Translate this page into:
Effects of berberine on intestinal flora of Non-alcoholic fatty liver induced by High-fat diet through 16S rRNA gene segmentation
⁎Corresponding author. zkx66165@sina.com (Yuzhen Wang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Background
Nonalcoholic fatty liver disease (NAFLD) is a hepatic manifestation of metabolic syndrome, which has become one of the globally recognized threats to health in 21st. The aim of this study was to elucidate the protective effects of berberine on intestinal mucosal barrier by regulating the intestinal flora in nonalcoholic fatty liver disease (NAFLD) rats. Methods: A total of 33 rats were randomly divided into the normal control group, high diet model group and berberine group. Changes of intestinal microbiota were detected by the 454 pyrosequencing of the V4 region of 16SrRNA genes. Morphological changes of liver and ileum tissue and serum biochemical markers were observed. Results: Bacteroidetes and Cyanobacteria of M group were significantly decreased as compared to N group, while Firmicutes and Cyanobacteria of B group were significantly decreased as compared to M group. Hepatocytes swelling and number of fat vacuoles of rats in B group were relieved, and hepatocytes were arranged neatly as compared to M group. The intestinal mucosal villi in B group were plump, and arranged tight and neatly, and the tight junctions and smaller gaps were also observed in B group compared to M group. Conclusions: High-fat diet (HFD) can interrupt the intestinal flora and damage intestinal barrier. Berberine can reduce gut permeability and improve intestinal barrier in NAFLD rats. Moreover, berberine can reduce the variability of intestinal flora in NAFLD rats. Our results suggest that berberine may be the protective effects on the intestinal mucosal barrier in NAFLD rats.
Keywords
Nonalcoholic fatty liver disease
Berberine
Intestinal flora
Gut microbiota
High-fat diet
1 Background
Nonalcoholic fatty liver disease (NAFLD) is a hepatic manifestation of metabolic syndrome, which begins with accumulation of lipids (Zhang et al., 2013). NAFLD includes nonalcoholic simple fatty liver (NAFL), nonalcoholic steatohepatitis (NASH), hepatocirrhosis and hepatocellular carcinoma (Cohen et al., 2011). The prevalence of NAFLD patients continuously increases all over the world (Kitabatake et al., 2017, Xue et al., 2017). The incidence was 20–30% in European and American countries, and about 15% in developed regions of China. NAFLD has become one of the globally recognized threats to health in 21st.
The pathogenesis of NAFLD has not been fully elucidated. The metabolic activity of intestinal flora can affect the balance of energy and the absorption of nutrients. Intestinal flora contributed to the occurrence and development of metabolic disease, such as the obesity, type II diabetes and cardiovascular disease (Lyra et al., 2010). Marshall (Marshall 1998) proposed gut–liver axis to explain the relationship between intestine and liver in the pathologic state. When intestinal mucosal barrier is injured, the intestinal bacteria, toxins and other harmful substances will enter the liver with the portal vein system. And the immune system, such as the Kupffer cell in the liver will be activated by these harmful substances, and release a series of inflammatory factors. These inflammatory factors not only cause liver damage, but also cause inflammation in other organs and the whole body.
Berberine is a quaternary ammonium salt from the protoberberine group of benzylisoquinoline alkaloids. Berberine has been used in the application of antibacterial and antidiarrheal drugs. In recent years, medical studies have found that berberine is more and more widely used in clinics, and its anti-inflammatory action, hypoglycemic and anti-hyperlipidemic effects have been reported. Berberine also have been proved to regulate the structure of intestinal flora and improve disease status (Xue et al., 2017). We hypothesize that berberine may have some effects on the treatment of NAFLD by improving the function of the intestinal mucosal barrier. However, comprehensive studies on the aspects of intestinal microbiota in NAFLD induced by berberine are lacking.
In the present study, we therefore established the HFD induced model of NAFLD, and elucidated the protective effects of berberine on the intestinal mucosal barrier and liver in the NAFLD rats by detecting the changes of intestinal mucosal structure, intestinal flora, hepatic steatosis, serum transaminase, serum lipid, endotoxin, intestinal fatty acid binding proteins (I-FABP) and D-Lactate by the treatment of berberine. Our findings provided theoretical basis for the treatment of NAFLD with berberine.
2 Materials and methods
2.1 Animal model establishment and experimental design
All experimental procedures in this study conform to the National Institutes of Health Guide for the Care, and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committee of Affiliated Hospital of our hospital. Thirty-three healthy male Sprague-Dawley rats weighted 200 ± 10 g were purchased from the Experimental Animal Center of Hebei Medical University (Certificate number: 1606071). Rats were fed in the animal laboratory of people’s hospital of Hebei province, with temperature at 18–26 °C and 40–70% humidity, and all rats were maintained on a 12/12 h light/dark cycle and unrestricted to food and water. The rats were randomly divided into three groups (11 rats per group) after 1 week of acclimation: normal control group (N group, n = 11), high diet model group (M group, n = 11) and berberine group (B group, n = 11). Rats in the N group were fed with a normal chow diet (3.48 kcal/g, 10.3% fat), while rats in the M group and B group were fed with high fat diet (5.01 kcal/g, 59.8% fat). One rat in each group was euthanized after 12 weeks to observe fat deposition of liver cell by using HE staining. After confirming the NAFLD model was successfully induced, rats in the B group received berberine (Northeast pharmaceutical, China) at a dose of 150 mg/(kg d) body weight/day by gavage, while rats in the N group and M group were administered an equal volume of normal saline by gavage for 4 weeks. After 16 weeks, all rats were fasted overnight and euthanized under anesthesia.
2.2 Collection and preparation of samples
Blood samples were collected from aortaventralis for 4–5 ml and placed in a vacuum blood collection tube without a heat source. Serum was recovered after clotting by centrifuged at 3000 r/min for 15 min and stored in EP tube and frozen in −80 °C refrigerator.
Three 5-μm thick sections of terminal ileum were collected. Two sections were rinsed thoroughly with PBS solution and then placed in 2.5% glutaraldehyde solution and 4% glutaraldehyde solution and stored at 4 °C, respectively. The other section was rinsed thoroughly with saline and then placed in 10% paraformaldehyde solution. The livers were immediately removed after blood samples collection. Two parts (about 0.5 cm × 0.5 cm × 0.5 cm) from the same section were collected and rinsed thoroughly with saline. One part was stored in EP tube and frozen in liquid nitrogen and then frozen at −80 °C. The other part was placed in a specimen bottle with 10% paraformaldehyde solution. Fresh dejection (1 g) of rats was collected after 16 weeks and placed in a 2 ml EP tube without RNase, and then stored quickly at −80 °C to detect the structural changes of intestinal microbiota.
2.3 Analytical procedures
Alanine aminotransferase (ALT), aspartate aminotransferase (AST), triglyceride (TG), and total cholesterol (Robertson et al., 2018) were measured by an automatic analyzer (Beckman X20, America). Endotoxin was detected by the Chromogenic TAL Endotoxin Assay Kit (Zhanjiang A&C Biological Ltd.; China), according to manufacturer’s instructions. Intestinal fatty acid binding proteins (I-FABP) and D-Lactate were detected by Rat I-FABP enzyme linked immunosorbent assay kits (Westang Biotechnology, Ltd., Shanghai, China), and Rat D-Lactate ELISA Kit (BioAssay Systems, America) respectively. All the procedures were followed the manufactures’ guidance. Liver and intestinal tissues were observed by HE staining. Intestinal mucosa was observed by scanning electron microscope and transmission electron microscope.
Changes of intestinal microbiota were detected by the 454 pyrosequencing of the V4 region of 16SrRNA genes. Fresh dejection of rats was selected, and DNA of fresh dejection was analyzed by PCR assays. Fecal DNA was sequenced using 16SrRNA-V3 region with 280 bp bacteria. The primer sequences were 520F (5′-barcode + GCACCTAAYTGGGYDTAAAGNG-3′) and 802R (5′-TACNVGGGTATCTAATCC-3′). Amplification products were examined by 2% agarose gel electrophoresis, and DNA extraction of the fecal pellets was carried out by Axygen’s gel recovery kit (AXYGEN, AP-GX-50, America). PCR products were quantified using Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen, P7589, America), and then mixed according to the amount of data required for each sample. The TruSeq Nano DNA LT Library Prep Kit was used to build library. For qualified libraries, the MiSeq Reagent Kit V3 (600 cycles) was used on the MiSeq machine to carry out 2 × 300 bp double-end sequencing.
2.4 Statistical analysis
Data were analyzed with SPSS21.0 software package. All results were presented as mean ± SD, and multiple comparisons between groups were conducted by One-way ANOVA and SNK test. P < 0.05 was considered statistically significant.
3 Results
3.1 Changes of body weight
When the NAFLD model was successfully induced, both M group and B group had increased body weight compared to N group after 12 weeks. After introduction of berberine at 16 weeks, weight gain of M group was greater than in B group (P < 0.05) (Table 1) and D-Lactate Compared to N group, M group and B group had higher endotoxin, I-FABP, and D-Lactate after 16 weeks (P < 0.05), while the endotoxin, I-FABP, and D-Lactate of B group were significantly decreased in comparison with M group (P < 0.05) (Table 3). Note: *P < 0.05 compared to N group; #P < 0.05 compared to M group. N, normal control group; M, high diet model group; B, berberine group. Note: *P < 0.05 compared to N group; #P < 0.05 compared to M group. N, normal control group; M, high diet model group; B, berberine group Note: *P < 0.05 compared to N group; #P < 0.05 compared to M group. N, normal control group; M, high diet model group; B, berberine group
Groups
12 weeks
16 weeks
N
387.2 ± 10.1
425.2 ± 16.3
M
471.8 ± 12.9*
524.8 ± 13.3*
B
470.6 ± 15.3*
492.8 ± 11.1*#
Groups
ALT (U/L)
AST (U/L)
TC (mmol/L)
TG (mmol/L)
N
44.21 ± 4.70
118.73 ± 8.17
1.13 ± 0.04
0.48 ± 0.08
M
82.18 ± 4.97*
201.97 ± 9.09*
1.79 ± 0.05*
1.09 ± 0.04*
B
63.76 ± 4.93*#
162.74 ± 9.14*#
1.53 ± 0.07*#
0.80 ± 0.04*#
Groups
Endotoxin (EU/ml)
I-FABP (pg/ml)
D-Lactate (mmol/l)
N
0.160 ± 0.005
118.7 ± 4.1
0.621 ± 0.013
M
0.373 ± 0.008*
279.1 ± 7.9*
0.997 ± 0.014*
B
0.268 ± 0.006*#
209.1 ± 5.1*#
0.768 ± 0.026*#
After 16 weeks, compared to N group, M group and B group had higher serum ALT, AST, TC, and TG levels (P < 0.05), while decreased level of ALT, AST, TC, and TG were observed in B group compared with M group (P < 0.05) (Table 2). Endotoxin, I-FABP, and D-Lactate Compared to N group, M group and B group had higher endotoxin, I-FABP, and D-Lactate after 16 weeks (P < 0.05), while the endotoxin, I-FABP, and D-Lactate of B group were significantly decreased in comparison with M group (P < 0.05) (Table 3).
3.2 Liver histology
After 16 weeks, compared with N group, rats in M group had a disordered structure of hepatic lobule, a malalignment and swelling of hepatocytes and different sizes of fat vacuoles. After intervention of berberine, hepatocytes swelling and number of fat vacuoles of rats in B group were relieved, and hepatocytes were arranged neatly (Fig. 1).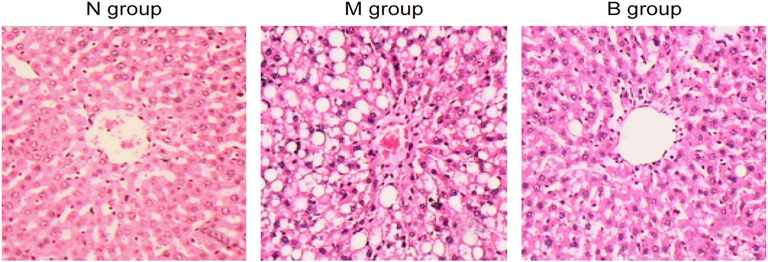
Histopathological change of liver in N group (n = 11), M group (n = 11) and B group (n = 11) (HE staining, ×200). N, normal control group; M, high diet model group; B, berberine group.
3.3 Intestine histology
The intestinal mucosal villi of N group were arranged tightly and the structure was integrated. The intestinal mucosal villi of M group became shorter and sparser, and partial intestinal mucosal villi were lost. The intestinal mucosal villi of group B were mildly edema and arranged relatively neatly and tightly in comparison with M group (Fig. 2A). The intestinal mucosal villi of rats in N group showed a broad blade, arranged tight and neatly. The intestinal mucosal villi in M group showed flat shape, arranged sparsely, and partial intestinal mucosal villi were broke. The intestinal mucosal villi in B group were plump, and arranged tight and neatly compared to M group (Fig. 2B). The intestinal mucosa in N group had extensive tight junctions, clear structure and small gap. The tight junctions disappeared and gap junctions increased in M group. The tight junctions existed and gap junctions became smaller in B group compared to M group (Fig. 2C).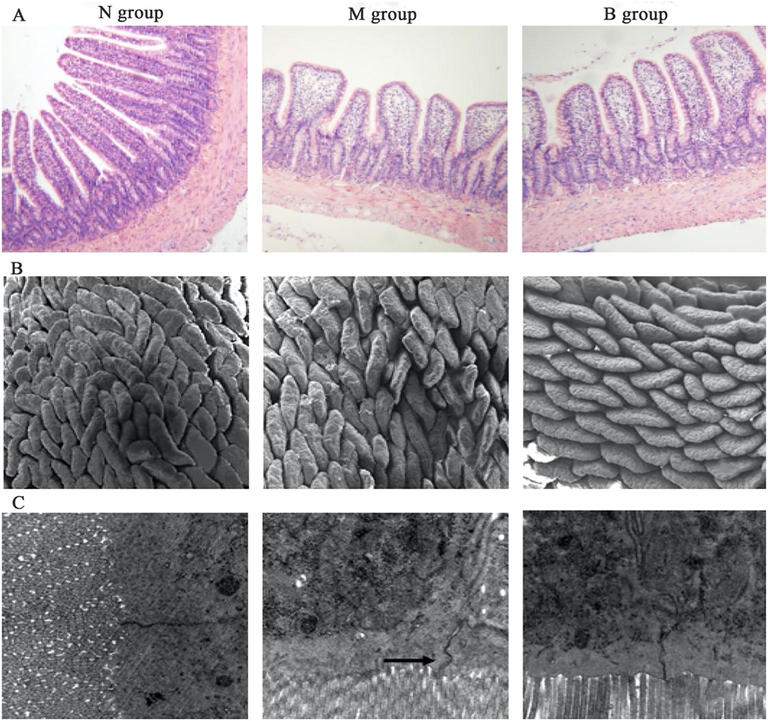
Intestinal tissue in N group (n = 11), M group (n = 11) and B group (n = 11) (HE staining, ×100). Scanning electron microscope observation of intestinal mucosa (×100) in N group, M group and B group. Transmission electron microscope observation of intestinal mucosa (×20 k) in N group, M group and B group. N, normal control group; M, high diet model group; B, berberine group.
3.4 Results of sequence analysis
Changes of intestinal microbiota were investigated by the 16rRNA gene sequence analysis. Among 261,541 high quality sequences, N group had 103,073 sequences, M group had 76,525 sequences, and B group had 81,943 sequences. The effective sequence length was 150 bp. The 261,541 sequences were delineated into 13,999 operational taxonomic units (OTUs) at the 97% similarity level. The OTUs of B group was significantly lower than N group and M group. The Chao 1 estimates and Shannon-Wiener diversity index revealed that the diversity of intestinal flora in B group were significantly lower than M group and B group (Table 4). It indicated that berberine decreased the bacterial diversity of intestinal flora. As shown in Fig. 3A, Firmicutes and Bacteroidetes were primary intestinal microbiota abundance of three groups, accounting above 90% for N group and M group, and above 70% for B group. Firmicutes and Proteobacteria of M group were significantly increased as compared to N group, while Bacteroidetes and Cyanobacteria of M group were significantly decreased. Bacteroidetes and Proteobacteria of B group were significantly increased as compared to M group, while Firmicutes and Cyanobacteria of B group were significantly decreased (Fig. 3C). As shown in Fig. 3B, about 100 bacterial genera were detected. In the N group, the first 3 dominant bacteria were Lactobacillus, Prevotella, and unknown genus of the Clostridium in the Firmicutes. In the M group, the first 3 dominant bacteria were 2 different unknown genera of the Clostridium in the Firmicutes, and unknown genus of S24-7 in the Bacteroidetes. Note: *P < 0.05 compared to N group; #P < 0.05 compared to M group. N, normal control group; M, high diet model group; B, berberine group
Groups
High quality sequence
OTU
Chao 1 index
Shannon index
N
103,073
1090 ± 158
1649 ± 233
6.93 ± 0.64
M
76,525
1176 ± 91
1783 ± 87
7.71 ± 0.26
B
81,943
533 ± 136*#
745 ± 187*#
5.68 ± 0.82*#
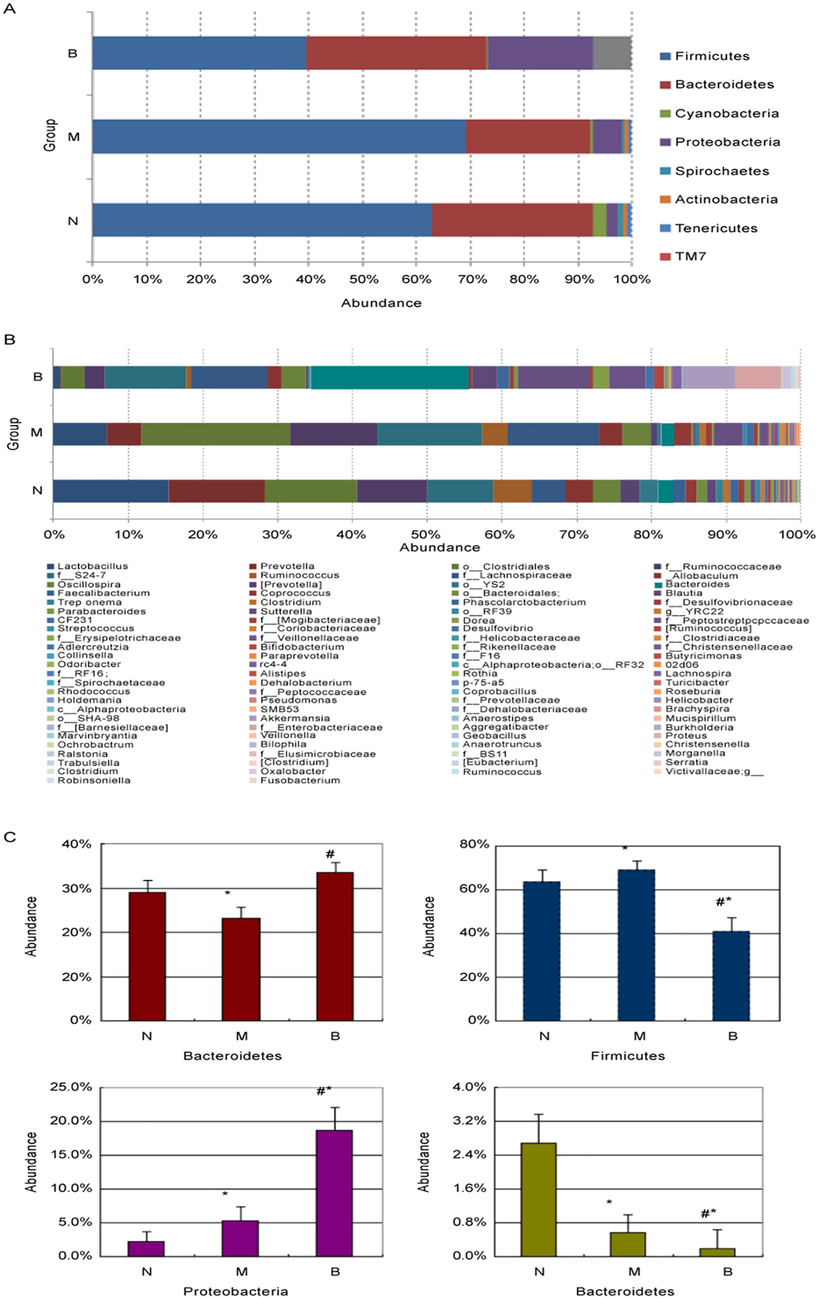
Changes of intestinal microbiota abundance at the family level. Changes of intestinal microbiota abundance at the genus level. Comparison of abundance of Bacteroidetes, Firmicutes, Proteobacteria and Cyanobacteria in the three groups (*P < 0.05 compared to N group; #P < 0.05 compared to M group). N, normal control group; M, high diet model group; B, berberine group.
In the B group, the first 3 dominant bacteria were Bacteroidetes, unknown genus of S24-7 in the Bacteroidetes, and unknown genera of the Lachnospiraceae in the Firmicutes. Lactobacillus and Prevotella were significantly decreased in M group as compared to N group (P < 0.05). Bacteroidetes of B group was significantly increased as compared to M group and N group (P < 0.05). Unknown genus of S24-7 in the Bacteroidetes and unknown genera of the Lachnospiraceae in the Firmicutes of B group were significantly decreased as compared to M group, while significantly increased as compared to N group (P < 0.05).
4 Discussion
The risk factors of NAFLD mainly include unhealthy lifestyle, insulin resistance, and metabolic syndrome (Paivarinta et al., 2016). The several studies showed that the body weight of rats was elevated induced by HFD (Zhang et al., 2012, Liu et al., 2016, Xue et al., 2017). Stoppeler et al used three different species of male rats (Lewis, Wistar, SD) exhibit hepatic steatosis after 3 weeks of a HFD (71% fat), however, only SD rats manifested fibrosis and hepatocyte damage and high sensitivity to HFD (Stoppeler et al., 2013). In our experiments, SD rats were fed with HFD (59.8% fat) and fat vacuole was increased in liver tissue. HFD was associated with increased intestinal permeability (Mangiola et al., 2016). Miele et al. (2009) provided the first evidence of the relationship between NAFLD and intestinal mucosal barrier. They demonstrated that gut permeability of NAFLD patients increased, and this abnormality was associated with disruption of intercellular tight junctions and overgrowth of small intestinal bacterial. On the other hand, occluding played an important role in keeping intestinal mucosa barrier, which was regarded as tight junction associated structural proteins in the intestines (Romero et al., 2015). In our experiments, the rats of M group had a disordered structure of hepatic lobule, a malalignment and swelling of hepatocytes and different sizes of fat vacuoles, the intestinal villi became shorter and sparser, and partial intestinal villi were lost, the tight junctions disappeared and gap junctions increased, which in accordance with previous studies(Xue et al., 2017). These results indicated that HFD can damage intestinal barrier and liver in NAFLD rats.
Endotoxin exists in the outer membrane of Gramneg, and its main ingredient is lipopolysaccharides (Yee et al., 2013). Endotoxin can damage intestinal mucosa epithelial cells, and destroy the structure of cells and the tight junctions between cells. It also destroys the intestinal mucosal barrier and increases the intestinal permeability. Moreover, a large amount of endotoxin enter into the blood through the damaged intestinal mucosa, and form the endotoxemia, which activate the immune system and release cytokines and inflammatory mediators, and then trigger inflammation and damage the liver, intestinal and other organs in different degrees. The changes in intestinal microbial composition were related to increased endotoxemia (Mangiola et al., 2016). In our experiments, the level of endotoxin was higher in M group compared to N group. It indicated that NAFLD was related to endotoxemia and intestinal mucosal barrier injury which in accordance with previous study (Ouelaa et al., 2017).
D-Lactate increases in liver disease in the early stages (Wang et al., 2017). A large number of bacteria in the gut D-Lactate will through the damaged intestinal mucosa into the blood circulation and reduce the intestinal mucosa permeability. FABPs were the protein in the histiocyte of mammal, which were related to the metabolism of fatty acids. I-FABP was found in the gastrointestinal mucosa cells, which increased in blood circulation when the intestinal mucosal barrier was injured (Viswanathan et al., 2010). In accordance with previous study (Wang et al., 2017), I-FABP and D-Lactate of M group and B group were significantly increased in comparison with N group, while berberine decreased the level of I-FABP and D-Lactate, indicating that HFD damaged the intestinal mucosal barrier and increased the intestinal mucosa permeability. Moreover, serum ALT, AST, TC, and TG of M group and B group were significantly increased in comparison with N group, while ALT, AST, TC, and TG of B group were significantly decreased in comparison with M group. It indicated that berberine can reduce the lipopolysaccharide (LPS) and hepatic inflammation, which in accordance with previous studies (Esposito et al., 2009, Xue et al., 2017).
Berberine is an intestinal antibacterial drug and is commonly used in diarrhea and other infectious diseases. After berberine intervention for 4 weeks, our findings were consistent with studies demonstrating that the intestinal mucosal villi of group B were mildly edema and arranged relatively neatly and tight compared to M group (Zhu et al., 2015). Moreover, the results of scanning electron microscope showed that the intestinal mucosal villi in B group were plump, and arranged tight and neatly compared to M group (Zhu et al., 2015). The results of transmission electron microscope showed that the tight junctions existed and gap junctions became smaller in B group compared to M group, which in accordance with previous study (Xue et al., 2017). These results indicated that berberine had protective effect of intestinal mucosa barrier, and regulated the intestinal endotoxemia in NAFLD rats.
Intestinal floras, known as the human second genome, which involved the forms of intestinal mucosa biological barrier, interacted with host and mutualism. A HFD can change the structure of intestinal flora (Katsube et al., 2019), and intestinal flora was associated with a large number of diseases or syndromes (Mariani et al., 2008; de Vos and de Vos, 2012). Zhu et al. (2015) demonstrated that the NAFLD group increased the ratio of Firmicutes and decreased the ratio of Bacteroidetes in comparison with normal diet group. In our experiments, the results of high throughput sequencing of fresh feces of rats showed that there was no difference of species of fecal bacteria in NAFLD rats and normal group, however, the proportion of different species of bacteria was significantly different. It indicated that HFD can induce the changes in intestinal flora, which mainly focused on the abundance of bacteria (Xue et al., 2017). In accordance with previous study, HFD exhibited increase in Bacteroides and decrease in Firmicutes in the feces and intestinal flora (Zhu et al., 2013). Mouzaki et al. (2013) found that the proportion of Bacteroides in the feces of NASH was decreased with the comparison of fecal microorganism of healthy controls. HFD reduced the Bifidobacterium, the main beneficial bacteria of the intestinal, while increased the endotoxemia, and the severity of endotoxemia was related to the number of Bifidobacterium (Katsube et al., 2019)
Berberine can influence the structure of intestinal microbiota as a kind of widely intestinal antimicrobial drugs. Xie et al. (2011) demonstrated that berberine reduced the proportion of Firmicutes and Bacteroidetes in the HFD rats received berberine at a dose of 200 mg/(kg d). Zhang et al. (2012) demonstrated that the proportion of the Actinobacteria and the Verrucomicrobia reduced in the HFD rats received berberine at a dose of 100 mg/(kg d). In our experiments, intestinal microbiota abundance of B group was significantly lower than M group by gene sequence analysis. Bacteroides is one of important symbiotic bacteria genera in the human gut microbes. Bacteroides ovatus of Bacteroides has a dedicated cross-feeding enzyme system, which digests polysaccharide and benefits to the host and other bacteria (Rakoff-Nahoum et al., 2016). Zhang et al. (2012) found that berberine can enrich the short-chain fatty acid (SCFA) producing strain of normal diet rats and HFD. SCFA can reduce inflammation and enhance the intestinal barrier (Peng et al., 2009). In this study, berberine increased the Bacteroidetes and decreased the Firmicutes in accordance with previous results (Zhang et al., 2012, Zhu et al., 2015). The SCFA strains may belong to the Bacteroides and have the protective effects on intestine. Moreover, berberine decreased the diversity of intestinal flora in this study. Membrez et al. (2008) found that gut bacteria growth is restrained, while fasting blood glucose and glucose tolerance of mice were improved in both ob/ob and diet-induced obese and insulin-resistant mice. Therefore, berberine can regulate the structure of intestinal flora and protect the liver.
The limitations in the study need to be mentioned. First, this study only compared the abundance of dominant bacteria in the results of the changes in the intestinal flora observed by high throughout sequencing technology. Secondly, the specific kind of bacteria played a clear positive role in the progress of the disease is difficult to confirm because of the effects of host, study research technique, environment and dietary. Thirdly, the sample size was small, and may influence the experiment results.
5 Conclusion
HFD can induce NAFLD in rats, and regulate the structure of intestinal flora and damage intestinal barrier. Berberine can reduce gut permeability, variability of intestinal flora and improve intestinal barrier in NAFLD rats. Our results suggested that berberine may be the protective effects on the intestinal mucosal barrier in NAFLD rats.
Acknowledgements
This study was supported by the protective effect of berberine on the protection of intestinal mucosal barrier in patients with nonalcoholic fatty liver and NAFLD project (Number: 16277777D).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Human fatty liver disease: old questions and new insights. Science. 2011;332(6037):1519-1523.
- [CrossRef] [Google Scholar]
- Role of the intestinal microbiome in health and disease: from correlation to causation. Nutr. Rev.. 2012;70(Suppl_1):S45-S56.
- [Google Scholar]
- Probiotics reduce the inflammatory response induced by a high-fat diet in the liver of young rats. J. Nutr.. 2009;139(5):905-911.
- [Google Scholar]
- Endoplasmic reticulum stress-induced cellular dysfunction and cell death in insulin-producing cells results in diabetes-like phenotypes in Drosophila. Biol. Open. 2019;8(12)
- [Google Scholar]
- Association between endotoxemia and histological features of nonalcoholic fatty liver disease. World J. Gastroenterol.. 2017;23(4):712-722.
- [Google Scholar]
- Effects of different diets on intestinal microbiota and nonalcoholic fatty liver disease development. World J Gastroenterol.. 2016;22(32):7353-7364.
- [Google Scholar]
- Intestinal microbiota and overweight. Benef. Microb.. 2010;1(4):407-421.
- [CrossRef] [Google Scholar]
- Gut microbiota in autism and mood disorders. World J. Gastroenterol.. 2016;22(1):361-368.
- [Google Scholar]
- Involvement of glutathione transferases, Gtt1and Gtt2, with oxidative stress response generated by H2O2 during growth of Saccharomyces cerevisiae. Redox Rep.. 2008;13(6):246-254.
- [Google Scholar]
- The gut as a potential trigger of exercise-induced inflammatory responses. Can. J. Physiol. Pharmacol.. 1998;76(5):479-484.
- [CrossRef] [Google Scholar]
- Gut microbiota modulation with norfloxacin and ampicillin enhances glucose tolerance in mice. FASEB J. Off. Publ. Feder. Am. Soc. Exp. Biol.. 2008;22(7):2416-2426.
- [Google Scholar]
- Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology (Baltimore, Md.). 2009;49(6):1877-1887.
- [Google Scholar]
- Intestinal microbiota in patients with nonalcoholic fatty liver disease. Hepatology (Baltimore, Md.). 2013;58(1):120-127.
- [Google Scholar]
- Citrulline decreases hepatic endotoxin-induced injury in fructose-induced non-alcoholic liver disease: an ex vivo study in the isolated perfused rat liver. Br. J. Nutr.. 2017;117(11):1487-1494.
- [Google Scholar]
- Changes in intestinal immunity, gut microbiota, and expression of energy metabolism-related genes explain adenoma growth in bilberry and cloudberry-fed Apc(Min) mice. Nutr Res.. 2016;36(11):1285-1297.
- [Google Scholar]
- Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr.. 2009;139-(9):1619-1625.
- [Google Scholar]
- The evolution of cooperation within the gut microbiota. Nature. 2016;533(7602):255-259.
- [Google Scholar]
- Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell. 2018;174(4):1033.
- [Google Scholar]
- The intestinal barrier function and its involvement in digestive disease. Rev. Esp. Enferm Dig.. 2015;107(11):686-696.
- [Google Scholar]
- Gender and strain-specific differences in the development of steatosis in rats. Lab. Anim.. 2013;47(1):43-52.
- [Google Scholar]
- Heart-type fatty acid-binding protein predicts long-term mortality and re-infarction in consecutive patients with suspected acute coronary syndrome who are troponin-negative. J. Am. Coll. Cardiol.. 2010;55(23):2590-2598.
- [Google Scholar]
- Wang, Chou, Chuang, Li, et al., 2017.Elevated levels of liver methylglyoxal and d-lactate in early-stage hepatitis in rats.
- Effects and action mechanisms of berberine and Rhizoma coptidis on gut microbes and obesity in high-fat diet-fed C57BL/6J mice. PloS One. 2011;6(9):e24520
- [Google Scholar]
- Probiotics may delay the progression of nonalcoholic fatty liver disease by restoring the gut microbiota structure and improving intestinal endotoxemia. Sci. Rep.. 2017;7:45176.
- [Google Scholar]
- Translating discovery in zebrafish pancreatic development to human pancreatic cancer: biomarkers, targets, pathogenesis, and therapeutics. Zebrafish. 2013;10(2):132-146.
- [Google Scholar]
- Autologous CIK cell immunotherapy in patients with renal cell carcinoma after radical nephrectomy. Clin. Dev. Immunol.. 2013;2013:1-12.
- [Google Scholar]
- Structural changes of gut microbiota during berberine-mediated prevention of obesity and insulin resistance in high-fat diet-fed rats. PloS One. 2012;7(8):e42529
- [Google Scholar]
- Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology (Baltimore, Md.). 2013;57(2):601-609.
- [Google Scholar]
- Effects of berberine on gut microbiota of rats with non-alcoholic fatty liver disease induced by high-fat diet. J. Shanghai Jiaotong Univ.. 2015;35(4):483-488.
- [Google Scholar]







