Effects of different host plants on population fitness of pea aphid (Acyrthosiphon pisum)
⁎Corresponding author at: Biocontrol Engineering Laboratory of Crop Diseases and Pests of Gansu Province, College of Plant Protection, Gansu Agricultural University, Lanzhou 730070, China.
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University. Production and hosting by Elsevier.
Abstract
Background
Host plants not only provide and living places and energy materials for insects, but also influence insect population parameters and population fitness.
Methods
This study examined the influence of various host plant species on the fitness of pea aphid (Acyrthosiphon pisum). The biological parameters and population parameters of pea aphid on 6 different host plants (Vicia fabae, Pisum sativum, Medicago sativa, Trifolium pratense, Onobrychis viciaefolia and Melilotus officinalis) were observed and counted by ecological experiments, which were carried out in a control chamber.
Results
The results showed that the developmental duration of 1st and 2nd instar nymphs of pea aphids on T. pratense and P. sativum was significantly prolonged, whereas that of 3rd and 4th instar nymphs on O. viciaefolia and M. officinalis was significantly shortened. Compared with the pea aphid on the V. faba, the longevity of adults on M. officinalis and P. sativum was significantly prolonged, but only the generation time on P. sativum was significantly prolonged. Moreover, the survival rate of nymphs was significantly lower on O. viciaefolia and M. sativa than on others. Net reproductive rate and mean generation time on V. faba were significantly higher than in other host plants. The intrinsic rate of increase (rm) and finite rate of increase (λ) of pea aphid feeding of A. pisum on P. sativum and O. viciaefolia decreased. However, those on the double population time on P. sativum and O. viciaefolia were significantly higher than the others.
Conclusion
The findings will clarify the population fitness of pea aphids on different hosts and guide the rational distribution of different host plants, and provide new references for aphid control strategies.
Keywords
Pea aphid
Host plant
Survival rate
Population parameter
Population fitness
1 Introduction
Aphids is an important kind of pest with piercing-sucking mouthparts, which can reduce crop yields by invading plant tissues and absorbing phloem sieve components. This results in stunted plant development and low growth. At the same time, aphids can spread plant virus diseases, causing infection and severe damage to crops. In addition, honeydew secreted by aphids not only affects the photosynthesis of plants, but also causes soot diseases of the plant (Gong et al. 2014, Patrick et al. 2018, Nalam et al. 2018). The pea aphid (Hemiptera: Aphididae), scientifically known as Acyrthosiphon pisum Harris, is a major pest across the world because it feeds on many different kinds of leguminous plants (Peccoud et al. 2009b, De Geyter et al. 2011, Peccoud et al. 2015). When introduced into a suitable host field, pea aphids can rapidly increase population size due to their parthenogenetic system and short generation time, resulting in significant economic losses. Furthermore, pea aphids are capable of spreading more than 30 plant viruses, such as pea streak virus, red clover vein mosaic virus, and bean yellow mosaic virus, which can be transmitted through aphids (Peccoud et al. 2009a, Goławska and Łukasik 2012, Congdon et al. 2017). Consequently, serious production losses occurred the destruction of alfalfa fields by pea aphids caused an average annual loss of 60 million US dollars in the United States (Harmon et al. 2009), and an annual economic loss of about 10%-30% in Northwest China (He et al. 2005).
Insect populations are affected by biotic and abiotic factors, including host plants, temperature, carbon dioxide, and concentration (Sun et al., 2016). Both plants and the insects that feed on plants are engaged in a strong competition for their own survival(Tesfaye et al., 2021). Host plants have created a wide variety of unique, poisonous, and insect-repelling compounds that serve as organic defenses against herbivorous insects (Li et al. 2017). These plants can synthesize a variety of secondary metabolites, such as phenolic compounds, phenols, saponins, flavonoids, and alkaloids (Heidel-Fischer and Vogel 2015). Secondary metabolites can repel phytophagous insects or have antifeedant, toxic and regulatory activities by increasing oxidative stress in insect tissues, thus affecting insect physiology (Woźniak et al. 2019, Goławska and Łukasik 2012). To maintain homeostasis, aphids have evolved complex adaptive mechanisms, such as detoxification enzymes against host plants' defense (Heiko and Celorio-Mancera, 2014)Li et al. 2020). Activities of insect detoxification enzymes (Pei et al. 2010), such as glutathione S-transferases (GSTs), cytochrome P450 (CYP450s), and carboxylesterases (CarEs), protect aphids under stress (Heidel-Fischer and Vogel 2015, Amezian et al. 2021). Changes in biochemical and morphological characteristics associated with plant defense have a significant affect on the expression of plants resistance to insect pests (Sharma et al. 2016b). In the last few years, the emergence of the global greenhouse effect, the frequent occurrence of extreme climates, and the incorrect use of chemical fertilizers and insecticides in agriculture have led to the significant expansion of the aphid population (Sharma et al. 2016a, Chen et al. 2019).
Climate change has increased the impact of irregular weather conditions, such as low and erratic precipitation, which can lead to drought stress and increase pest population density, adversely affecting crop production (Sharma et al. 2016a, Chen et al. 2019). Sap-sucking insects are among the most significant economic pests of crops and cause substantial damage to agricultural production all over the world (Nguyen et al., 2017). Globally, farmers consider pea aphids a more serious economic pest than defoliators. These aphids cause extensive plants damage by feeding, honeydew production, and transmission of the virus. As a result, a variety of synthetic pesticides are still employed to manage agricultural pests. Such a method has seriously endangered the health of farmers, animals, and food consumers while also greatly increasing environmental pollution and pesticide resistance. Hence, the identification of aphid-resistant cultivars is critical to agricultural production. The hypothesis behind this research is that the natural defense of different host plants will affect the performance of pea aphids. As a result, the study aimed to evaluate how various host plant species affect the population fitness of pea aphids and to identify its ecological phenotypes on different hosts. This study serves as a basis for further research on the interactions between pea aphids and host plant species.
2 Materials and Methods
2.1 Aphids culture
Pea aphids were collected from the alfalfa experimental field of Gansu Agricultural University in Lanzhou, China (36.03°N, 103.40°E). The parthenogenesis of one pea aphid led to the establishment of a single asexual line, which was used for further tested materials. The aphid populations were cultured on broad bean Vicia fabae under a 16-h ligh:8-h dark photoperiod at 22 ± 1 ℃ with 70–80% relative humidity in the laboratory. Aphid cultures were maintained for at least 3 generations before being used in the experiment.
2.2 Host plants
The experiment involved six host plants, including broad bean Vicia fabae (primary host plant), pea (Pisum sativum), alfalfa (Medicago sativa), clover (Trifolium pratense), red bean grass (Onobrychis viciaefolia) and melilotus (Melilotus officinalis). All host plants were used for further experiments in the laboratory to study the effects of different host plants on the population fitness of pea aphids. The experimental populations of different host plants of pea aphids were established in the laboratory with six host plants of at least 3 generations and then used in the experiment.
2.3 Effects of different plants on the growth and reproduction of pea aphid
To investigate how different host plants impact the growth, development, and fecundity of pea aphids, the experiment utilized detached leaves-feeding method. This involved placing fresh and clean leaves on a piece of filter paper in a Petri dish (10 cm). The petioles of leaves were wrapped with absorbent cotton balls, and sufficient ddH2O was added to keep the cotton ball and filter paper wet. Then one aphid was put into a Petri dish within 6 h after birth and fed on the leaves of the corresponding six plant species. The Petri dishes were placed in an artificial climate box (RZX, Ningbo Jiangnan Co. Ltd., Ningbo, China) with a temperature of 22 ± 1 °C, 70–80% relative humidity, and a 16-h ligh:8-h dark photoperiod. The fresh leaves were added every 3 days. A total of aboat 50 aphids were used per plant. The number of dead aphids, the molting time, and frequency was observed and recorded every 12 h, and the molting dander was picked out with camel brush. Each nymph was counted every day until the death of the adult aphids. The biological parameters of pea aphids on six host plants were calculated, such as nymph survival rate, nymph developmental duration, aphid mortality, adult fecundity, and adult longevity.
2.4 Statistical analysis
The biological parameters (developmental duration, adult longevity, and generation time) of a single aphid were used as a biological replicate for statistical analysis. 17 aphids were randomized into one group, and each group was established as a biological replicate for statistical analysis of the time-dependent life table and nymph survival rate. The experiment was repeated three times. Population parameters of different host plants were calculated as: Net reproductive rate: R0 = ∑ lxmx; Mean generation time: T = ∑ xlxmx ∕ ∑ lxmx; Intrinsic rate of increase: rm = lnR0 ∕T; Finite rate of increase: λ = erm; Population doubling time: Dt = ln2∕rm; where x is a time interval in days, lx denotes the survival probability of female during the period of x, and mx indicates the average numbers of new nymphs during the period of x (Gou et al.2021, Govindan and Hutchison 2020). Excel 2019 was used for data sorting, and Sigmaplot 12 (Systat Software Inc., San Jose, CA, USA) was used to draw diagrams. Statistical analysis was performed using IBM SPSS Statistics version 20.0 (SPSS 20.0) (IBM, Armonk, NY, USA). Tukey' S HSD was used in the variance analysis (ANOVA) to indicate significant differences among different treatments. Nymphal survival data was performed arcsine transformation and then analyzed with one-way ANOVA.
3 Results
3.1 Effects of different host plants on the developmental duration of pea aphid
Six host plants have different effects on the development duration of the pea aphid. The development duration of the 1st instar nymph of pea aphid on the T. pratense was the longest, which was significantly different from the other five hosts (Fig. 1A, F(5, 300) = 11.027, P < 0.001). The developmental duration of the 2nd instar nymph was significantly shorter on M. officinalis than that of P. sativum (Fig. 1B, F(5, 300) = 6.014, P < 0.001). The nymph developmental time of the 3rd and 4th instar pea aphids was the same in the six host plants. The development duration of pea aphid on O. viciaefolia was obviously longer than that of V. faba, P. sativum, and on T. pretense (Fig. 1C, F(5, 300) = 9.891, P < 0.001; Fig. 1D, F(5, 300) = 2.991, P < 0.05).
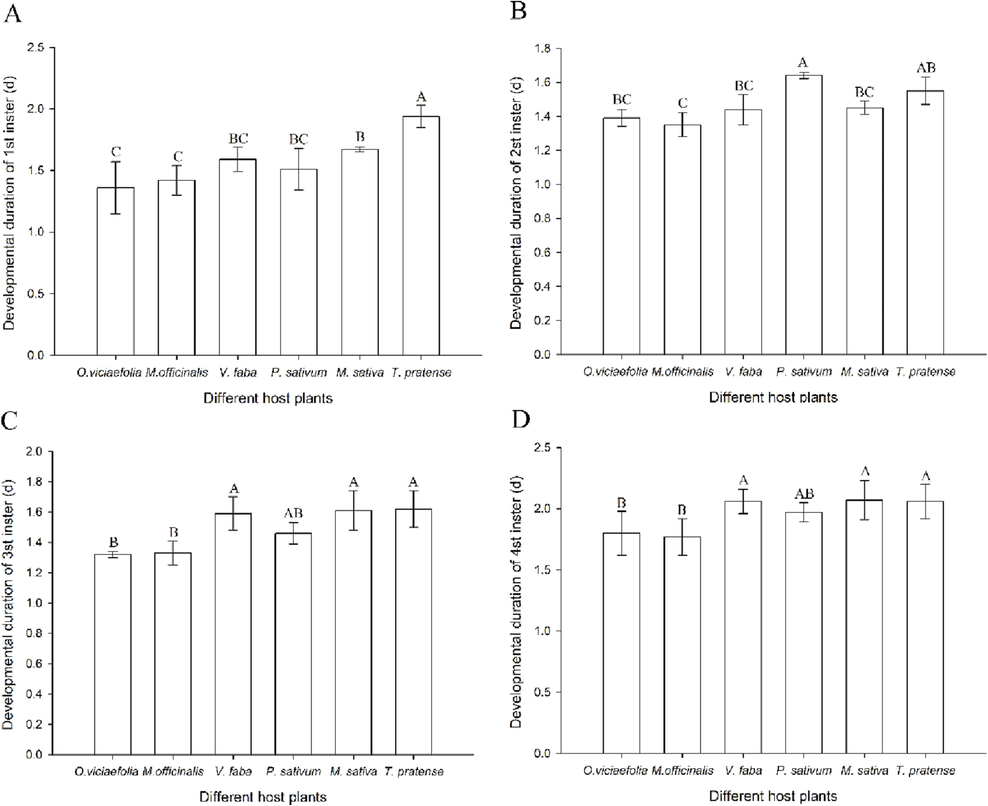
- Effects of different host plants on the developmental duration of pea aphid. (A) developmental duration of 1st instar; (B) developmental duration of 2nd instar; (C) developmental duration of 3rd instar; (D) developmental duration of 4th instar; The bars displayed mean ± SE, different letters above the bars indicate significant differences (P ≤ 0.05).
3.2 The survival rate of pea aphid nymph on different host plants
The survival rate of pea aphid nymph was the highest on the T. pretense, compared with other host plants. There was no significant difference in the survival rates of pea aphid nymphs fed on M. officinalis, T. pratense, P. sativum, and V. faba. However, the nymph survival rates fed on O. viciaefolia and M. sativa had no statistically significant difference but had a significant difference when fed on other plants (Fig. 2, F(5, 12) = 43.185, P < 0.001).
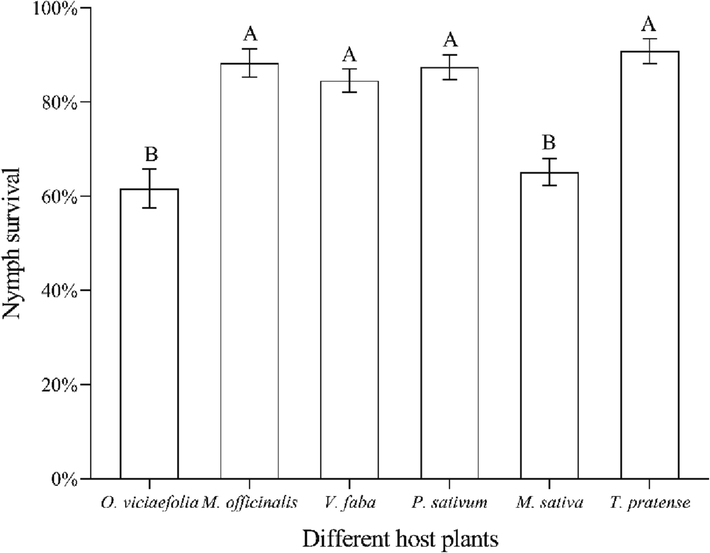
- Effects of different host plants on the nymph survival of pea aphid. Nymphal survival data were performed arcsine transformation and then were analyzed with one-way ANOVA. The bars displayed mean ± SE, different letters above the bars indicate significant differences (P ≤ 0.05).
3.3 Effects of different host plants on the adult longevity and generation time of pea aphid
The adult longevity of pea aphids on M. officinalis and P. sativum was longer than that of the other four host plants (Fig. 3A, F(5, 300) = 210.435, P < 0.001). However, the effect of host plants on the generation duration of pea aphids differed from that of adult longevity. The generation time of pea aphid on T. pratense was the shortest, which was significantly different from that of the P. sativum and M. officinalis, but had no significant difference with that of the other three host plants (Fig. 3B, F(5, 300) = 30.12, P < 0.001).
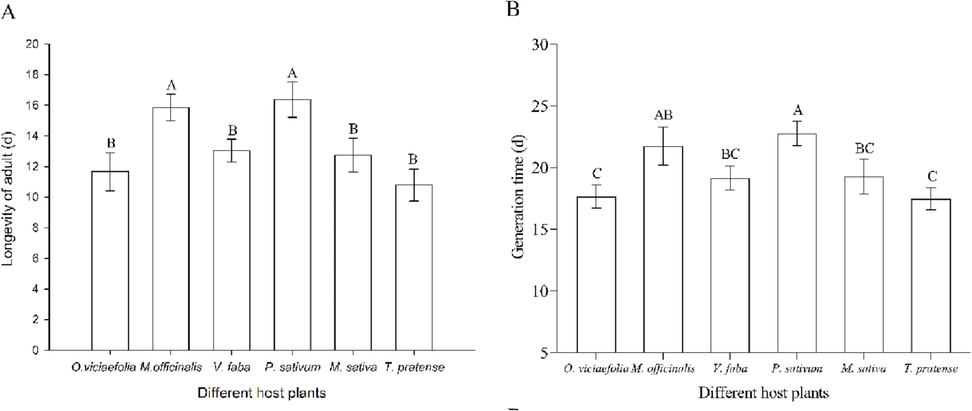
- Effects of different host plants on the longevity of adult and generation time of pea aphid. (A) longevity of adult; (B) generation time; The bars displayed mean ± SE, different letters above the bars indicate significant differences (P ≤ 0.05).
3.4 Effects of different host plants on the survival curve of pea aphid
The nymph survival and survival rate of pea aphids were different in all host plants. The nymph survival on P. sativum and M. officinalis were significantly lower than that on V. faba (Fig. 4). The survival curve of pea aphid on V. faba was significantly different from that of the other five host plants (log-rank test, O. viciaefolia vs V. faba: χ2 = 23.98, P < 0.001; M. officinalis vs V. faba: χ2 = 19.54, P < 0.001; P. sativum vs V. faba: χ2 = 18.54, P < 0.001; M. sativa vs V. faba: χ2 = 23.12, P < 0.001; T. pratense vs V. faba: χ2 = 15.61, P < 0.001). However, the survival curves of pea aphids on five host plants (except for V. faba) were not significantly different from each other, indicating that the population fitness costs of different hosts were different.
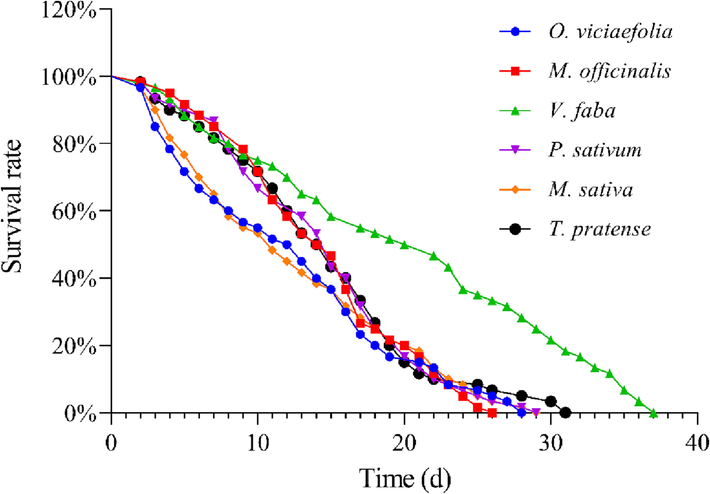
- Effects of different host plants on survival rate of pea aphid. Survival rate datum of pea aphid were analyzed with log-rank test.
3.5 Effects of different host plants on population parameters of pea aphid
Host plants showed significant effects on the population parameters of pea aphids. The population characteristics of pea aphids differed significantly across the six host plants examined. The highest net reproduction rate and mean generation time were seen in aphids feeding on V. faba, which was statistically distinct from the other plants. However, the least net reproductive rate and mean generation time of aphids occurred on O. viciaefolia and M. officinalis, respectively (Fig. 5A, F(5, 12) = 22.465, P < 0.001;Fig. 5B, F(5, 12) = 7.863, P < 0.002). The intrinsic rate of increase of aphids fed on P. sativum was the highest and showed no significant difference compared with the populations fed on V. faba, T. pratense, and M. officinalis. However, they were significantly different from those that fed on O. viciaefolia and M. sativa (Fig. 5C, F(5, 12) = 21.421, P < 0.001). The highest doubling time occurred on aphids fed on M. sativa. There was no significant difference compared with the population fed on O. viciaefolia. However, the doubling time significantly deferred among the populations fed on V. faba, T. pratense, M. officinalis, and P. sativum (Fig. 5D, F(5, 12) = 21.2, P < 0.001). The highest finite rate of increase also occurred in the population fed on P. sativum, which was significantly different from the population fed on V. faba, M. sativa, and O. viciaefolia (Fig. 5E, F(5, 12) = 13.424, P < 0.001).
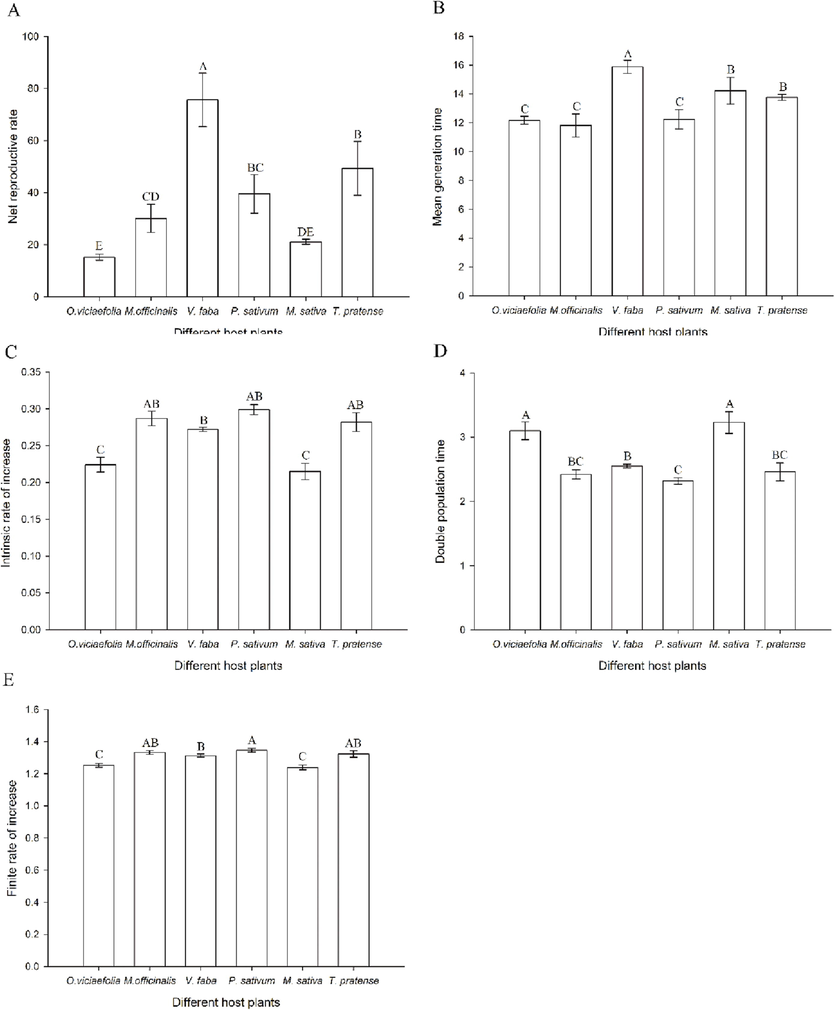
- Effects of different host plants on population parameters of pea aphid. (A) net reproductive rate; (B) mean generation time; (C) intrinsic rate of increase; (D) double population time; (E) finite rate of increase; The bars displayed mean ± SE, different letters above the bars indicate significant differences (P ≤ 0.05).
4 Discussion
Aphids are sap-sucking insect pests, causing economic loss to crops (Nalam et al. 2018). Aphids have evolved complex adaptive mechanisms, such as the defense of detoxification enzymes against host plants (Elzinga and Jander 2013, Will and Vilcinskas 2015, Kaloshian and Walling 2016, Van and Torsten 2016). The global greenhouse effect, frequent occurrences of harsh weather, improper use of chemical fertilizers and pesticides in agriculture, and other factors have all contributed to the recent considerable increase in the aphid population (Sharma et al. 2016a, Chen et al. 2019). On the other hand, several defense mechanisms in plants were developed at the same time. These defense mechanisms included anti-xenobiotic factors, which have a negative impact on the fecundity, survival, growth, and development of aphids (Nalam et al. 2018). Host plants affect not only the quality of nutrition provided to insects but also their interactions, thus affecting insects' biological characteristics and population parameters.
In the present study, all six host plants had different effects on the developmental duration of pea aphids. Among the tested host plants, the 1st instar nymphs had the most extended developmental duration on the T. pratense. The developmental duration of the 2nd instar nymph on M. officinalis was significantly shorter than that of on P. sativum. All six host plants showed the same effect on the developmental duration of the 3rd and 4th instar nymphs. The developmental duration of pea aphid on O. viciaefolia was significantly longer than that of on V. faba, P. sativum, and T. pretense. It implies that the pea aphid has a certain adaptability to host plants. Our research reveals that different host plants can affect the plasticity of aphids in host utilization, which is supported by relevant references (Balog and Schmitz 2013, Barman et al. 2017, Mehrparvar et al. 2019). According to research carried out by Tesfaye (2013), pea aphids are more attracted to field peas than broad beans. The reason for the opposite results of the two experiments may be that the experimental environmental conditions and host plant species are different. The present study was conducted under laboratory conditions with six different host plants, while the research of Tesfaye was conducted under field conditions with four legume crops. Furthermore, the maternal effect is a critical determinant of aphid fitness, which suggests that the performance of offspring is the result of the mother's experience. Because aphids have overlapping telescopic generations, it can be expected that there will be a a significant maternal effect in subsequent offspring generations. Eliminate any biases brought on by the existence of maternal effects, aphids may need to be monitored throughout many generations in novel habitats (Olivares-Donoso et al. 2007, Tariq et al. 2010, Chung et al. 2013). In this study, different host plant had different affects on the nymph survival and survival rate of pea aphids nymphs. The nymph survivals on P. sativum and M. officinalis were significantly lower than that on V. faba. The adult longevity of pea aphids on M. officinalis and P. sativum was significantly longer than that of the other four host plants.
The chemical composition of host plants can be modified due to stress, which can positively or negatively impact the aphid's performance or, in some cases, have no effect. The nutritional conditions and secondary metabolites of host plants will influence the biological parameters of insects. The compositions of the plant epicuticle have been proven to promote the feeding of pea aphids. The relationship between the quality of host plant and the reproductive performance of aphid has also been verified in pea aphids, which provide better nutrients of V. faba can help pea aphids that produce more offspring. Furthermore, V. faba can provide a better plant surface for all aphid host races that are more conducive to aphid growth and reproduction (Friedemann et al. 2015). In this study, the pea aphid raised on fava beans had a higher net reproductive rate and mean generation time, which was more favorable for the growth of the pea aphid population than the other five host plants. Moreover, plants are known to contain secondary metabolites that are capable of affecting the survival of aphids (Balog and Schmitz 2013, Barman et al. 2017, Mehrparvar et al. 2019). This demonstrates that various host plants may have varied effects on the functioning of pea aphids due to differing chemical compositions. The dynamics of herbivore populations may be significantly impacted by changes in the physical and chemical makeup of hosts (Lee and Lee 2013, Kuczyk et al. 2021). This affects the formation and growth of aphids on cucumber and watermelon plants (Moran 1981). Plants' secondary metabolites, known as “plant protectants”, can influence both the biological and phenotypic traits of aphids. The secondary metabolites associated with plant resistance mainly include indirection (phenolic compounds) and end-products (flavonoids, lignin, and isoflavones) (Wu et al. 2021). This indicates that compared with other host plants, P. sativum and M. officinalis may contain secondary metabolites that inhibit growth and development. Although our current research did not involve the effect of host plant secondary metabolites on the growth, development and population parameters of pea aphids. Literature research shows significant differences in the metabolic fingerprints of four leguminous species (M. sativa, T. pratense, P. sativum and V. faba) studied before aphid infestation, which is related to the performance of the aphid (Sanchez-Arcos et al. 2019).
The life table parameter values (R0, rm, and λ) can reflect the ability of the insect population to proliferate and forecast future trends in population rise (Gou et al. 2021). The greatest net reproduction rate and mean generation time were found in the V. faba species in the current investigation. The susceptibility of the V. faba to pea aphids may be due to the lack of noxious compounds or secondary metabolites in the plant, although it exhibited a poor net reproductive rate. The population parameters of pea aphids on different host plants can provide a reference for the reasonable planting layout of six different plants or have a certain significance for selecting artificial restoration plants in grassland and for the rational distribution of crops in the interlaced areas of agriculture and animal husbandry.
The population adaptability of insects was affected by many factors, such as insect symbiotic bacteria, Bacterial symbiosis can also affect the adaptability of insect populations, and it plays an important role in the interaction between insects and hosts (Weinert et al. 2015). Symbiosis can affect the fitness of the hosts by reducing the density of symbiont (Scott et al. 2022), and Cardinium can increase the female yield by increasing maternal adaptability and egg size, thus improving fertilization rate and offspring adaptability (Katlav et al. 2022). In this study, Pea aphids on different host plants have different reproductive capacity, We will study and analyze the correlation between pea aphid and obligate endosymbionts Buchnera to to better explain the effects of host plants on the adaptability of insects.
5 Conclusions
Host plants are critical for the aphid growth and development. Compared with the other host plant, Pea aphid was more conducive to development and reproduction feeding on V. faba, while pea aphid was least conducive to reproduction feeding on O. viciaefolia, reproduction of pea aphid feeding on the other host plants was between V. faba and O. viciaefolia. Pea aphid exhibits different fintness on different host plants, which will provide theoretical basis for the prevention and control of pea aphids by the rational utilization of crop layout, and it provide reference for the selection of legumes in artificial restoration of degraded grassland.
Acknowledgment
We are grateful to Yu-ping Gou and Peter Quandahor for comments and assistance. N. L., Q.-Y. Y. and C.-C. L. conducted the experiments; N. L. and T.-W., Z. performed the statistical analyses; N.L. and C.-Z.L. conceived and designed the study; N.L., T.-W., Z. and C.-C. L. wrote and revised the manuscript. All authors read and gave final approval the final manuscript for publication. The authors extend their appreciation to the Researchers Supporting Project number (RSPD2023R745), King Saud University, Riyadh, Saudi Arabia.
Funding
This work was supported by the National Natural Science Foundation of China (31960351, 31660522). The authors extend their appreciation to the Researchers Supporting Project number (RSPD2023R745), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Comparative analysis of the detoxification gene inventory of four major Spodoptera pest species in response to xenobiotics. Insect Biochem. Molec.. 2021;138:103646
- [Google Scholar]
- Predation determines different selective pressure on pea aphid host races in a complex agricultural mosaic. PLoS One.. 2013;8(2):e55900.
- [Google Scholar]
- Plasticity in host utilization by two host-associated populations of Aphis gossypii Glover. B. Entomol. Res.. 2017;108(03):360-369.
- [Google Scholar]
- Effects of host plants reared under elevated co2 concentrations on the foraging behavior of different stages of corn leaf aphids Rhopalosiphum maidis. Insects.. 2019;10(6):182.
- [Google Scholar]
- The effect of second generation populations on the integrated colors of metal-rich globular clusters in early-type galaxies. Astrophys. J. Lett.. 2013;769:L31.
- [Google Scholar]
- Establishing alighting preferences and species transmission differences for Pea seed-borne mosaic virus aphid vectors. Virus Res.. 2017;241:145-155.
- [Google Scholar]
- Triterpene saponins of Quillaja saponaria show strong aphicidal and deterrent activity against the pea aphid Acyrthosiphon pisum. Pest Manag. Sci.. 2011;68(2):164-169.
- [Google Scholar]
- The role of protein effectors in plant–aphid interactions. Curr. Opin. Plant Bio.. 2013;16(4):451-456.
- [Google Scholar]
- Attachment forces of pea aphids (Acyrthosiphon pisum) on different legume species. Ecol. Entomol.. 2015;40(6):732-740.
- [Google Scholar]
- Antifeedant activity of luteolin and genistein against the pea aphid. Acyrthosiphon pisum. J. Pest Sci.. 2012;85(4):443-450.
- [Google Scholar]
- Oral Delivery mediated RNA Interference of a carboxylesterase gene results in reduced resistance to organophosphorus insecticides in the cotton aphid. Aphis gossypii Glover. PLoS One.. 2014;9(8):e102823.
- [Google Scholar]
- Effects of four constant temperatures on the development of two Bradysia (Diptera: Sciaridae) species. J. Appl. Entomol.. 2021;145(5):449-457.
- [Google Scholar]
- Influence of temperature on age-stage, two-sex life tables for a minnesota-acclimated population of the brown marmorated stink bug (Halyomorpha halys) Insects.. 2020;11:108.
- [Google Scholar]
- Species response to environmental change: impacts of food web interactions and evolution. Science.. 2009;323:1347-1350.
- [Google Scholar]
- 2005, History, achievement and prospect of alfalfa insect pest research in China. Pratacultural Science. 2005;22:75-78.
- [Google Scholar]
- Molecular mechanisms of insect adaptation to plant secondary compounds. Curr. Opin. Insect Sci.. 2015;8:8-14.
- [Google Scholar]
- Transcriptome responses in herbivorous insects towards host plant and toxin feeding. Annual Plant Reviews.. 2014;47:197-234.
- [Google Scholar]
- Hemipteran and dipteran pests: effectors and plant host immune regulators. J. Integr. Plant Biol.. 2016;58(4):350-361.
- [Google Scholar]
- Common endosymbionts affect host fitness and sex allocation via egg size provisioning. P. Roy. Soc. B-Biol. Sci.. 2022;1971(289):20212582.
- [Google Scholar]
- Population-specific responses of an insect herbivore to variation in host-plant quality. Ecol. Evol.. 2021;11(24):17963-17972.
- [Google Scholar]
- Molecular and morphological characterization of two aphid genera, Acyrthosiphon and Aulacorthum (Hemiptera: Aphididae) J. Asia-Pac. Entomol.. 2013;16(1):29-35.
- [Google Scholar]
- Information theory tests critical predictions of plant defense theory for specialized metabolism. Sci. Adv.. 2020;6(24):eaaz0381.
- [Google Scholar]
- Transcriptional responses of detoxification genes to four plant allelochemicals in aphis gossypii. J. Econ Entomol.. 2017;110(2):624-631.
- [Google Scholar]
- ‘Bottom-up’ effects in a tritrophic plant–aphid–parasitoid system: Why being the perfect host can have its disadvantages. B. Entomol. Res.. 2019;109(6):831-839.
- [Google Scholar]
- Intraspecific variability in herbivore performance and host quality: a field study of Uroleucon caligatum (Homoptera: Aphididae) and its Solidago hosts (Asteraceae) Ecol. Entomol.. 1981;6(3):301-306.
- [Google Scholar]
- Independent cytoplasmic incompatibility induced by Cardinium and Wolbachia maintains endosymbiont co-infections in haplodiploid thrips populations. Evo.. 2017;71(4):995-1008.
- [Google Scholar]
- Contrasting performances of generalist and specialist Myzus persicae (Hemiptera: Aphididae) reveal differential prevalence of maternal effects after host transfer. B. Entomol. Res.. 2007;97(1):61-67.
- [Google Scholar]
- Chemical ecology and sociality in aphids: opportunities and directions. J. Chem. Ecol.. 2018;44(9):770-784.
- [Google Scholar]
- A continuum of genetic divergence from sympatric host races to species in the pea aphid complex. P. Nati. Acad. Sci. USA. 2009;106(8):7495-7500.
- [Google Scholar]
- Post-Pleistocene radiation of the pea aphid complex revealed by rapidly evolving endosymbionts. P. Nati. Acad. Sci. USA. 2009;106(38):16315-16320.
- [Google Scholar]
- Genetic characterisation of new host-specialised biotypes and novel associations with bacterial symbionts in the pea aphid complex. Insect Conserv. Diver.. 2015;8:484-492.
- [Google Scholar]
- Effects of host plants on insecticide susceptibility and carboxylesterase activity in Bemisia tabaci biotype B and greenhouse whitefly. Trialeurodes vaporariorum. Pest Manag. Sci.. 2010;63:365-371.
- [Google Scholar]
- Untargeted metabolomics approach reveals differences in host plant chemistry before and after infestation with different pea aphid host races. Front. plant Sci.. 2019;10:188.
- [Google Scholar]
- Context dependence in the symbiosis between Dictyostelium discoideum and Paraburkholderia. Evol. Biol.. 2022;6(3):245-254.
- [Google Scholar]
- Elevated CO2 influences host plant defense response in chickpea against Helicoverpa armigera. Arthropod-Plant Inte.. 2016;10(2):171-181.
- [Google Scholar]
- Plant–aphid interactions under elevated co2: some cues from aphid feeding behavior. Front. plant sci.. 2016;7:520.
- [Google Scholar]
- Maternal host plant effects on aphid performance: contrasts between a generalist and a specialist species on Brussels sprout cultivars. Agr. Forest Entomol.. 2010;110(2):624-631.
- [Google Scholar]
- Acyrthosiphon pisum (Harris) (Homoptera: Aphididae) feeding preference and performance on cool-season food legumes in northwestern Ethiopia. Int. J. Pest Manage.. 2021;59(4):319-328.
- [Google Scholar]
- Functional evaluation of proteins in watery and gel saliva of aphids. Front. plant sci.. 2016;7:1840.
- [Google Scholar]
- The incidence of bacterial endosymbionts in terrestrial arthropods. Proc. R. Soc. B.. 2015;282:20150249.
- [Google Scholar]
- The structural sheath protein of aphids is required for phloem feeding. Insect Biochem. Molec.. 2015;57:34-40.
- [Google Scholar]
- Oxidative stress links response to lead and Acyrthosiphon pisum in Pisum sativum L. J. Plant Physiol.. 2019;240:152996
- [Google Scholar]
- Physiological and biochemical response of different resistant alfalfa cultivars against thrips damage. Physiol. Mol. Biol. Pla.. 2021;27(3):649-663.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102764.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







