Translate this page into:
Effectiveness of silicon on immature stages of the fall armyworm [Spodoptera frugiperda (J. E. Smith)]
⁎Corresponding author. liuchzh@gsau.edu.cn (Changzhong Liu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
Fall armyworm (Spodoptera frugiperda) is a polyphagous pest causing economic losses to various crops. Commonly, chemical pesticides are used to control this pest, but these pesticides have a lot of side effects. Therefore, alternate environment-friendly method are inevitable for the management of S. frugiperda. Silicon (Si) supplementation to crop plants develops induced resistance against pests.
Methods
As the crop damage is done by the larval stage of the Fall armyworm, a laboratory experiment was carried out to investigate the impact of Si on immature stages of S. frugiperda. Treatments consisted of silicon dioxide (SiO2) and potassium silicate (K2SiO3) with two methods of application (foliar spray and soil application). No Si was the control treatment.
Results
The current results showed that all Si treatments significantly (p ≤ 0.05) affected the immature stages of S. frugiperda. The foliar spray of SiO2 caused the highest larval mortality (40.33 ± 3.45 %) compared to K2SiO3 (29.33 ± 3.26 %) and control (4.67 ± 1.03 %). The greatest effect of Si treatments was observed in the early stages of larvae as insect mortality was significantly higher in 1st (19.67 ± 2.33 %) and 2nd (12.67 ± 1.03 %) instar of larval growth. Similarly, a significantly higher larval mortality was recorded one day (10.67 ± 2.07 %) and two days (19.67 ± 2.33 %) after hatching. Among Si sources, larval mortality was affected more in SiO2 than K2SiO3 and control. Meanwhile, the larval mortality was significantly more affected in Si's foliar spray (26.67 ± 17.26 %) than soil application (22.89 ± 14.46 %). A similar effect was observed in larvae to pupa ratio (59.67 ± 3.45 %), adult fecundity (146.67 ± 18.36 eggs), and neonate emergence (37.83 ± 4.62 %). In the pupa to adult ratio and adult sex ratio, no significant impact of Si sources or application methods was observed.
Conclusions
The current study results showed a significantly negative impact of Si on immature stages of S. frugiperda. Hence, Si application may diminish S. frugiperda colonization and initial damage in maize because it can decrease the fecundity of S. frugiperda and significantly increase the mortality of newly emerging larvae.
Keywords
Silicon dioxide
Potassium silicate
Foliar spray
Soil application
Fall armyworm
1 Introduction
The fall armyworm (Spodoptera frugiperda) (J. E. Smith) is a noctuid pest causing considerable economic losses to a number of plants, but prefers to infest maize crop (Casmuz et al., 2017). Spodoptera frugiperda can result in 15 to 73% worldwide economic losses for maize alone (Guo et al., 2018). Spodoptera frugiperda is a year-round inhabitant of the Americas' tropical and subtropical regions, and it makes its way quite far northern as temperate North America every year. S. frugiperda was first reported in Western Africa in January 2016, and it has spread at an incredible rate since then. S. frugiperda was reported in other African countries within two years of entering West Africa (Abrahams et al., 2017, Stokstad 2017). Subsequently, it moved to the eastern side (Njeru 2017), reaching Asian countries like Thailand, India and Myanmar in 2018 (Kalleshwaraswamy et al., 2018). S. frugiperda was first recorded in China in January 2019, and in autumn 2019, it spread to nearly all of China's southern regions (Center 2019, Wu et al., 2019). Maize is cultivated in all provinces of China, making it the world's second-largest maize producer (Li et al., 2020). As a result, if S. frugiperda can regularly reach the main maize growing regions, Chinese crop output and food security would be jeopardized. S. frugiperda danger to China will keep growing as globalization and connectivity develop due to global trade (Early et al., 2018).
When maize is infested with S. frugiperda, yield losses range from 15 to 73% (Ranaweera et al., 2021). The annual economic losses in Ghana and Zambia have reached US$177.3 million and US$159.3 million, respectively (Abrahams et al., 2017). Collectively, maize, rice, sorghum, and sugarcane, have suffered total economic crop losses of US$13 billion per annum in sub-Saharan Africa (Abrahams et al., 2017). Estimation of the potential financial loss of maize in China caused by S. frugiperda indicates a range from US$5.4–47 billion per annum (Song et al., 2020).
Spodoptera frugiperda rapid local spread is caused mainly by natural long-distance movement (Ren et al., 2019). Because of its strong flight, S. frugiperda is rapidly expanding. It can fly 100 km in a single night. Furthermore, the S. frugiperda is active all year and does not hibernate in the winter (Garcia et al., 2018). Even at temperatures as low as 18˚Celsius, S. frugiperda can complete the development process. These unique characteristics have made fall armyworm more damaging (Barfield et al., 1978).
To measure this risk and thus develop a biosecurity plan, it is essential to figure out some management practices for S. frugiperda. As a result, various researchers have mainly engaged in the development of biopesticides to combat insect pests as part of insect’s management to prevent crop losses and boost yield (Idrees et al., 2016, Luo et al., 2018, Ali et al., 2021, Qadir et al., 2021). On the other hand, farmers have become increasingly reliant on synthetic insecticides to manage S. frugiperda, which is inefficient and detrimental to the environment and natural enemies, resulting in insecticide resistance (Cai et al., 2017, Gu et al., 2018). Methomyl, lindane, methyl parathion, and endosulfan are examples of insecticides that have been used to control S. frugiperda and are well-known for being extremely hazardous (Bateman et al., 2021), with many of these insecticides being prohibited in many states (Humphreys et al., 2008). Furthermore, due to their toxicity, pesticides are not safe for farmers, and the majority of farmers have no expertise or information on the precautions to take when applying these pesticides in their fields. As a result, it is urgently needed to find safe, ecologically friendly, and cost-effective alternatives to synthetic insecticides for long-term control of S. frugiperda.
Induced plant resistance is one of the areas that can be used for the management of S. frugiperda. Resistance of host plants to insect pests and the chemical elicitors to stimulate host plants' defences have become more prevalent in research worldwide (Hamm et al., 2010). Plant signaling networks regulate most of these defenses, including plant growth regulators and other modifications. The plants produce various hormones, and exogenic administration of a few causes plants to respond to various stresses. They also affect insect biology, limiting growth and development (Haq et al., 2021).
Silicon (Si) is the 2nd most prevalent element in soil, accounting for around a quarter of the earth's crust (wt/wt) (Acevedo et al., 2022). Even though Si is not considered a compulsory element for most plants, it has beneficial impacts on plant growth, disease resistance, and defense mechanisms against insect pests and diseases have been thoroughly reported in various plants.
Foliar spray of Si has been proven to affect plant development, produce, and Si content in various crops like sugar beet (Artyszak 2018), finger millet (Kim et al., 2017), soybean (Sandhya et al., 2020), wheat (Sattar et al., 2020), manage pests and other stresses in Oryza stiva (Rezende et al., 2009, Stout et al., 2009), tomato (Kedarnath et al., 2016), grape (Shivaraj et al., 2022), cucumber (Nagaratna et al., 2022) and coffee (Lopes et al., 2013) and induction of resistance to environmental factors in crops like Triticum (Sattar et al., 2020) Glycine max (Lee et al., 2010), potato (Pilon et al., 2014) and rice (Wang et al., 2015). Plant resistance to diseases and insect pests is now well documented, and Si helps to reduce plant damage inflicted by these pests (Ma 2004). (Nagaratna et al., 2022) experimented with checking the impact of silicic acid on biological parameters and fitness of S. frugiperda. (Pereira et al., 2021) supplemented silicon on maize plants to study its effect on the colonization of S. frugiperda and the attraction of its predator. (Assis et al., 2015) evaluated resistance in sunflower against caterpillars using SiO2. (Gomes et al., 2005) reported that the application of calcium silicate induced resistance in wheat against aphids. (Abbasi et al., 2020) carried out a laboratory trial to check the effect of Si applications on biological parameters and oviposition of whitefly. (Hou and Han 2010) evaluated the resistance of rice to Asiatic Rice Borer by applying Si. (Acevedo et al., 2021) also reviewed the effectiveness of Si in elevating the resistance of different crops against insect pests.
Ulina et al. (2022) investigated the efficacy of biosilica fertilizer against FAW incidence and its effect on maize production. (Jeer et al., 2022) monitored the impact of these Si sources and potassium on total sugars, total phenols, anti-oxidant, and defense enzymes in wheat stem tissues along with their impact on pink stem borer damage and yield. (Sousa et al., 2022) characterized the impact of Si fertilization regarding priming, induced resistance, and tolerance to FAW in maize's landrace variety and hybrid. In another study, the effects of potassium silicate (K2SiO3) on the survival, development and reproduction of Aphis gossypii Gloverat various life stages were tested under laboratory conditions. Efficacy of foliar spraying with K2SiO3 compared to lambda-cyhalothrin (LCH) against the field strain of A. gossypii third nymphal instar under greenhouse conditions as well as its impacts on the activity of antioxidant enzymes were also evaluated (Tawfeek and Eldesouky 2022).
Despite various research demonstrating that Si deposition in plants affects the larvae of S. frugiperda (Nascimento et al., 2014, Nascimento et al., 2018, Acevedo et al., 2021, Nagaratna et al., 2022), there is little information on the impact of Si supplementation on the early phases of insect infestation in the literature. As maximum crop damage is done by the larval stage of S. frugiperda, therefore, this study aims to check the effect of SiO2 and K2SiO3 with different application methods on the early stages and the adult stage, especially female sex ratio and fecundity of S. frugiperda.
2 Materials and methods
2.1 Experimental site and treatments
The current experiment was conducted in laboratory conditions at the College of Crop Protection Gansu Agricultural University Lanzhou, China (36.0915˚ N, 103.7006˚ E), 2020–21. The experiment was conducted in a completely randomized design (CRD) under a factorial arrangement with three replicates. Treatments consisted of Silicon dioxide (SiO2) and Potassium silicate (K2SiO3) applied with two different methods of application (Foliar spray and soil application). Water was used as a foliar spray and soil application in the control treatment.
2.2 Insect materials
Eggs of S. frugiperda were collected from insect colonies maintained in the laboratory and kept for hatching in an incubator at 25 °C and 65 % R. H. Newly emerging larvae were transferred into Petri dishes and were supplied with discs of maize leaves treated with Si daily till pupation. Newly emerged adults were transferred to rectangular cages. White sheets were used to cover the cages' interior walls, which served as oviposition sites. Sterilized cotton balls soaked in a 15% honey solution were also provided as a food source in the cages. The sterile cotton balls and white papers were replaced daily to ensure homogenous egg ages.
2.3 Silicon applications
Maize seeds of the variety Nonghua (农化) 816 were sown in the plastic pots containing potting soil. Pots were watered daily to maintain moisture levels. Available studies demonstrate that silicon dioxide and potassium silicate have moderate to low acute toxicity. These pesticides have been placed in Toxicity Category III for acute oral and dermal effects (Toxicity Category I indicating the highest degree of toxicity, and IV the lowest). Similarly, an inhalation study and eye and dermal irritation studies suggest moderate to low toxicity. Therefore, SiO2 and K2SiO3 were purchased from Shanghai Macklin Biochemical Co., Ltd, Shanghai, China. 800 ppm concentration solutions were prepared for both Si compounds by dissolving in water before application. A hand sprayer was used for foliar Si applications, with the plants' bases wrapped with plastic sheets. Drenches were injected into the soil around the plant's base in soil application treatments.
2.4 Data collection
The impact of Si was checked on larvae percentage mortality (from 1st to 6th instar), larvae percentage mortality after one day, two days, three days, seven days, fifteen days, and total larvae mortality, larvae to pupa ratio, pupa to adult ratio, adult sex ratio, total fecundity, eggs development period and subsequent larvae emergence ratio. Corrected percentage mortality was calculated using Abbot's formula. Freshly laid eggs were collected from the cages and put in clean Petri dishes (diameter, 9 cm). The number of eggs laid overnight was counted with a microscope's aid. This repeated daily until the females died.
Corrected mortality is = [x(c) - x(t)]/x(c).
x (t) is the proportion of the treatment group that survives and x(c) is the proportion of the control group that survives, both groups started at the same size.
2.5 Data analysis
The collected data on percentage larval mortality, pupation rate, adult sex ratio, fecundity and fertility were subjected to analysis of variance (factorial design up to two-way interaction) to test the effect of Si application methods and Si sources. All the data were analyzed using SPSS statistics software (IBM, SPSS Version 19, United States). LSD test at p ≤ 0.05 was used to separate the significant difference between treatment means. All statistical analyses were carried out using. GraphPad Prism version 7.00 was used to make graphs.
3 Results
3.1 Percentage mortality of S. Frugiperda larvae (1st to 6th instar)
The current study revealed a significant (P ≤ 0.05) impact of all the Si applications on the percentage mortality of all larval instars of S. frugiperda compared to control. Mean percentage mortality in 1st instar larvae was recorded in the foliar spray of SiO2 (21.33 ± 1.15 %) followed by soil application of SiO2 (18.00 ± 2.00 %) and foliar spray of K2SiO3 (17.33 ± 1.15 %) (Fig. 1). Among application methods percentage mortality in 1st instar larvae was significantly (P ≤ 0.05) higher in SiO2 (19.67 ± 2.34 %) compared to K2SiO3 (15.67 ± 1.96 %) and control (3.67 ± 0.82 %). Moreover, a statistically (P ≤ 0.05) higher mortality of 1st instar larvae of S. frugiperda was recorded in foliar sprays (14.22 ± 7.90 %) compared to soil application (11.77 ± 6.67 %) (Table 1). A similar trend was observed in the percentage mortality of 2nd instar larvae of S. frugiperda. Foliar spray of SiO2 resulted in a statistically (P ≤ 0.05) higher mortality (13.33 ± 1.15 %) of 2nd instar larvae, followed by soil application of SiO2 (12.00 ± 0.00 %) and foliar spray of K2SiO3 (9.33 ± 1.15 %) (Fig. 1). SiO2 resulted in statistically (P ≤ 0.05) higher mortality (12.67 ± 1.03 %) of 2nd instar larvae compared to K2SiO3 (9.00 ± 1.09 %) and control (1.00 ± 1.09 %). Among application methods, the mean percentage mortality in the foliar spray and soil application was (7.78 ± 5.69 %) and (7.33 ± 4.79 %), respectively (Table 1). Mean percentage mortality in 3rd instar larvae of S. frugiperda was significantly (P ≤ 0.05) affected by all Si applications compared to control. Mean percentage mortality was (6.00 ± 0.00 %), (5.33 ± 1.15 %), (4.00 ± 0.00 %), (4.00 ± 0.00 %), and (0.00 ± 0.00 %) recorded in foliar spray of SiO2, soil application of SiO2, foliar spray of K2SiO3, soil application of K2SiO3, and control respectively (Fig. 1). Mean percentage mortality in 3rd instar larvae was significantly (P ≤ 0.05) higher in SiO2 (5.67 ± 0.82 %) compared to K2SiO3 (4.00 ± 0.00 %) and control (0.00 ± 0.00 %). Whereas no significant (P ≤ 0.05) difference was observed in the foliar spray (3.33 ± 2.64 %) and soil application (3.11 ± 2.47 %) on mean percentage mortality of 3rd instar larvae of S. frugiperda. In the 4th instar larvae of S. frugiperda, significantly higher percentage mortality was detected in the foliar spray of SiO2 (2.67 ± 1.15 %) followed by drenching application of SiO2 (2.00 ± 0.00 %) and foliar spray K2SiO3 (1.33 ± 0.00 %). No percentage mortality of 4th instar larvae was observed in soil application of K2SiO3 and control (Fig. 1). In the 5th and 6th instar larvae of S. frugiperda, no percentage mortality was observed in all the treatments (Table 1). Comparatively, a significantly higher percentage of mortality was observed in 1st instar larvae (19.67 ± 2.33 %), followed by 2nd, 3rd, and 4th instar larvae of S. frugiperda (Fig. 1). Means within a column followed by different lower-case letters are significantly different at P ≤ 0.05 (LSD test).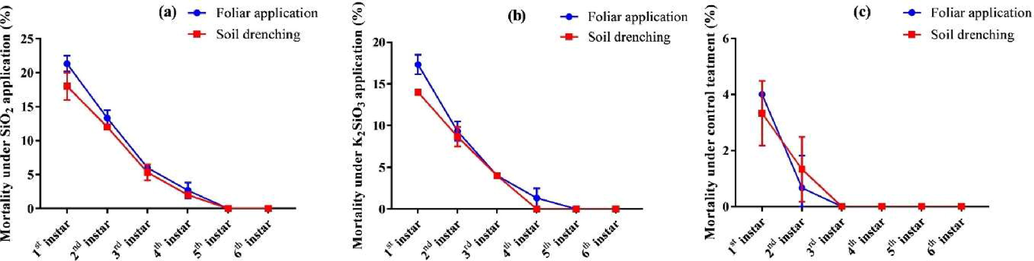
Effect of Si on percentage mortality of different instars of S. frugiperda larvae. Means were compared using the LSD test at p ≤ 0.05. Vertical bars indicate SE.
Treatments
1st Instar
2nd Instar
3rd Instar
4th Instar
5th Instar
6th Instar
Silicon Sources
SiO2
19.67 ± 2.34a
12.67 ± 1.03a
5.67 ± 0.82a
2.33 ± 0.82a
0.00 ± 0.00
0.00 ± 0.00
K2SiO3
15.67 ± 1.96b
9.00 ± 1.09b
4.00 ± 0.00b
0.67 ± 1.03b
0.00 ± 0.00
0.00 ± 0.00
Control
3.67 ± 0.82c
1.00 ± 1.09c
0.00 ± 0.00c
0.00 ± 0.00b
0.00 ± 0.00
0.00 ± 0.00
Application Methods
Foliar Application
14.22 ± 7.90a
7.78 ± 5.69a
3.33 ± 2.65a
1.33 ± 1.4a
0.00 ± 0.00
0.00 ± 0.00
Soil Drenching
11.78 ± 6.67b
7.33 ± 4.79a
3.11 ± 2.47a
0.67 ± 1.00b
0.00 ± 0.00
0.00 ± 0.00
3.2 Percentage mortality of S. Frugiperda larvae after one day, two days, three days, seven days, fifteen days of emergence, and total larvae mortality
The Si sources and Si application methods statistically (P ≤ 0.05) affected larval mortality of S. frugiperda at one day, two days, three days, seven days, and fifteen days after emergence. After one day of emergence, foliar spray of SiO2 statistically (P ≤ 0.05) affected the percentage mortality of S. frugiperda compared to K2SiO3 and control. Statistically (P ≤ 0.05), higher values of percentage mortality were recorded in foliar sprays of SiO2 (12.00 ± 2.00 %), followed by soil application of SiO2 (9.33 ± 1.15 %) and foliar spray of K2SiO3 (9.33 ± 1.15 %). Minimum percentage mortality was recorded in control (2.67 ± 1.15 %). Among both methods of Si application, statistically (P ≤ 0.05) higher mortality was recorded in SiO2 (10.67 ± 2.06 %) than in K2SiO3 (8.67 ± 1.63 %) and control (2.67 ± 1.03 %). Similarly, higher values of percentage mortality after one day of emergence were recorded in the foliar spray (8.00 ± 4.35 %) compared to soil application (6.67 ± 3.32 %) of both Si sources. Percentage mortality of fall armyworm larvae after two days of emergence was (21.33 ± 1.15 %), (18.00 ± 2.00 %), (17.33 ± 1.15 %), (14.00 ± 0.00 %), and (4.00 ± 0.00 %) in the foliar spray of SiO2, soil application of SiO2, foliar spray of K2SiO3, soil application of K2SiO3, and control, respectively. Moreover, the maximum percentage of mortality after two days of emergence was recorded in SiO2 (19.67 ± 2.33 %) compared to K2SiO3 (15.67 ± 1.96 %) and control (3.67 ± 0.82 %). Foliar sprays (14.22 ± 7.90 %) of both Si sources caused a statistically (P ≤ 0.05) higher mortality than soil application (11.78 ± 6.67 %). A similar trend of percentage mortality was observed after three days of emergence in larvae of S. frugiperda. Foliar spray of SiO2 resulted in significantly higher mortality (29.33 ± 1.15 %) than soil application of SiO2 and K2SiO3 (Fig. 2). Minimum percentage mortality was recorded in control. Among Si sources, the percentage mortality after three days of emergence was (27.00 ± 2.75 0%) in SiO2 and (20.67 ± 2.42 %) in K2SiO3 compared to control (4.67 ± 1.03 %). The percentage of larval mortality was (18.89 ± 11.09 %) in the foliar spray and (16.00 ± 8.94 %) in soil application of Si (Table 2). The mortality of S. frugiperda larvae after seven days of emergence was statistically (P ≤ 0.05) higher in the foliar spray of SiO2 (40.67 ± 1.15 %) than in soil application of SiO2 (34.67 ± 1.15 %) and foliar spray of K2SiO3 (29.33 ± 1.15 %) (Fig. 2). Overall, a higher percentage of mortality was observed in the foliar sprays (24.88 ± 15.97 %) of Si compared to soil application (21.56 ± 13.33 %). A statistically (P ≤ 0.05) higher percentage of mortality was recorded in SiO2 (37.67 ± 3.44 %) after seven days of larval emergence compared to K2SiO3 (27.33 ± 2.42 %) and control (4.67 ± 1.03 %) (Table 2). Results indicated that after fifteen days of emergence, larval mortality was statistically (P ≤ 0.05) affected by all Si applications compared to untreated control. The maximum percentage of mortality was recorded in the foliar spray of SiO2 (43.33 ± 1.15 %), followed by soil application of SiO2 (37.33 ± 1.15 %), foliar spray of K2SiO3 (32.00 ± 2.00 %), and soil application of K2SiO3 (26.67 ± 1.15 %) (Fig. 2). Among Si sources, the percentage of mortality was (40.33 ± 3.44 %), (29.33 ± 3.26 %), and (4.67 ± 1.03 %) in SiO2, K2SiO3, and control, respectively (Table 2). It can be seen from Fig. 2 that the total percentage of mortality of S. frugiperda is similar to the percentage of mortality after fifteen days of emergence. Means within a column followed by different lower-case letters are significantly different at P ≤ 0.05 (LSD test).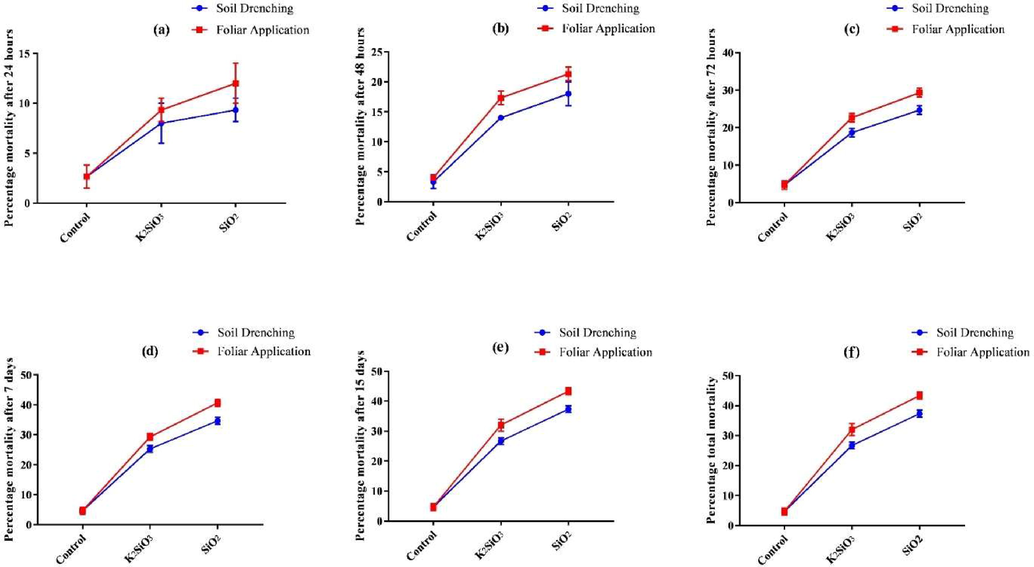
Effect of SiO2 and K2SiO3 applied with two application methods (Foliar application and soil drenching) on percentage mortality of S. frugiperda larvae after (a) one day, (b) two days, (c) three days, (d) seven days, (e) fifteen days of emergence, and (f) total larvae mortality. Means were compared using the LSD test at p ≤ 0.05. Vertical bars indicate SE.
Treatments
One day
Two days
Three days
Seven days
Fifteen days
Total mortality
Silicon sources
SiO2
10.67 ± 2.06a
19. 67 ± 2.33a
27 ± 2.75a
37.67 ± 3.45a
40.33 ± 3.44a
40.33 ± 3.44a
K2SiO3
8.67 ± 1.63b
15. 67 ± 1.96b
20.67 ± 2.42b
27.33 ± 2.42b
29.33 ± 3.26b
29.33 ± 3.26b
Control
2.67 ± 1.03c
3.67 ± 0.81c
4.67 ± 1.03c
4.67 ± 1.03c
4.67 ± 1.03c
4.67 ± 1.03c
Application methods
Foliar application
8 ± 4.35a
14.22 ± 7.90a
18.89 ± 11.09a
24.89 ± 15.97a
26. 67 ± 17.26a
26. 67 ± 17.26a
Soil drenching
6.67 ± 3.31a
11.78 ± 6.67b
16 ± 8.94b
21.56 ± 13.33b
22. 89 ± 14.46b
22. 89 ± 14.46b
3.3 Larvae to pupa and pupa to adult ratio of S. Frugiperda under different Si applications
Fig. 3 shows the effect of Si applications on larvae to pupa ratio and pupa to adult ratio of S. frugiperda. The current study showed that all the Si applications significantly (P ≤ 0.05) affected the larvae to pupa ratio of S. frugiperda. Significantly (P ≤ 0.05) lower values of larvae to pupa ratio were observed in the larvae fed on maize leaves treated with SiO2 (59.67 ± 3.44 %) compared to K2SiO3 (70.67 ± 3.26 %) and control (95.33 ± 1.03 %) (Table 3). The lowest larvae to pupa ratios were observed in the foliar spray (56.67 ± 1.15 %) of SiO2, which were significantly (P ≤ 0.05) similar to soil application of SiO2 (62.67 ± 1.15 %), while larvae to pupa ratios were significantly (P ≤ 0.05) lower in the foliar spray of K2SiO3 (68.00 ± 2.00 %) than soil application of K2SiO3 (73.33 ± 1.15 %). Overall, lower larvae to pupa ratios were observed in the foliar spray (73.33 ± 17.26 %) than in Si sources' soil application (77.11 ± 14.46 %). Means within a column followed by different lower-case letters are significantly different at P ≤ 0.05 (LSD test).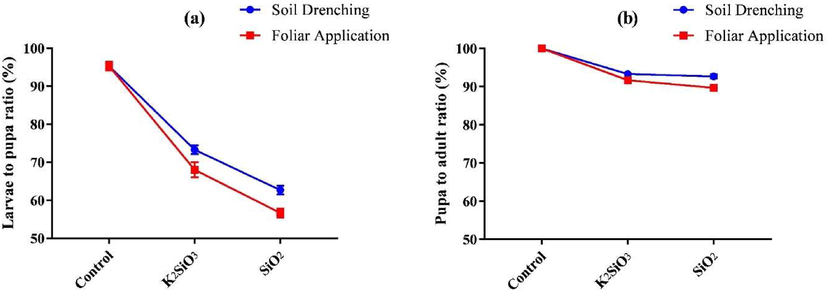
Effect of SiO2 and K2SiO3 applied with two application methods (Foliar application and soil drenching) on(a) larvae to pupa ratio (%) and (b) pupa to adult ratio (%) of S. frugiperda. Means were compared using the LSD test at p ≤ 0.05. Vertical bars indicate SE.
Treatments
Larvae to pupa ratio (%)
Pupa to adult ratio (%)
Male sex ratio (%)
Female sex ratio (%)
Silicon sources
SiO2
59.67 ± 3.45c
91.17 ± 1.72c
57.78 ± 3.72a
42.22 ± 3.71a
K2SiO3
70.67 ± 3.26b
92.5 ± 1.04b
59. 72 ± 4.33a
40.28 ± 4.33a
Control
95.33 ± 1.03a
100 ± 0.0a
57. 92 ± 4.58a
42.08 ± 4.58a
Application methods
Foliar application
73.33 ± 17.26b
93.78 ± 4.76b
58.80 ± 4.48a
41.20 ± 4.48a
Soil drenching
77.11 ± 14.46a
95.33 ± 3.54a
58.14 ± 3.86a
41.86 ± 3.86a
In the pupa to adult ratio, Si applications significantly (P ≤ 0.05) showed lower ratios than control. Pupa to adult ratio in the foliar spray of SiO2 (89.67 ± 0.57 %) was significantly (P ≤ 0.05) similar to foliar spray of K2SiO3 (91.67 ± 0.57 %); similarly, soil application of SiO2 (92.67 ± 0.57 %) was also significantly (P ≤ 0.05) identical to soil application K2SiO3 (93.33 ± 0.57 %). The pupa to adult ratio in control was (100.00 ± 0.00 %). Among Si sources, papa to adult ratios was (91.17 ± 1.72 %), (92.05 ± 1.04 %), and (100.00 ± 0.00 %) in SiO2, K2SiO3, and control, respectively (Table 3).
3.4 Effect of Si on the sex ratio of S. Frugiperda
Fig. 4 shows the effect of SiO2 and K2SiO3 and their application methods on the sex ratio of S. frugiperda. The results revealed no significant (P = 0.55) impact of Si sources on the male and female sex ratio. The male sex ratio was (57.78 ± 3.71 %) in SiO2, (59.72 ± 4.33 %) in K2SiO3, and (57.92 ± 4.58 %) in control; similarly, the female sex ratio was (42.22 ± 3.72 %) in SiO2, (40.28 ± 4.33 %) in K2SiO3, and (42.08 ± 4.58 %) in control. The results also showed no significant impact of Si application methods on the sex ratio of S. frugiperda adults. The male and female sex ratios in the foliar spray were (58.80 ± 4.48 %) and (41.20 ± 4.48 %), respectively; similarly, in soil application, ratios were (58.14 ± 3.86 %) and (41.86 ± 3.86 %) (Table 3).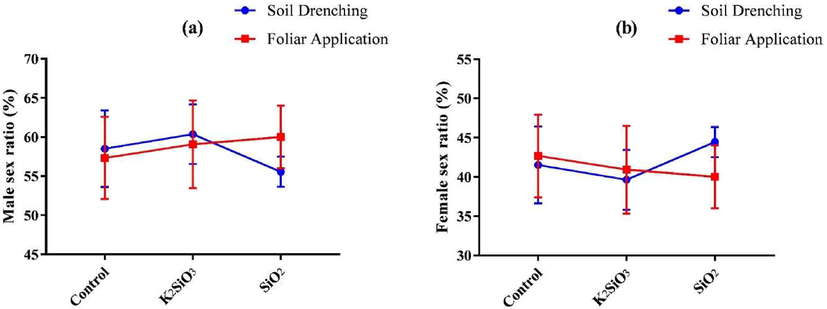
Effect of SiO2 and K2SiO3 applied with two application methods (Foliar application and soil drenching) on(a) male sex ratio (%) and (b) female sex (%) of S. frugiperda. Means were compared using the LSD test at p ≤ 0.05. Vertical bars indicate SE.
3.5 Effect of Si on fecundity, eggs development period, and larvae emergence of S. Frugiperda
Fig. 5 shows the effect of SiO2 and K2SiO3 and their application methods on total fecundity, egg development period, and larvae emergence of S. frugiperda. All the Si applications significantly (P ≤ 0.05) affected the total fecundity of S. frugiperda. A considerably (P ≤ 0.05) lower fecundity was observed in the foliar spray of SiO2 (130 ± 2.65 eggs), followed by the foliar spray of K2SiO3 (141.67 ± 0.57 eggs), and soil application of SiO2 (163.33 ± 1.53 eggs) and K2SiO3 (168 ± 2.65 eggs). Si application methods also showed a significant (P ≤ 0.05) impact on the fecundity of S. frugiperda. Total fecundity was (154.33 ± 28.28 eggs) in the foliar spray and (175.00 ± 14.28 eggs) in soil application of Si. Among Si sources, lower fecundity was observed in SiO2 (146.67 ± 18.35 eggs) compared to K2SiO3 (154.83 ± 14.52 eggs) and untreated control (192.50 ± 2.88 eggs).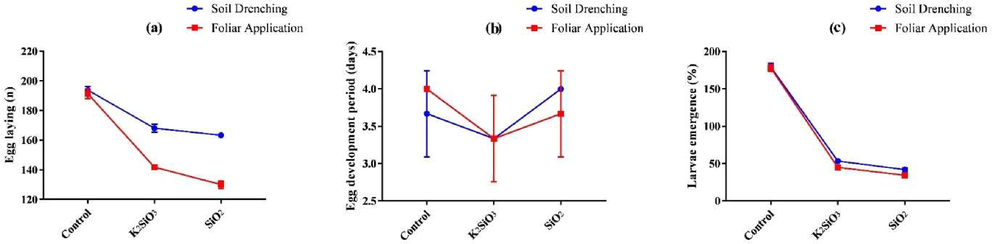
Effect of SiO2 and K2SiO3 applied with two application methods (Foliar application and soil drenching) on (a) egg-laying (n), (b) eggs development period (days), and (c) larvae emergence (%) of S. frugiperda. Means were compared using the LSD test at p ≤ 0.05. Vertical bars indicate SE.
The current study results showed no significant impact of Si sources (P = 0.20) or Si application methods (P = 1.00) on the egg development period of S. frugiperda. The egg's development periods were (3.83 ± 0.41 days), (3.33 ± 0.52 days), and (3.83 ± 0.41 days) in SiO2, K2SiO3, and control, respectively. Similarly, the egg development period in the foliar spray and soil application was (3.67 ± 0.5 days) and (3.67 ± 0.5 days), respectively (Table 4). Means within a column followed by different lower-case letters are significantly different at P ≤ 0.05 (LSD test).
Treatments
Egg-laying (n)
Egg development (days)
Larvae emergence (%)
Silicon sources
SiO2
146.67 ± 18.36c
3.83 ± 0.41a
37.83 ± 4.62c
K2SiO3
154.83 ± 14.52b
3.33 ± 0.51a
49.0 ± 5.17b
Control
192.5 ± 2.88a
3.83 ± 0.40a
178.67 ± 4.27a
Application methods
Foliar application
154.33 ± 28.28b
3.67 ± 0.50a
85.56 ± 69.53b
Soil drenching
175 ± 14.28a
3.67 ± 0.50a
91.44 ± 66.18a
Fig. 5 showed the impact of SiO2 and K2SiO3 and their application methods on larvae emergence of S. frugiperda. The larvae emergence was significantly (P ≤ 0.05) lower in soil application of SiO2 (25.46 ± 1.35 %), followed by the foliar spray of SiO2 (26.13 ± 1.06 %) and foliar spray of K2SiO3 (31.4 ± 1.51 %). No significant impact (P = 0.74) of Si application methods was observed on the percentage larvae emergence of S. frugiperda. The percentage of larvae emergence was (50.18 ± 32.22 %) and (49.91 ± 32.12 %) in the foliar spray and soil application of Si, respectively. Moreover, among Si sources, the percentage of larvae emergence was significantly (P ≤ 0.05) lower in SiO2 (25.79 ± 1.15 %) compared to K2SiO3 (31.57 ± 1.39 %) and control (92.76 ± 1.85 %) (Table 4).
4 Discussion
Our findings revealed that Si treatments increased the mortality of S. frugiperda larvae compared to controls (No silicon). Reduced digestibleness or poor nutritional value of Si supplemented plants could reason for reduced survival of newly emerging larvae (Lee et al., 2003; Hall et al., 2019). The poorer digestibility of Si supplemented plants may be attributed to the plant tissue's increased abrasiveness or improved plant defenses due to the synergistic effects with the pathways influenced by jasmonic acid (Massey and Hartley 2009, Reynolds et al., 2016, Haq et al., 2021). These findings show that early infestation is less likely in the plants supplemented with Si than in non-supplemented maize plants. A similar impact of Si application on S. frugiperda larvae has been reported by (Pereira et al., 2021), who demonstrated the larval mortality of S. frugiperda feeding on Si supplemented differently from those on non-supplemented plants. After 48 h of being fed, they observed that larval mortality was about six times higher on plants receiving Si applications than plants with no Si.
Jeer et al. (2022) reported that plant stem borer damage was significantly reduced (66% over control) in wheat plots treated with K and Si when compared with untreated control (T1) and insecticidal check (T6). Both, K and Si treated plots recorded lower pink stem borer damage than control, while soil applied Si had significant influence in reducing Plant stem borer damage. (Nagaratna et al., 2022) observed a substantial effect of Si and plant growth regulators on larval survival, with the foliar Si application and Gibberellic acid resulting in the lowest (70 %) larval survival. All treatments were statistically different from the control, with a maximum larval survival rate of 90%. They further speculated that the detrimental effect of exogenic Si on S. frugiperda larval growth could be due to Si deposition in the cell walls of the plants, which improved the stiffness of leaves and increased the synthesis of secondary defensive chemicals such as tannins and phenols. For treatments in which plants were supplied with silicon, a reduction in the total number of aphids was observed after two and three days (Gomes et al., 2008). At 72 h, leaves of plants that received silicon application were less colonized by aphids. The treatment with aphid pre-infestation and silicon fertilization had the lowest rate of aphid population increase, while the control had the highest rate of population increase. Si is usually applied to the soil or sprayed on the plant leaves in agricultural systems (Reynolds et al., 2016). The physical barrier made on the leaf surface, pH effects and changes in osmotic pressure have been attributed to Si-protecting effects via foliar spray (Liang et al., 2005). Foliar applied Si reduced the number of immature and adult of thrips on peanuts plants and enhanced crop yields (Dalastra et al., 2011). After Si sprays, an increase in Si content in leaf tissues of Glycine max was linked to a reduction in the severity of Phakopsora pachyrhizi (Rodrigues et al., 2009). Increased leaf Si concentration was similarly linked to reduced defoliating insect harm in potatoes (Aparecida De Assis et al., 2012).
The current study results indicated that the pupa to adult ratio was significantly similar in all Si treatments but different from the control. Similar findings are reported by Mondego et al. (2018), who stated that the survival rate of the pupa developed from larvae fed with Si treated maize leaves was 92.5 %, which supports the current study results. The current results agree with the results of Nagaratna et al. (2022), who stated that the mean survival percentage of S. frugiperda pupa was 87 % with Si applications. The current study showed no significant impact of Si applications on the sex ratio of S. frugiperda. These results are supported by (Nagaratna et al., 2022), who observed the substantial impacts of Si and plant growth regulators on adult parameters of S. frugiperda except for the sex ratio. Similar results are described by Nascimento et al. (2018). They stated that Si application significantly affected all adult parameters of S. frugiperda except the sex ratio. Thabet et al. (2020) checked the effect of silica nanoparticles on population parameters and American serpentine leaf miner gene expression. They also stated that th sex ratio was not significantly different among SiO2NP concentrations.
The silicon effect is most noticeable in insect fertility. The observed drop in fertility in this study shows a negative impact of Si treatments, which could result in lower insect population density and, as a result, less potential for plant damage. In the current study, a lower fecundity and fertility were observed in the adults derived from larvae fed on Si-treated plants compared to control. Silicon has been shown to diminish the reproduction of S. frugiperda on maize plants in the past (Alvarenga et al., 2017). Nagaratna et al. (2022) stated that the foliar spray Si decreased the fecundity of S. frugiperda up to 57.36%. Silicon treatment of rice plants also lowered egg-laying and viability in S. frugiperda (He et al., 2015, Nascimento et al., 2018). Adult of S. frugiperda developed from larvae fed on plants treated with Si also deposited fewer eggs (Silva et al., 2014). Silicon also induced resistance against green peach aphids in potatoes by lowering female fertility (Gomes et al., 2008). In the current study, the negative effect of silicon on fecundity and fertility could be due to silicon deposition in plants, which can activate and boost the synthesis of defense metabolites (Tatagiba et al., 2014). High Si content in leaf tissue could inhibit the larvae from consuming enough nutrients and water (Nagaratna et al., 2022), and S. frugiperda's fitness was affected due to the physiological alterations. As a result, Si's sub-lethal effect on S. frugiperda growth could limit the species' reproduction rate. On the plants treated with foliar and soil treatments of Si, a lower number of eggs on the first and second days of egg-laying were observed. In addition, compared to control, soil application reduced egg viability by 50% on the first and second days of egg-laying (Nascimento et al., 2018). Tawfeek and Eldesouky (2022) reported that Treatments with K2SiO3 significantly reduced the longevity of adult aphids, as the values were 15.45 days (LC10), 14.82 days (LC25), 11.23 days (LC50), and 17.69 days (control). Compared to the control, Si treatment dramatically lowered the intrinsic rate of rising, the finite rate of increase, and the net reproduction rate of Nilaparvata lugens. The high Si treatment significantly shortened the mean generation time compared to the control. The time it took for the population to double in Si modified treatments was longer than in control treatments (Yang et al., 2017).
5 Conclusion
According to our findings, Si foliar spray to maize was as effective as soil drenching in increasing resistance against S. frugiperda. However, it is unknown if the resistant impacts of foliar application of Si would endure the same results as soil-applied Silicon. Because it reduces fecundity by S. frugiperda and reduces the survival rate of newly emerging larvae, our findings suggest that Si treatment may diminish adoption and early damage by S. frugiperda in crops. Furthermore, larvae that survive on Si-treated plants are likely to become adults with poor fertility. These impacts of Si applications on the biology and behaviour of S. frugiperda could help minimize this serious pest's population.
Acknowledgement
The authors extend their appreciation to the Researchers Supporting Project number (RSP2022R483), King Saud University, Riyadh, Saudi Arabia.
Funding
This work is supported by the Science Project of Agriculture and Rural Department of Gansu Province; grant number GZB20191105. The authors extend their appreciation to the Researchers Supporting Project number (RSP2022R483), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Fall armyworm: impacts and implications for Africa. Evidence note (2). Center for Agriculture Bioscience International-CABI; 2017.
- Silicon-mediated enhancement of herbivore resistance in agricultural crops. Frontiers in Plant Science.. 2021;12
- [Google Scholar]
- The impact of different plant extracts on population suppression of Helicoverpa armigera (Hub.) and tomato (Lycopersicon esculentum Mill) yield under field conditions. PLOS ONE.. 2021;16(12)
- [CrossRef] [Google Scholar]
- Induction of resistance of corn plants to Spodoptera frugiperda (JE Smith, 1797)(Lepidoptera: Noctuidae) by application of silicon and gibberellic acid. Bulletin of entomological research.. 2017;107(4):527-533.
- [Google Scholar]
- Inducers of resistance in potato and its effects on defoliators and predatory insects. Revista Colombiana de Entomología.. 2012;38(1):30-34.
- [Google Scholar]
- Effect of silicon fertilization on crop yield quantity and quality—A literature review in Europe. Plants.. 2018;7(3):1-17.
- [Google Scholar]
- Induction of caterpillar resistance in sunflower using silicon and acibenzolar-S-methyl. Journal of Agricultural Science Technology.. 2015;17(3):543-550.
- [Google Scholar]
- A temperature-dependent model for fall armyworm development. Annals of the Entomological Society of America.. 1978;71(1):70-74.
- [Google Scholar]
- Updated assessment of potential biopesticide options for managing fall armyworm (Spodoptera frugiperda) in Africa. Journal of Applied Entomology.. 2021;145(5):384-393.
- [Google Scholar]
- The optimal age and radiation dose for Bactrocera dorsalis (Hendel)(Diptera: Tephritidae) eggs as hosts for mass-reared Fopius arisanus (Sonan)(Hymenoptera: Braconidae) Biological control.. 2017;108:89-97.
- [Google Scholar]
- Review of the host plants of fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae) Revista de la Sociedad Entomológica Argentina.. 2017;69(3):209-231.
- [Google Scholar]
- Center, N. A. T. E. S., 2019. Major pest Spodoptera frugiperda have invaded in Yunnan, and all areas should immediately strengthen investigation and monitoring. Plant pathogen pest information.
- Silicon as a resistance inducer controlling the silvering thrips Enneothrips flavens Moulton (Thysanoptera: Thripidae) and its effects on peanut yield. Ciência e Agrotecnologia.. 2011;35:531-538.
- [Google Scholar]
- Forecasting the global extent of invasion of the cereal pest Spodoptera frugiperda, the fall armyworm. NeoBiota.. 2018;40:25-50.
- [Google Scholar]
- Delimiting strategic zones for the development of fall armyworm (Lepidoptera: Noctuidae) on corn in the state of Florida. Journal of economic entomology.. 2018;111(1):120-126.
- [Google Scholar]
- Uso de silício como indutor de resistência em batata a Myzus persicae (Sulzer)(Hemiptera: Aphididae) Neotropical Entomology.. 2008;37(2):185-190.
- [Google Scholar]
- Resistance induction in wheat plants by silicon and aphids. Scientia Agricola.. 2005;62(6):547-551.
- [Google Scholar]
- The response of four braconid parasitoid species to methyl eugenol: Optimization of a biocontrol tactic to suppress Bactrocera dorsalis. Biological Control.. 2018;122:101-108.
- [Google Scholar]
- Potential invasion of the crop-devastating insect pest fall armyworm Spodoptera frugiperda to China. Plant Protection.. 2018;44(6):1-10.
- [Google Scholar]
- Hall, C. R., Waterman, J. M., Vandegeer, R. K., Hartley, S. E., & Johnson, S. N., 2019. The role of silicon in antiherbivore phytohormonal signalling. Frontiers in plant science, 1132.
- Herbivore-and elicitor-induced resistance in rice to the rice water weevil (Lissorhoptrus oryzophilus Kuschel) in the laboratory and field. Journal of chemical ecology.. 2010;36(2):192-199.
- [Google Scholar]
- Silicon-based induced resistance in maize against fall armyworm [Spodoptera frugiperda (Lepidoptera: Noctuidae)] Plos one.. 2021;16(11):e0259749.
- [Google Scholar]
- High levels of silicon provided as a nutrient in hydroponic culture enhances rice plant resistance to brown planthopper. Crop Protection.. 2015;67:20-25.
- [Google Scholar]
- Silicon-mediated rice plant resistance to the Asiatic rice borer (Lepidoptera: Crambidae): effects of silicon amendment and rice varietal resistance. Journal of economic entomology.. 2010;103(4):1412-1419.
- [Google Scholar]
- Outcomes of the California ban on pharmaceutical lindane: Clinical and ecologic impacts. Environmental health perspectives.. 2008;116(3):297-302.
- [Google Scholar]
- Acaricidal potential of some botanicals against the stored grain mites, Rhizoglyphus tritici. J. Entomol. Zool. Stud.. 2016;4:611-617.
- [Google Scholar]
- Silicon Supplementation Along with Potassium Activate Defense Reaction in Wheat Plants and Reduce the Impact of Pink Stem Borer Incidence. Silicon 2022
- [CrossRef] [Google Scholar]
- First report of the fall armyworm, Spodoptera frugiperda (JE Smith)(Lepidoptera: Noctuidae), an alien invasive pest on maize in India. Pest Manage in Hortic Ecosyst.. 2018;24:23-29.
- [Google Scholar]
- In vitro evaluation of silicon sources against late blight (Phytophthora infestans) of tomato. Int J Sci Nat.. 2016;7(4):881-884.
- [Google Scholar]
- Silicon regulates antioxidant activities of crop plants under abiotic-induced oxidative stress: a review. Frontiers in Plant Science.. 2017;8:510.
- [Google Scholar]
- A correlation between macronutrient balancing and insect host-plant range: evidence from the specialist caterpillar Spodoptera exempta (Walker) Journal of Insect Physiology.. 2003;49(12):1161-1171.
- [Google Scholar]
- Effect of silicon on growth and salinity stress of soybean plant grown under hydroponic system. Agroforestry systems.. 2010;80(3):333-340.
- [Google Scholar]
- Prediction of migratory routes of the invasive fall armyworm in eastern China using a trajectory analytical approach. Pest Management Science.. 2020;76(2):454-463.
- [Google Scholar]
- Effects of foliar-and root-applied silicon on the enhancement of induced resistance to powdery mildew in Cucumis sativus. Plant Pathology.. 2005;54(5):678-685.
- [Google Scholar]
- Effect of foliar application of potassium silicate on the progress of coffee leaf rust. Tropical Plant Pathology.. 2013;38(6):547-551.
- [Google Scholar]
- Molecular identification of cultivable bacteria in the gut of adult Bactrocera tau (Walker) and their trapping effect. Pest management science.. 2018;74(12):2842-2850.
- [Google Scholar]
- Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil science plant nutrition.. 2004;50(1):11-18.
- [Google Scholar]
- Physical defences wear you down: progressive and irreversible impacts of silica on insect herbivores. Journal of Animal Ecology.. 2009;78(1):281-291.
- [Google Scholar]
- Effect of resistance elicitors on the biology and feeding preference of'Spodoptera frugiperda'(JE Smith)(Lepidoptera: Noctuidae) in corn. Australian Journal of Crop Science.. 2018;12(11):1774-1781.
- [Google Scholar]
- Effect of Silicon and Plant Growth Regulators on the Biology and Fitness of Fall Armyworm, Spodoptera frugiperda, a Recently Invaded Pest of Maize in India. Silicon.. 2022;14(3):783-793.
- [Google Scholar]
- Silicon application promotes rice growth and negatively affects development of Spodoptera frugiperda (JE Smith) Journal of applied entomology.. 2018;142(1-2):241-249.
- [Google Scholar]
- Não preferência a Spodoptera frugiperda (Lepidoptera: Noctuidae) induzida em arroz pela aplicação de silício. Revista Brasileira de Ciências Agrárias.. 2014;9(2):215-218.
- [Google Scholar]
- Njeru, R., 2017. Report on stakeholders consultation meeting on: Fall Armyworm in Africa: status and strategy for effective management.
- Silicon supplementation of maize impacts Fall Armyworm colonization and increases predator attraction. Neotropical Entomology.. 2021;50(4):654-661.
- [Google Scholar]
- Foliar or Soil Applications of Silicon Alleviate Water-Deficit Stress of Potato Plants. Agronomy Journal.. 2014;106(6):2325-2334.
- [Google Scholar]
- Effectiveness of Different Soft Acaricides against Honey Bee Ectoparasitic Mite Varroa destructor (Acari: Varroidae) Insects.. 2021;12(11):1032.
- [Google Scholar]
- Ranaweera, G. K. M. M. K., Mubarak, A. N. M., Kumara, A. D. N. T., Musthapa, M. M., Thariq, M. G. M., & Majeed, U. L. A., 2021. Tapping the Latent Resistant Potential of Traditional Maize Germplasm: An Attempt to Combat the Invasive Fall Armyworm Spodoptera Frugiperda. Available at SSRN 4043595. http://dx.doi.org/10.2139/ssrn.4043595.
- Fighting against fall armyworm by using multiple genes pyramiding and silencing (MGPS) technology. Sci China Life Sci.. 2019;62(12):1703-1706.
- [Google Scholar]
- Silicon: potential to promote direct and indirect effects on plant defense against arthropod pests in agriculture. Frontiers in Plant Science.. 2016;7:744.
- [Google Scholar]
- Effect of root and foliar applications of silicon on brown spot development in rice. Australasian Plant Pathology.. 2009;38(1):67.
- [Google Scholar]
- Foliar application of potassium silicate reduces the intensity of soybean rust. Australasian Plant Pathology.. 2009;38(4):366.
- [Google Scholar]
- Effect of foliar silicic acid on growth, nutrient uptake and blast disease resistance of finger millet (Eleusine coracana (L.) Gaertn.) Int. J. Curr. Microbiol. Appl. Sci.. 2020;9(4):2111-2121.
- [Google Scholar]
- Foliar applied silicon improves water relations, stay green and enzymatic antioxidants activity in late sown wheat. Silicon.. 2020;12(1):223-230.
- [Google Scholar]
- Outstanding questions on the beneficial role of silicon in crop plants. Plant Cell Physiology.. 2022;63(1):4-18.
- [Google Scholar]
- Biologia de Spodoptera frugiperda (JE Smith)(Lepidoptera: Noctuidae) em algodoeiro de fibra colorida tratado com silício. EntomoBrasilis.. 2014;7(1):65-68.
- [Google Scholar]
- Intrusion of fall armyworm (Spodoptera frugiperda) in sugarcane and its control by drone in China. Sugar Tech.. 2020;22(4):734-737.
- [Google Scholar]
- Characterization of priming, induced resistance, and tolerance to Spodoptera frugiperda by silicon fertilization in maize genotypes. Journal of Pest Science. 2022;95(3):1387-1400.
- [Google Scholar]
- New crop pest takes Africa at lightning speed, American Association for the. Advancement of Science. 2017;356(6337):473-474.
- [Google Scholar]
- Direct induced resistance in Oryza sativa to Spodoptera frugiperda. Environmental entomology.. 2009;38(4):1174-1181.
- [Google Scholar]
- Physiological responses of rice plants supplied with silicon to Monographella albescens Infection. Journal of Phytopathology.. 2014;162(9):596-606.
- [Google Scholar]
- Influence of Potassium Silicate on the Survival, Development and Reproduction of Aphis gossypii Glover (Hemiptera: Aphididae) Alexandria Science Exchange Journal.. 2022;43(1):1-9.
- [CrossRef] [Google Scholar]
- Silica nanoparticle effect on population parameters and gene expression of an internal feeder. Biology (Pre-print): American serpentine leafminer; 2020.
- Biosilica Fertilizer Reduces Fall Armyworm Damage. IOP Conf. Ser.: Earth Environ. Sci.. 2022;985(1):012049.
- [Google Scholar]
- Foliar application with nano-silicon alleviates Cd toxicity in rice seedlings. Environmental Science Pollution Research.. 2015;22(4):2837-2845.
- [Google Scholar]
- Analysis of migration routes of the fall armyworm Spodoptera frugiperda (JE Smith) from Myanmar to China. Plant Protection.. 2019;45(2):1-6.
- [Google Scholar]
- Silicon amendment to rice plants impairs sucking behaviors and population growth in the phloem feeder Nilaparvata lugens (Hemiptera: Delphacidae) Scientific Reports.. 2017;7(1)
- [CrossRef] [Google Scholar]







