriate dose is caculated (Riviere and Papich, 2017). Animals that are hypersensitive to diminazene aceturate or phenazone as well as patients with impaired renal and hepatic function should not be administered the drug (Fussanger, 1995). Diminazene aceturate is used in the treatment of trypanosomosis in dogs at a dose of 3.6-7 mg/kg intramuscularly every two weeks, and in horses, cattle, sheep and goats at 3.5 mg/kg intramuscularly once, and in the treatment of babesiosis in dogs at 3.5 mg/kg, repeated after 24 h respectively. Diminazene aceturate is used in the treatment of cytauxzoonosis in cats at a dose of 3–5 mg/kg intramuscularly at a time or 2 mg/kg intramuscular injection repeated in one week (Holman and Snowden, 2009). The drug also has an anti-inflammatory property which is yet to be fully proven (Trapp et al., 2006).
Piroxicam is a non-steriodal anti-inflammatory drug (NSAID) used to treat pain, fever and inflammation. It is highly potent with half-life of over 50 h, administered once daily. It exists in two different interconvertible crystal polymorphs with melting point of 196–198 °C and 199–201 °C, respectively. Piroxicam inhibits cycloxygenase (COX), the enzyme that catalyzes the conversion from arachidonic acid to prostaglandins (PGs) that mediate pain. Piroxicam crosses the blood brain barrier which makes it very potent in reducing fever (Baltoyiannis et al., 2001). However there is no information on the effect of diaminazene aceturate on kinetics of piroxicam in Nigeria indigenous dogs. Akogwu et al. (2017a,b,c) carried out similar work in goats using piroxicam and sulphadimidine. Immunogenic potential and different physicochemical properties of the two drugs could be of therapeutic benefit. The present study was carried out, with an intent to identifying effect of diaminazene aceturate on kinetics of piroxicam, following intramuscular administration in dogs.
2 Materials and methods
2.1 Drugs
Diminazene aceturate and piroxicam produced by Hambet, Shandong, China were used for the studies at single dose of 3.5 mg/kg body weight.
2.2 Determination of therapeutic dose of piroxicam
Human equivalent dose formula was used to determine therapeutic dose of piroxicam (3.5 mg/kg) in dogs (Saganuwan, 2012).
But: Human BSA = H0.528 × W0.528 × K.
Dog BSA = H0.528 × W0.528 × K (multiply height by 2).
Km = metabolic constant; BSA = Body surface area; K (constant)=0.14; H =height; W = weight.
2.3 Experimental animals
The study was conducted in the Department of Veterinary Phamacology and Toxicology laboratory, Collage of Veterinary Medicine, Federal University of Agriculture Makurdi, Nigeria. Ten apparently healthy Nigerian indigenous dogs of both sexes, aged 8 ± 2 months, and weighing 10 ± 0.5 kg were purchased from dog market in Makurdi, and used for the experiment. The animals divided into two groups of 5 each, were kept in a clean kernel, and fed normal rice, beans, semovita, fish and meat thrice daily. Clean water was provided ad libitun. The dogs acclamatized for 14 days were handled according to the international guiding principles on biomedical research involving the use of animals (CIONs and ICLAS, 2012), as approved by the Ethical Committee, Collage of Veterinary Medicine, Federal University of Agriculture Makurdi, Nigeria.
2.4 Experimental design
Modified randomized cross-over controlled design was adopted for this study. Each of the animals was randomly picked for administration of the drugs. The group of dogs administered piroxicam was used as control (Akogwu et al., 2017a).
2.5 Drug administration and sampling
Ten dogs (5males; 5 females) were injected piroxicam (3.5 mg/kg) in the thigh muscles. After one month, the same group of dogs was injected in the separate thigh muscles, diminazene aceturate (3.5 mg/kg), followed by piroxicam at 3.5 mg/kg body weight. Modified arithmetic method was used for blood sampling (Akogwu et al., 2017b). Blood samples were collected ten minutes before drug administration, from the cephalic vein using a 23G needle, and 5 ml syringe into ethylene diamine tetra acetate (EDTA) bottles at 0.0, 0.08, 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 24, 48, 72, 96 h, respectively, for analysis of piroxicam. The samples collected were immediately centrifuged at 5000 revolution per minute (rpm) for 5 min. Plasma was obtained using a micropipette, placed in cryogenic vials, and stored at −20 °C until analyzed.
2.6 Preparation of standard
Piroxicam (1,000 mg/ml) stock solution was diluted with acetonitrile to obtain serial dilutions containing 10, 20, 30, 40, 50, 60, 70, 80, 90 and 100 µg of piroxicam in 1 ml of solution. One millitre of each was again diluted with 5 ml of acetonitrile. One millitre of plasma was added to each of the solutions. The tubes were centrifuged at 2500 rpm for 15 min, and 1 ml of supernatant was removed and 0.1 ml of 1.47 M aqueous perchloric acid (HCLO4) added. A spectrophotometer at 330 nm was used to measure the absorbance against a blank prepared in same manner with piroxicam and piroxicam/diminazene aceturate. The absorbance was plotted against concentration of piroxicam and diminazene aceturate (Akogwu et al., 2017a,b).
2.7 Analysis of piroxicam
The revised method of Nagabhushanam and Sudha (2010) was adopted. Acetonitrile 2.5 ml was added to 1 ml of serum in centrifuge tubes, mixed gently and properly. The tubes were centrifuged at 2500 rpm for 15 min, and 1 ml of the supernatant was added to 0.1 of 1.47 M aqueous perchloric acid solution. The mixture was properly shaken and measured at 330 nm using a spectrophotometer, to obtain the absorbance against a blank prepared in the same manner with plasma. Minimum limit of detection was 2 µg/ml (Akogwu et al., 2017a,b).
2.8 Calculation of pharmacokinetic parameters
The pharmacokinetic parameters for individual animals were calculated manually using established non-compartmental pharmacokinetic equations (Baggot, 2001) as modified by Saganuwan (2020). The fractions of doses absorbed were determined according to the method of Saganuwan (2012).
-
Cmaxwas determined from graph
-
Tmaxwas determined from graph
-
Absorption rate constant (α) was calculated as follows;
-
Absorption half-life (T1/2) =
-
Mean absorption time (MAT)=
-
Elimination rate constant (β) was calculated as: β=
-
The values of microconstant were used to determine the following parameters; Elimination half- life (T1/2β) =
-
Area under curve: AUC0-n = {Cp1 + Cp2 (t2-t1) +{Cp2 + Cp3 (t3-t2)} +……..(mg/L/hr)
-
Area under the curve Zero to infinity (AUC0-∞) = AUC0-96 + (mg/L/h)
-
Body clearance (Clb = Vd × β (L/kg/hr)
-
Volume of distribution area (Vd) =
-
Mean residence time (MRT) =
-
Area under moment curve = MRT AUC (mg/L)
-
Fraction of absorbed drug (Fad) = = 6 h)
Fractions of piroxicam absorbed from zero to 96 h and zero to infinity were calculated and translated to percent drug absorbed.
Xv. Bioavalability of absorbed fraction (Faf) =.
2.9 Statistical analysis
Plasma kinetic data were presented both in graphical and tabular form. Plasma concentrations and pharmacokinetic parameters for piroxicam and piroxicam/diminazene aceturate were presented as mean standard error of mean (SEM). Kinetic parameters were analyzed using students t test paired at 5% level of significance. The concentrations of piroxicam and piroxicam/diminazene aceturate were analyzed using two-way analysis of variance (ANOVA), and least significant difference was detected at 5% level (Zar, 2008).
3 Results
3.1 Plasma concentration–time profile of piroxicam alone and piroxicam co-administered with diminazene aceturate in Nigerian indigenous dogs following intramuscular administration
The concentration of piroxicam and piroxicam/diminazene aceturate over a range of time is presented in Table 1. The concentrations were not different, significantly (p > 0.05) at 0.08 and 0.25 h. However, the concentration was significantly higher (p < 0.05) at 0.5 h (8.270 ± 0.98), 2 h (8.870 ± 1.47), 3 h (10.260 ± 0.36 µg/ml) in piroxicam/ diminazene aceturate group as compared to 4.70 ± 0.95, 5.500 ± 1.49 and 6.680 ± 0.87 µg/ml in the piroxicam group respectively. Nevertheless at 12 h, the concentration of piroxicam /diminazene aceturate dropped significantly (p < 0.05) to 5.780 ± 1.2 µg/ml as compared to piroxicam group (7.730 ± 1.65 µg/ml), respectively (Table1). Plasma concentration–time profile of piroxicam alone (Fig. 1), piroxicam/diminazene aceturate (Fig. 2) and combination of piroxicam and piroxicam/diminazene aceturate (Fig. 3) obey two compartmental model of kinetics that is flip-flop in nature.
Time (hr)
Piroxicam alone
Diminazene aceturate/Piroxicam
0.08
4.960 ± 0.77
5.030 ± 0.94
0.25
5.240 ± 0.99
4.630 ± 1.56
0.5
4.740 ± 0.95
8.270 ± 0.98a
1
5.830 ± 0.92
5.470 ± 1.02
1.5
9.190001 ± 1.79
6.160 ± 1.45b
2
5.800 ± 1.49
8.870 ± 1.47a
3
5.720 ± 0.93
10.260 ± 0.36a
4
6.850 ± 0.87
5.910 ± 0.78b
6
6.680 ± 0.87
10.230 ± 0.36a
8
9.460 ± 3.34
8.700 ± 0.99
10
8.816 ± 2.43
8.060 ± 1.26
12
7.730 ± 1.65
5.780 ± 1.26b
24
7.060 ± 1.25
7.640 ± 0.95
48
7.860 ± 1.00
6.660 ± 1.13
72
8.700 ± 2.74
7.800 ± 1.54
96
9.050 ± 1.32
9.940 ± 0.56
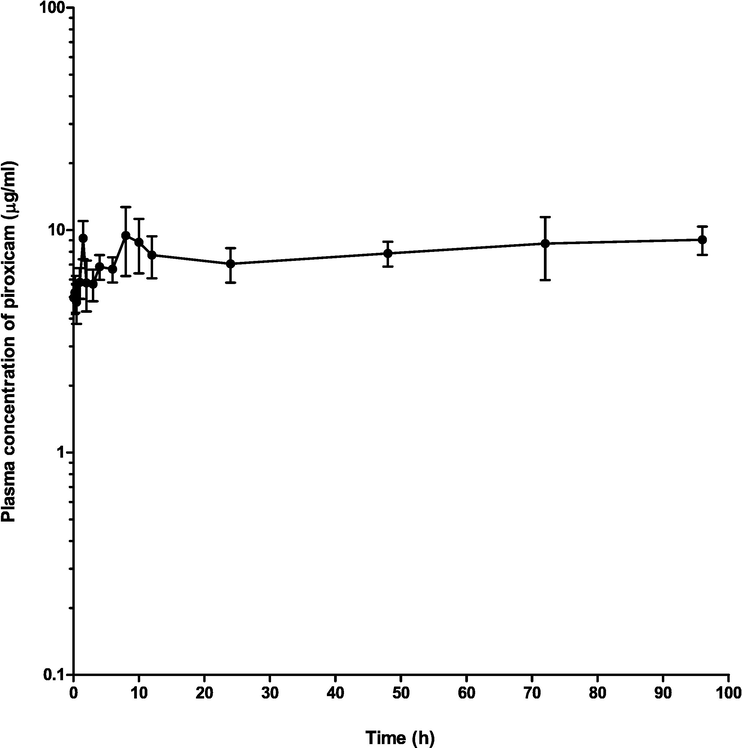
Mean plasma concentration–time curve of 3.5 mg/kg intramuscular piroxicam alone in dogs (n = 10).
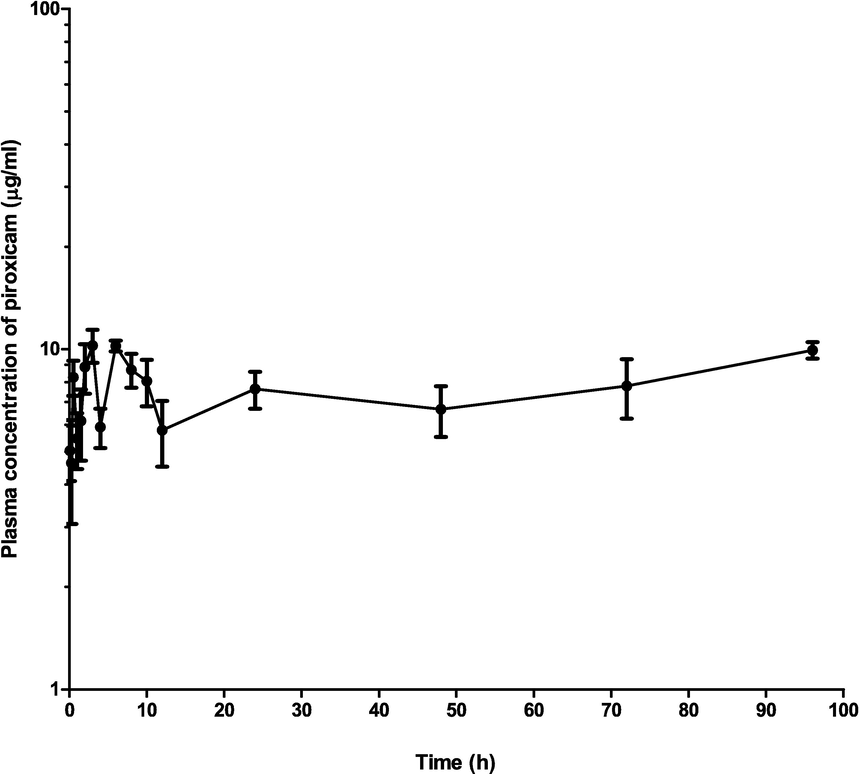
Mean plasma concentration–time curve of 3.5 mg/kg intramuscular piroxicam coadministered with intramuscular diminazene aceturate (3.5 mg/kg) in dogs (n = 10).
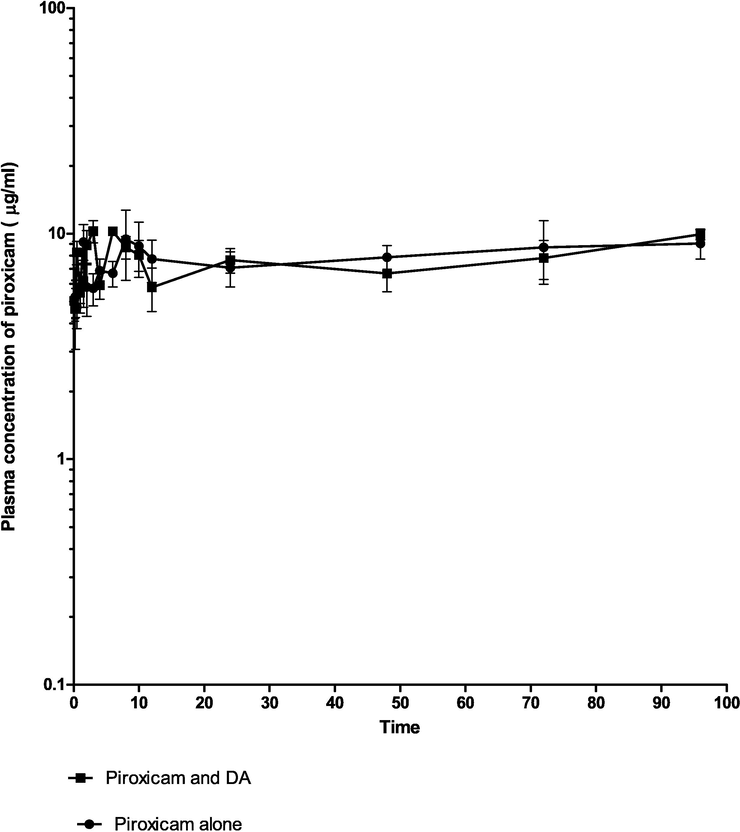
Mean plasma concentration–time curve of 3.5 mg/kg intramuscular piroxicam, and 3.5 mg/kg of piroxicam/diminazene aceturate (3.5 mg/kg) in dogs respectively (n = 20).
3.2 Pharmacokinetic prarameters of intramuscular piroxicam and piroxicam/diminazene aceturate in Nigerian indigenous dogs
The calculated pharmacokinetic parameters of piroxicam and piroxicam/diminazene aceturate are presented in Table 2. Plasma concentration maximum (Cmax = 12.659 ± 0.85 µg/ml), time peak (Tmax = 13.675 ± 9.21 h), absorption half-life (T1/2α = 0.016 ± 0.04 h), elimination rate constant (β = 0.137 ± 0.10 h−1), area under curve from zero to infinity (AUC0-∞=778.885 ± 66.99 mg/L/h) and area under moment curve (AUMC = 98200.140 ± 5.33 mg/h2/L) were significantly lower (p < 0.05) in piroxicam/diminazene aceturate treated group as compared to Cmax (18.560 ± 2.97 µ/mg), Tmax(45000 ± 11.49 h), T1/2α(0.250 ± 0.00 h), β(0.935 ± 0.75 h−1) AUC0-∞(814.472 ± 86.43 mg/L/h) and AUMC(36274640 ± 9010 mg/h2/L) in the piroxicam treated group respectively. However, absorption rate constant (α = 7.405 ± 1.75 h−1), mean absorption time (MAT = 0.658 ± 0.45 h), area under curve AUC0-96h (734.832 ± 63.20 mg/L/h), volume of distribution (Vd = 0.311 ± 0.16 L/kg) and mean residence time (MRT = 106.561 ± 54.84 h) were significantly higher (p < 0.005) in piroxicam/diminazene aceturate group as compared with α(5.823 ± 1.46 h−1), MAT (0.361 ± 0.11 h) Vd (0.206 ± 0.08 L/kg) and MRT (49.793 ± 13.43 h) in the group treated with piroxicam alone respectively (Table 2).
Kinetic parameters
Piroxicam alone
Diminazene aceturate/piroxicam
Cmax (µ/ml)
18.560 ± 2.97
12.659 ± 0.85b
Tmax(h)
45.000 ± 11.49
13.675 ± 9.21b
α(h-1)
5.823 ± 1.46
7.405 ± 1.75a
T1/2α (h)
0.250 ± 0.08
0.016 ± 0.04b
MAT(h)
0.361 ± 0.11
0.658 ± 0.45a
β(h-1)
0.935 ± 0.75
0.137 ± 0.10b
T1/2β (h)
33.634 ± 9.34
34.850 ± 11.94a
AUC0-96(mg/L/h)
768.370 ± 9.34
734.832 ± 63.20a
AUC0-∞(mg/L/h)
814.472 ± 86.43
778.885 ± 66.99b
CL(L/kg/h)
0.0034 ± 0.00
0.003 ± 0.00
Vd(L/kg)
0.2062 ± 0.08
0.311 ± 0.16a
MRT(h)
49.793 ± 13.43
106.561 ± 54.84a
AUMC(mg/h2/L)
36274.840 ± 9010.44
9820.140 ± 5.33b
Fab0-96 h (%)
7.848 ± 0.00
7.505 ± 0.00b
Fab(0-∞%)
8.319 ± 0.04
7.955 ± 0.03b
Faf(%)
–
95.63 ± 8.22
4 Discussion
The slow elimination rate of piroxicam and piroxicam/diminazene aceturate in the present study agrees with the report, indicating that antinflammatory and antibiotic agents could display flip-flop kinetics, which occurs when the absorption rate is slower than the elimination rate, hence a long duration sampling is required. Dosage formulations, chemistry of drugs, excipient and extravascular physiology could cause flip-flop phenomenon (Yanaz et al., 2011), characterized by slower absorption rate constant (5.823 ± 1.46 h) and elimination rate constant (0.935 ± 0.75 h) for piroxicam alone, as compared to 7.405 ± 1.75 h and 0.137 ± 0.10 h for piroxicam/diminazene aceturate, respectively. The rate-limiting factor of intramuscular absorption is the deposit site from which drugs are diffused (Pintand et al., 1992). Reported is flip-flop kinetics of meclofenamic acid (horse), indomethacin (poultry), acetaminophen (rat) (Baggot, 2001), benzimidazole (horse), cefazolin (horse) (Baggot, 2001; Sams and Ruoff, 1985), amoxicillin/clavulaninic acid (goat), amprolium (chicken) and sulphadimethoxine (ungulates), respectively (Baggot, 2001; Chatfield et al., 2001). Pathophysiological conditions that could cause flip-flop principle are congestive heart failure and liver cirrhosis (Brater et al., 1984; Fredrick et al., 1991). Different routes of drug administration could be responsible for flip-flop phenomenon (Yanaz et al., 2011). The decreased Cmax, Tmax, T1/2α, β, AUC0-∞ and AUMC as well as increased α, MAT, AUC0-96h,Vd and MRT in the group administered piroxicam with diminazene aceturate are suggestive of non-linear mixed kinetics. Steady state concentration could yield optimal therapeutic regimen (Saganuwan, 2020), that maximum plasma concentration, maximum time reached, elimination half-life and volume of distribution are the most important kinetic parameters (Akogwu et al., 2017a,b, c). The disposition kinetics of piroxicam and piroxicam/diminazene aceturate in the present study disagrees with the report of Akogwu et al. (2017c) indicating that elimination of piroxicam in goats was independent of absorption. The higher elimination half-life (33.634 ± 9.34 h) of piroxicam and piroxicam/diminazene aceturate (34.85 ± 11.94 h) as compared to that of goat, mice, rat and rhesus monkey (2–9 h) show that piroxicam elimination is not absorption dependent in these species of animals (Akogwuet al., 2017a; Milone and Twomey, 1980). Elimination half-life of 45 h has been reported for beagle (Hobbs and Twomey, 1981). The differences in species variation, route of administration and sex could be responsible for differences in the kinetic parameters. Therefore combination of the two drugs may be useful for the treatment of trypanosomosis, leishmniasis, amoebiasis, pneumocystis associated with fever, inflammation and pain (Oliveira and de Freitas, 2015).
Wagner-Nelson principle may be used to determine linear segment of absorption rate. Curved or curve linear segment of absorption may yield component of absorption rate over time (Wagner and Nelson, 1964). Hence a two compartment model could be collapsed into one compartment model. When absorption is first order in one compartment open model, but stops abruptly, Guggenhein method may be applied for calculation of absorption phase. Nevertheless, estimation error of elimination rate constant could lead to Wagner-Nelson absorbed fraction of kinetic greater than unity (Wang and Nedelman, 2002). Slow absorption rate of piroxicam may be due to low dose of piroxicam administered, invariably preventing side effects. High dose of administered piroxicam could be distributed faster than low doses (Saganuwan, 2016).
The slow release of piroxicam in dogs may be due to mechanochromic nature of the drug. Piroxicam is converted to zwitterionic compound via recrystallization and intermolecular proton transfer (Cheng and Choi, 2000). The breakdown of a weak C—O bond results in chemical change (Frank et al., 1996). Monohydrate form is more wettable, hence dissolves faster (Cini, et al., 2007) than other forms which are lypophilic (Saganuwan, 2016). Microencapsulated form has higher capacity of release in the plasma, as co-crystal formation with carboxylic acid increases its bioavailability (Vrecer et al., 2003). Cubic crystal better solubility and low extent of bioavailability (Liu et al., 2010), is related to protein binding capacity and sex difference (Akogwu et al., 2017a). High plasma level of piroxicam for long period correlates with its analgesic activity (Milone and Twomey, 1980). Piroxicam dissolved in polyvinylpypyrrolidine is highly viscous, hence released slowly (Takaca-Novak et al., 2004). Acidity of dog urine and enolic function of piroxicam with pKa of 2.68 (Christifis et al., 2005), may be responsible for its delay elimination. The acid-base property of piroxicam is responsible for its structure–activity relationship (Manewka et al., 2014). High piroxicam concentration decreases water molecule in the body, becoming apolar, that can result in concentration- independent high-energy shift of the absorption maximum (Shah, 2003). Hence dog administered piroxicam should be given large quantity of water for faster absorption and elimination. Piroxicam embedded in hydrophilic polymer may be released faster (Saganuwan, 2016). Addition of sodium or potassium to piroxicam enhances its pharmacokinetic and pharmacodynamic activity, and electrospinning increases dissolution rate (Lombardino and Lowe, 2004). A combined intramuscular formulation of piroxicam and diminazene aceturate of 3.5 mg/kg each could be a potent, high efficacious polypharmacy against trypanomosis and babesiosis. Many parenteral drugs are outside physiological pH range, hypertonic, and lack aqueous vehicles. Hence the absorption is erratic, causing tissue irritation and pain at the site of injection. Ampicillin, cephradine, dicloxacillin, quinidine, phenytoin and digoxin are not suitable for intramuscular injection. Propylene glycol cause drug precipitation at intramuscular injection site as seen with diazepam and phenytoin. Oily solution and aqueous suspension of drug are absorbed slowly as seen for ceftiofur (Baggot, 2001).
5 Conclusion
Piroxicam and piroxicam coadministered with diminazene aceturate showed flip-flop mechanism of absorption, invariably leading to long elimination half-life in dogs. Hence the combination may be good for the treatment of trypanosomosis, babesiosis, leishmaniasis, amoebiasis and pneumocytis in dogs. The combination therapy could be once or twice and be repeated when necessary.
Authors’ contributions
SAS designed the study, did the statistical analysis and wrote the manuscript, OFE and AJO carried out the study, and all the authors proofread the manuscript.
Funding
The authors funded the research using their monthly emoluments.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the manuscript.
Acknowledgements
The authors sincerely thank Mr. Vincent Upev, Department of Veterinary Physiology and Biochemistry, Federal University of Agriculture Makudi for his assistance in various capacities.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Effects of piroxicam on pharmacokinetics of sulphadimidine in West African dwarf male and female goat (Capra hircus) Pharmacent. Anal. Acta. 2017;8(7):1-7.
- [Google Scholar]
- Effects of piroxicam on tissue distribution of sulphadimidine in West African dwarf goats. Human Exp. T oxicol.. 2017;35(12):1-8.
- [Google Scholar]
- Comparartive pharmacokinetics of piroxicam in male and female West African dwarf goats. Congent Food Agric.. 2017;2017(3):1-7.
- [Google Scholar]
- The physiological Basis of Veterinary Clinical Pharmacology. Oxford, UK: Blackwell Science; 2001. p. :298.
- A comparative experimental study of the effects of diclofenac and ketoprofen on the small-bowel muscosa of canines. Res. Exp. Med.. 2001;200(2):125-135.
- [Google Scholar]
- Disposition of sulfadimethoxine in camels (Camelus dromedarius) following single intravenous and oral doses. J. Zoo Wildlife Med.. 2001;32(4):430-435.
- [Google Scholar]
- Enhanced percutaneous absorption of piroxicam via salt formation with ethanolamines. Pharm. Res.. 2000;19(9):1-7.
- [Google Scholar]
- Mononuclear metal complexes with piroxicam. Synthesis, structure and biological activity. J. Inorg. Biochem.. 2005;99(11):2197-2210.
- [Google Scholar]
- Unusual coordinating behavior by three non-steriodal anti-inflammatory drugs from the piroxicam family towards copper (II). Synthesis, x-ray structure complexes, and cytotoxic activity for a copper (II)- piroxicam complex. J. Inorg. Biochem.. 2007;101(8):1140-1152.
- [Google Scholar]
- CIOMS, ICLAS, 2012. http://www.cioms.ch/index.php/12-newsflash/326-cioms-and-clas-reelease-the new-international-guiding-principles-for-biomedical-research-involving-animals (accessed 12 December 2015).
- Quantum chemical investtigation of the thermal and photoninduced proton-transfer reaction of 2-(2', 4'- dinitrobenzyl) pyridine. J. Phys. Chem.. 1996;100(40):16187-16194.
- [Google Scholar]
- Furosemide absorption in patients with cirrhosis. Clin. Pharmacol. Ther.. 1991;49(3):241-247.
- [Google Scholar]
- Berenil in Veterinary Medicine: Report From Chemotherapy Institute. United State: Institute of Faberwerke Hoechst; 1995.
- Metabolism of piroxicam in laboratory animals. Drug Metab. Dispos.. 1981;9:114-118.
- [Google Scholar]
- Canine hepatozoonosis and babesiosis, and feline cytauxzoonosis. Vet. Clin. N. Am. Small Anim. Pract.. 2009;39(6):1035-1053.
- [Google Scholar]
- Influence of structure on the spectroscopic properties of the polymorphs of piroxicam. J. Phys. Chem.. 2010;114(49):16641-16649.
- [Google Scholar]
- Discovery of piroxicam (1962–1980) from the following article: the role of the medicinal chemistry in drug discovery then and now. Nat. Rev. Drug Disc.. 2004;3:853-862.
- [Google Scholar]
- The interaction of new piroxicam analogues with lipid-bilayers – a calorimetric and fluorescience spectroscopic study. Acta Poloniae Pharm. Drug. Res.. 2014;71(6):1004-1012.
- [Google Scholar]
- The analgesic properties of piroxicam in animals and correlation with experimentally determined plasma levels. Agent Act. 1980;10(1–2):31-37.
- [Google Scholar]
- Nagabhushanam, M.V., Sudha, R.A., 2010. Evaluation of pharmacokinetics parameters of solid dispersions of piroxicam. Inkollu: DCRM pharmacy college, Department of Pharmacentics. pp. 333–343.
- Diminazene aceturate- an antiparasitic drug of antiquity: advances in pharmacology and therapeutics. Pharmacol. Res.. 2015;102:138-157.
- [Google Scholar]
- Nonlinearity of amoxicillin absorption kinetics in human. Eur. J. Clin. Pharmacol.. 1992;43(3):283-288.
- [Google Scholar]
- Riviere, J.E., Papich, M.G., 2017. Veterinary Pharmacology and Therpeutics, 10th ed., p. 1552.
- Principles of Pharmacogological Calculations (1st ed.). Zaria: Ahmadu Bello University Press; 2012. p. :529.
- Physicochemical and structure -activity properties of piroxicam – a mini review. Comp. Clin. Pathol.. 2016;25:941-945.
- [Google Scholar]
- Unique pharmacokinetic and pharmacodynamic parameters of antimicrobials in goats. In: Kukovics S., ed. Goat (Capra) from Ancient to Modern. London: Intechopen; 2020. p. :135-158.
- [Google Scholar]
- Pharmacokinetics and bioavailability of cefazolin in horses. Am. J. Vet. Res.. 1985;46:348-352.
- [Google Scholar]
- Drug delivery to the central nervous system: a review. Pharmacy Pharmaceut. Sci.. 2003;6(2):252-273.
- [Google Scholar]
- Microscopic protonation/deprotonation equilibria of the anti-inflammatory agent piroxicam. Halvetica Chem. Acta. 2004;78(3):553-562.
- [Google Scholar]
- Trapp, S., Messick, J.B., Vidotto, O., Jojima, F.S., de Morais, H.A. Babesia gibsoni genotype Asia in dogs fromPa Brazil. Vet. Parasitol. 141;(1-2):177-180.
- Characterization of piroxicam crystal modifications. Int. J. Pharmaceut.. 2003;256(1–2):3-15.
- [Google Scholar]
- The kinetic analysis of blood levels and urinary excretion in the absorptive phase after single doses of drug. J. Pharmacent. Sci.. 1964;53:1392-1403.
- [Google Scholar]
- Early clinical development of pharmaceuticals for Type 2 Diabetes Mellitus. J. Clin. Endocrinol. Metab.. 2002;87(12):5362-5366.
- [Google Scholar]
- Flip-flop pharmacokinetics delivering a reversal of disposition: Challenges and opportunities during drug development. Ther. Deliv.. 2011;2(5):643-672.
- [Google Scholar]
- Biostatistical Analysis (4th ed.). New Delhi, India: Pearson Education; 2008. p. :663.
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102914.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







