Effect of two protein elicitors extracted from Alternaria tenuissima and Beauveria bassiana against rice leaf folder (Marasmia exigua)
⁎Corresponding author at: Department of Plant Pathology, Agriculture College, Guizhou University, Guiyang 550025, China. yongwangbis@aliyun.com (Yong Wang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The rice leaf folder, Marasmia exigua (Butler), is dangerous pests and negatively affecting the rice production worldwide. Protein elicitors are considered to be bio-factors that induce rice resistance against herbivores. The present study was performed with two protein elicitors, Hrip 1 (extracted from Alternaria tenuissima) and PebB1 (from Beauveria bassiana) to study their efficacy against the rice leaf folder. Three different concentrations of Hrip 1 (68.88, 46.69 and 28.44 µg/ml) and PebB1 (56.66, 39.76 and 32.62 µg/ml) were assigned to evaluate the developmental time, survival rate, and fecundity of M. exigua. Both elicitors were applied to the three-leaf stage of the rice plant where newly emerged adult insects were released. Bioassay results showed that after the application of protein elicitors, the life cycle and survival rate of the rice leaf folder were prolonged, while fecundity decreased. The concentration of protein had a significant (p < 0.05) influence on ontogeny. In addition to ontogeny, the expression profile of jasmonic acid, salicylic acid, and ethylene associated with signalling pathway, which indicated that exogenous application of both elicitors produced a significant up-regulation in all the genes associated in signalling pathways of the plant. The plants treated with elicitors produced resistance against M. exigua (as compared to the control). This in vitro study invites new research into Hrip 1 and PebB1 as having prophylactic potential against rice leaf folder, and suggest that both protein elicitors can be used as a novel biological control tool for M. exigua.
Keywords
Plants resistance
Protein elicitors
Rice leaf folder
Signalling pathway
1 Introduction
Rice is considered as one of the most important staple food crops all over the world (Yu et al., 2002). In China, due to proper agronomic practices and high yield varieties, rice production is steadily increasing, but it is at risk because of a group of lepidopteron insect pests in general and rice leaf folder in particular (Ye et al., 2003). The excessive use of synthetic fertilizers, especially nitrogenous fertilizers, is the main cause of lepidopteron pest outbreaks (Graf et al., 1992; Punithavalli et al., 2013). The pests are distributed widely in all rice-growing areas of the temperate and tropical regions of Asia, Africa, and Oceania. In some rice-growing areas, three different types of rice leaf folders, viz. Cnaphalocrocis medinalis (Guenée), Marasmia exigua (Butler), and M. patnalis (Bradley) are often considered as the major pests. The morphological and biological characteristics of the rice leaf folders have been studied by several researchers (Khan et al., 1988; Barrion et al., 1991; Bale et al., 2002; Huang and Chi, 2012; Yang et al., 2015) for control of these insect pests. The impact of temperature and ecology-related information remains scarce because their development, survival, fecundity, and population parameters are still unknown (Maffei et al., 2012).
An elicitor is a chemical or biological factor used by the plant after being attacked by insect pests as a signal molecule to enhance systemic resistance against herbivores by activating numerous defence-related pathways (Alborn et al., 1997; Garcia-Brugger et al., 2006). Microbial Associated Molecular Patterns (MAMPs) and Herbivore-Associated Molecular Patterns (HAMPs) have been considered in microbial control of insects, such as Lepidoptera, Orthoptera, And Diptera (Garcia-Brugger et al., 2006). Some elicitors and eliciting components lead to resistance against insect pests by acting as resistant protein- and nucleotide-binding factors in plants (Smith and Boyko, 2007; Botha et al., 2006).
Different types of elicitors have been isolated from fungi, bacteria, viruses, and herbivores (Paré et al., 2005; Zhao et al., 2005; Sharathchandra et al., 2006), and their chemical nature (include proteins, glycoproteins, lipids, and oligosaccharides) have been identified (Veit et al., 2001). Defence pathways are activated by protein elicitors, proteinaceous inducers from microbes, and induced systemic acquired resistance (SAR) in plants (Shao et al., 2008). Various types of protein elicitors have been identified from various fungi, and some researchers have reported different types of elicitors, for example, Pep-13 of Trichoderma and endo-β-1, 4-xylanases from Phytophthora as well as bacterial elicitors such as flg22 from bacterial flagella (Dean et al., 1989; Felix et al., 1999; Brunner et al., 2002; Mao et al. 2010). Alternaria tenuissima is a common plant pathogen that causes disease in a number of plants and produces some toxins harmful for plants, animals, and human health (Gannibal et al., 2007). A heat-stable and acidic protein, PeaT1, was purified from the mycelium of A. tenuissima as a new protein elicitor (Mao et al., 2010). Recently, Sokea et al. (2019) reported that Hrip 1 protein was also obtained from A. tenuissima. These protein elicitors had played a tremendous role in controlling plant pathogens but their role had not been elaborately studied in controlling insect pests (Basit et al., 2019).
Various microbes, including bacteria, entomopathogenic fungi, nematodes, and viruses, have been shown to be effective against various insect pests (Burges, 2012; Ruiu, 2018). Almost 750 species of entomopathogenic fungi have been used as biological control agents for insect pests worldwide. Genera of Isaria, Beauveria, Metarhizium, and Lecaniciilum, in particular, have shown excellent pathogenicity for many insect pests due to their residual activity, high host specificity, and mammalian toxicity (Wraight and Carruthers, 1999; Quesada-Moraga et al., 2006; Zimmermann, 2007). Rather than insecticides, developing and identifying bioactive metabolites in entomopathogenic fungi that manifest (sub)lethal effects against insect pests to cause a delay in life cycle improved their toxicity (Hegedus and Khachatourians, 1995; Ortiz-Urquiza et al., 2010). Some fungi have been introduced to secrete various insecticidal and toxic bioactive substances as antifeedants in culture media (Quesada-Moraga et al., 2006; Ortiz-Urquiza and Keyhani, 2013).
Numerous entomopathogenic fungi from plant tissue have been isolated and exposed to plants to induce acquired systemic resistance against different biotic and abiotic stresses in various herbivores (Jaber and Ownley, 2018; Basit et al., 2019). Recently, various protein elicitors derived from fungi have been evaluated to evoke systemic resistance and defence responses in plants against different phytophagous insect pests and numerous pathogens (Thomma et al., 2011; Zhang et al., 2011). Plants produce resistance against related attackers (insect pests and pathogens) at very early stages through the plant immune system (Chisholm et al., 2006; Thomma et al., 2011; Zhang et al., 2011; Basit et al., 2019). Through gene expression and metabolic changes, all signal molecules might be involved in regulating the downstream signalling molecule against the defence system response (Vandelle, et al., 2006). After an attack by insect pests and pathogens, the plant defence system is usually regulated by numerous signalling pathways, such as salicylic acid, jasmonic acid and ethylene (Chisholm et al., 2006). The SA and JA are the most important signalling molecules that enhance plant defence responses to herbivory and necrotrophic pathogen infestations (Thaler et al., 2012).
Keeping in view the aforementioned role of entomopathogenic fungus the present in vitro study was conducted to evaluate the putative role and molecular characterization of two protein elicitors, Hrip 1 (extracted from A. tenuissima) and PebB1 (from Beauveria bassiana) derived from the microbial associated molecular pattern against rice leaf folder. Furthermore, the expression of key genes associated with SA, JA, and ET signalling pathways was quantified by reverse transcriptase quantitative polymerase chain reaction (RT-qPCR).
2 Material and methods
2.1 Plant and insect culture
Rice (Oryza sativa L.) was grown in pots under laboratory conditions in a growth chamber. Three common species of rice leaf folders (Cnaphalocrocis medinalis, Marasmia exigua, and M. patnalis) were reared on rice plants at 25 ± 2 °C and a relative humidity of 80 % with a photoperiod time of 10 D:14 L. After the emergence of the adults, 3–5 pairs were released on each plant, and leaves were covered with insect cages.
2.2 Purification of Hrip 1
Yeast peptone dextrose (YPD) was used to express the Hrip 1 protein elicitor gene. Hrip 1 was cultured in 25 mL liquid YPD medium with 1 % dextrose, 0.5 % yeast extract, and 1 % peptone. YPD medium was shaken at 200 rpm at 30 °C overnight, and then transferred to 1 mL liquid medium of Buffered Glycerol Complex Medium (Millipore,Crop., Billerica,MA,USA) (BMGY) with 100 mM KH2PO4 and 100 mM K2HPO4 (pH = 7.0). The medium was placed on a shaker at 200 rpm until its absorbance reached 600 nm. The pellet was collected by centrifuging the medium at 5000 rpm for 10 mins at 25 °C. The pellet was re-suspended in a liquid medium of 100 mL of Buffered Methanol-complex Medium (BMMY) medium supplemented with 1.3 g yeast and incubated in a shaker at 200 rpm for 72 h at 29 °C. The protein supernatant was filtered with a syringe filter with a 0.22 μm membrane pore size. The further purification was carried out using a His-Tag Purification column (GE Healthcare, Waukesha, WI, USA). Three buffers were used to elute the protein elicitor: buffer A (50 mM Tris-HCl + 200 mM NaCl) to remove the impurities and to bind the proteins in the columns, Buffer B (50 Mm Tris-HCl + 200 Mm NaCl + 20 Mm Imidazole) to balance the columns, and Buffer C (50 mM Tris-HCl + 200 mM NaCl + 500 mM imidazole) to elute the protein elicitor. The obtained protein was then centrifuged using a desalting tube. The desalting columns were washed three times with a buffer (50 mM Tris-HCl, pH 8.0) to remove the concentrated salt.
2.3 Purification of PeBb1
For the purification of PeBb1, B. bassiana spores were grown in 50 mL liquid broth (LB) medium (Millipore,Crop., Billerica,MA,USA) and shaken at 37 °C for 4 h. When the optical density (OD) value reached 0.6–0.8, 200 μM isopropyl β-D-1-thiogalactopyranoside was added to obtain the subsequent recombinant protein. The protein was shaken at 16 °C at 200 rpm for 14–16 h, and then centrifuged to obtain the pellet, which were re-suspended in buffer (50 mM Tris-HCl, 200 mM NaCl, pH 8.0). Then, the cells in the pellets were disrupted by ultrasonic sound and the combatant protein was collected after centrifuging at 5000 rpm for 20 min. Purification was also performed using His-Trap Hp columns (GE Healthcare, Waukesha, WI, USA), and the three buffers were used for protein elution, as described above.
2.4 Characterization of protein elicitors
The concentrations of Hrip 1 and PeBb1 were checked by protein assay II using the QuantiKit from BCA, (Pierce, Rockford, IL, USA). and then both were stored at −80 °C until further analysis. The protein elicitors were diluted 25, 50, and 100 times. The characteristics of the protein elicitors were observed at three concentrations: Hrip 1 (68.88, 46.69 and 28.44 µg/ml) and PebB1 (56.66, 39.76, and 32.62 µg/ml).
2.5 Effect of protein elicitors on the lifecycle of rice leaf folder
To check the efficiency of protein elicitors against rice leaf folder, 3 mL of Hrip 1 (68.88, 46.69 and 28.44 µg/ml) and PebB1 (56.66, 39.76, and 32.62 µg/ml) were applied at the third leaf stage of rice and 50 mM Tris-HCl was used as the control. After spraying the protein elicitors, plants were allowed to dry for 12 h. Then, three to five freshly moulted larvae were released on a sprayed leaf and restricted with an insect cage. The larval developmental time of each instar was recorded. Each treatment was replicated 10 times, and the efficiency of the protein elicitors was assessed three times.
2.6 Effect of protein elicitors on survival rate and fecundity of rice leaf folder
To check the efficacy of the protein elicitors on the survival rate of rice leaf folder, three to five adults were released on the leaf and restricted with insect cages. The adults were removed, but three active larvae were maintained on each plant. The survival rate of each larva was observed on a daily basis from 6 h post application to pupation. Fecundity was regularly observed in surviving rice leaf folder adults for seven consecutive days, and average fecundity was then measured. Each concentration of Hrip 1 and PebB1 was replicated ten times.
2.7 Isolation of RNA and cDNA synthesis
Total RNA of the treated and control plant leaves after feeding of rice leaf folder was isolated separately using the plant RNA ER301-01 kit (Trans Gen Biotech, Beijing, China), following the manufacturer’s protocol. The concentration of RNA was quantified using a nano-photometer (NP80 Touch, Implen Inc., Westlake Village, CA, USA). A One-Step cDNA Removal and cDNA Synthesis AT341-01 kit (TransGen Biotech, Beijing, China) was used for cDNA synthesis.
2.8 Reverse transcription–quantitative PCR (RT-qPCR)
RT-qPCR was performed to quantify the expression profile of the key genes associated with JA, SA, and ET pathways. The feeding of rice leaf folder on plants treated with protein elicitors was considered a treatment, and plants treated with buffer were considered controls. For amplification of RT-qPCR, 12 gene primer pairs (Table 1) were used with the Applied Biosystems, USA (ABI 7500) system. All reactions were performed using the SYBR Premix Ex Taq II kit ((TransGen Biotech, Beijing, China), in a 20 μL total sample volume (2.0 μL cDNA, 10.0 μL SYBR Premix Ex Taq II, 1.8 μL of primers, and 6.2 μL of distilled deionised water). The qRT-PCR procedure was as follows: pre-denaturation at 95 °C for 10 min, denaturation at 95 °C for 15 s, and annealing at 60 °C for 15 s, with a total of 40 cycles. Standard curves were run simultaneously.
| Target Gene | Forward Sequence (5′ → 3′) | Reverse Sequence (5′ → 3′) |
|---|---|---|
| LOC_Os12g37350.1 | CTCCATGGTTGGTGGAACGA | TAGGGGTACTGGCCGAAGTT |
| LOC_Os11g39220.1 | GCTCACACTTGCGGAATCAC | GGCTTTGTTTGGGGCAACAT |
| LOC_Os06g23760.1 | AGCTCAGGTCACCGACTTTG | ATGAAACGGGAATTCGGCCT |
| LOC_Os08g39850.1 | GAGATGAGGAGTTCGCGAGG | ACGGCAAGAAGAGGTCATGG |
| LOC_Os11g15040.4 | TTCAATGCAGGAGGGACGAC | AGTCATGCATGCGGTTCTCA |
| LOC_Os01g56380.1 | GCATCAACGTCGTGCCTTTC | GATCGGAGCAGTAGACGACG |
| LOC_Os03g53200.1 | TCTTCGACAAGAACGGCGAT | AGGCCAAGAGAACGAGTCAC |
| LOC_Os05g41210.1 | GCGACGGTTGCATCACTACT | GCCTCAGTTGGGTTCTGACC |
| LOC_Os11g08380.1 | TAGCAATGGCCGCTTCAAGA | CTTGAAGCTCGGGTAGTCGG |
| LOC_Os03g01130.1 | GCGGAGCTGTACCTCAACAT | CTTGGAAGACTCCGCTGGTT |
| LOC_Os01g10940.1 | CGGAGACGTTCCTCTTCACC | CTTCTCGTAGTCGACGCTGG |
| LOC_Os03g37710.1 | TGAGAGGAGCCATAGGTGGT | GTAGCGGCTCATGTCGAAGT |
2.9 Statistical analysis
Data were analysed using the Statistics 8.1 software (Analytical Software, Tallahassee, FL, USA). Significant differences between Hrip 1 and PeBb1 concentrations over time were found by one -way factorial analysis using least significant differences (LSD) test at a 0.05 level of probability. Gene expression analysis was performed by comparative analysis of computed tomography CT (2-ΔΔCT) (Basit et al 2020). The Ct values obtained from two different experimental RNA samples were directly normalized to a housekeeping gene and fold changes between buffers and treated samples were calculated using Student’s t-test at a significance level of 0.05.
3 Results
3.1 Efficacy of both protein elicitors Hrip 1 and PebB1 on nymph development time
Factorial analysis showed a significant differences in the overall developmental time of rice leaf folder after Hrip 1 and PeBb1 application (Figs. 1 and 2). The nymph development time was enhanced by the protein concentrations of both the elicitors. In the control experiment, the 1st instar took 3 d to become a 2nd instar. However, after the application of Hrip 1, it took for 3.4, 3.2, and 3.1 days (F1 = 0.31, p < 0.01) at the concentrations of 68.88 µg/ml, 46.69 µg/ml, and 28.44 µg/ml, respectively. In the case of PebB1, the time was 3.3, 3.15, and 3.1 days (F1 = 0.20, p < 0.00) at concentrations of 68.88 µg/ml, 46.69 µg/ml, and 28.44 µg/ml. At higher concentration the larval developmental time is high and at lower concentration larval developmental time is low.
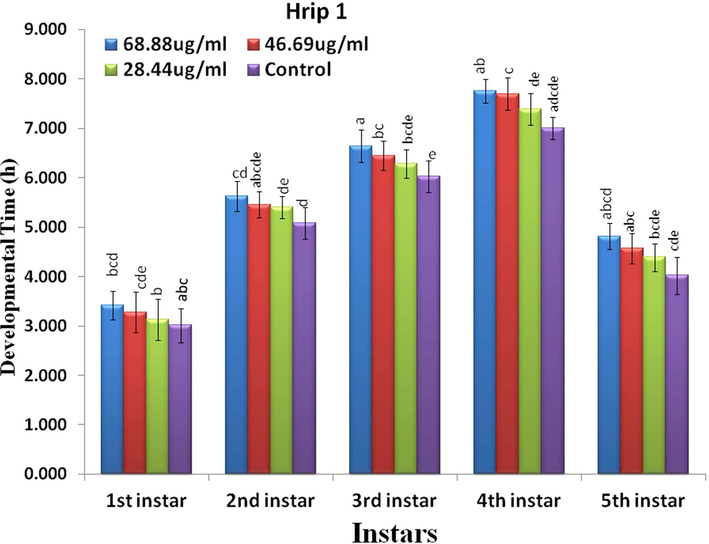
- Efficacy of Hrip 1 on the nymph development time of rice leaf folder (n = 10) at different concentration.
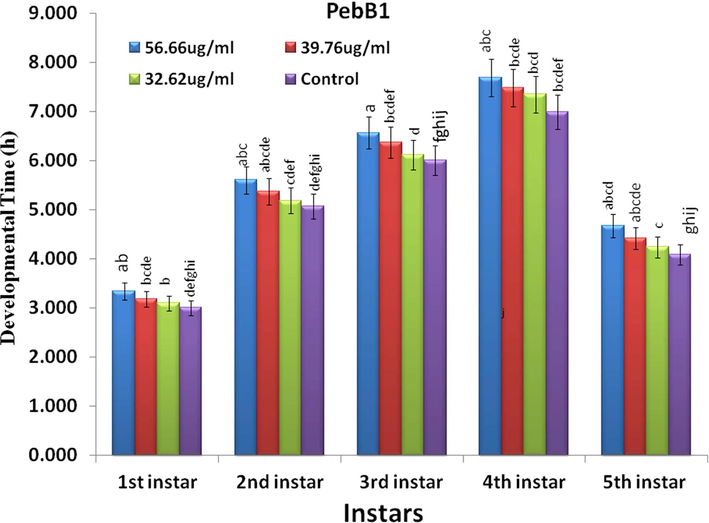
- Efficacy of PebB1 on the nymph development time of ice leaf folder (n = 10) at different concentration.
The 2nd nymphal instar needed more than five days in both the elicitors, while after treatment with Hrip 1, it took 5.6, 5.4, and 5.3 days (F2 = 1.24, p < 0.02), but with PebB1 it was 5.4, 5.3, and 5.2 days (F2 = 0.31p < 0.01). For the 3rd nymphal instar, after treatment with both the elicitors, the time changed to longer than six days: Hrip was 6.6, 6.4, and 6.2 days (F3 = 0.24, p < 0.00) but PebB1 was 6.5, 6.3, and 6.1 days (F3 = 0.58, p < 0.03). The 4th nymphal instars took 7 d to become the 5th instar without treatment with both elicitors, while, after treatment with Hrip 1, they needed 7.6, 7.4, and 7.2 d (F4 = 1.3p < 0.02) and 7.7, 7.6, and 7.3 days (F4 = 2.87, p < 0.05) for PebB1. The 5th instar took 4.1 days for both the elicitors, while after the application of Hrip 1, it took 4.8, 4.5, and 4.3 days (F5 = 1.15, p < 0.05) and in the case of PebB1, it took 4.6, 4.4, and 4.2 days (F5 = 0.6, p < 0.05). Maximum elongation time was found at the highest concentration of Hrip 1 and PeBb1, while the minimum was found at the lowest concentration (Figs. 1 & 2).
3.2 Efficacy of both protein elicitors Hrip 1 and PebB1 protein elicitors on survival rate
The bioassay revealed that the overall survival rate of rice leaf folder decreased in the treated plants as compared to the control for Hrip 1 and PebB1 (Figs. 3 and 4). Significant effects were quantified by the mean survival rate of rice leaf folder at different concentrations of Hrip 1 (F1 = 25.76, p < 0.00). The time interval (F2 = 178.00, p < 0.00) and interaction showed non-significant results (F1 = 0.43, p < 0.91) (Table 2). The survival rate increased with protein concentration (Fig. 3).
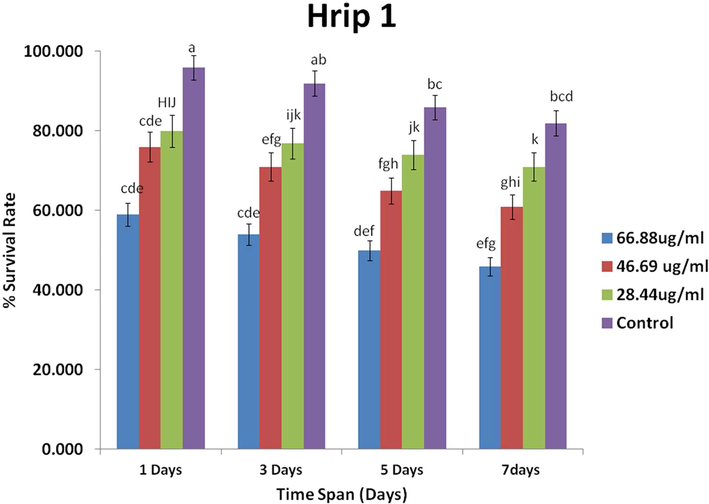
- Efficacy of Hrip 1 protein on the mean survival rate of the rice leaf folder (±SE; n = 10) at different concentration levels.
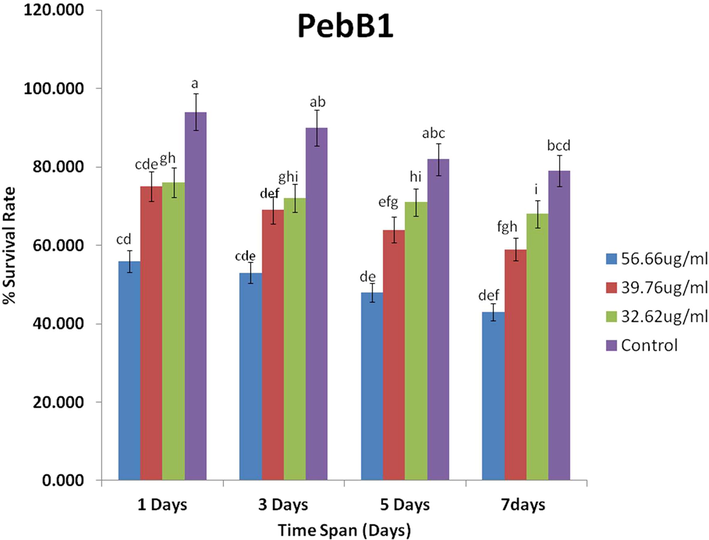
- Efficacy of PebB1 protein on the mean survival rate of the rice leaf folder (±SE; n = 10) at different concentration levels.
| S.O.V | DF | SS | MS | F-Value | P-Value |
|---|---|---|---|---|---|
| Replication | 9 | 4777.5 | 530.83 | ||
| Time | 3 | 3985.0 | 1328.33 | 25.76 | 0.00 |
| Treatment | 3 | 27665.0 | 9221.67 | 178.80 | 0.00 |
| Time*Treatment | 9 | 200.0 | 22.22 | 0.43 | 0.91 |
| Error | 135 | 6962.5 | 51.57 | ||
| Total | 159 | 43590.0 |
PebB1 showed a significant effect on the treated plants as compared to the control (Fig. 4). The survival rate was lower than that of the control rice leaf folder at different concentrations (F1 = 19.23, p < 0.00). However, the time interval (F2 = 148, p < 0.00) and interaction showed no significant result (F3 = 0.46, p < 0.90) (Table 3).
| S.O.V | DF | SS | MS | F-Value | P-Value |
|---|---|---|---|---|---|
| Replication | 9 | 4777.5 | 530.83 | ||
| Time | 3 | 3985 | 1328.33 | 25.76 | 0 |
| Treatment | 3 | 27,665 | 9221.67 | 178.8 | 0 |
| Time*Treatment | 9 | 200 | 22.22 | 0.43 | 0.91 |
| Error | 135 | 6962.5 | 51.57 | ||
| Total | 159 | 43,590 |
3.3 Efficacy of both protein Hrip 1 and PebB1 protein elicitors on fecundity
After the application of both protein elicitors, fecundity was decreased compared to the control (Figs. 5 & 6). Fecundity was quantified on a regular basis until the 5th day. After the application of Hrip 1, maximum fecundity was observed at the highest protein concentration, while the lowest was recorded at the lowest concentration, but fecundity decreased with time (Fig. 5). Analysis of variance for time (F1 = 134, p < 0.00), concentration (F2 = 425, p < 0.00), and the interaction (F3 = 11.93, p < 0.00) showed a significant result (Table 4).
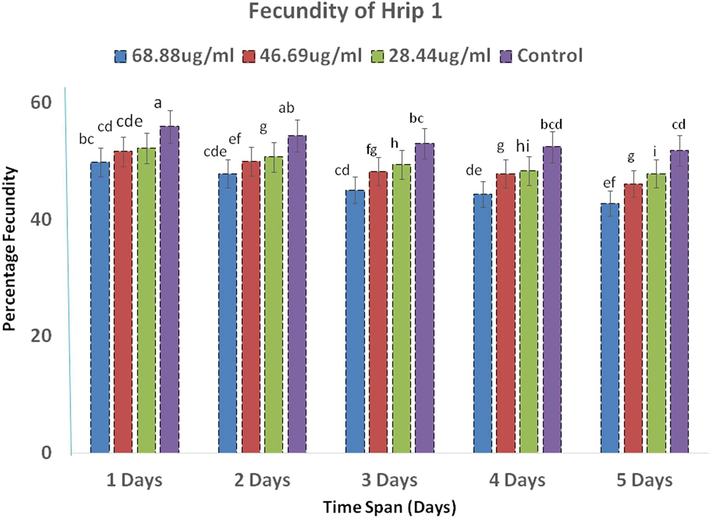
- The average of the fecundity after the application of Hrip 1.
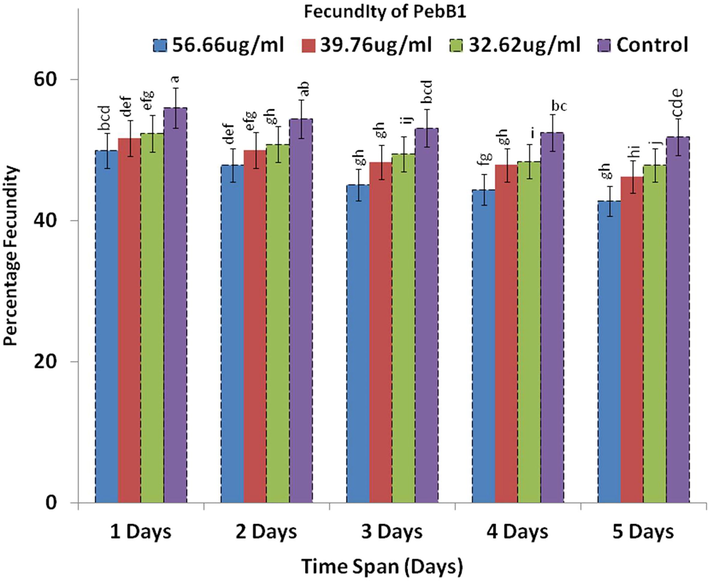
- The average of the fecundity after the application of PebB1.
| S.O.V | DF | SS | MS | F -Value | P -Value |
|---|---|---|---|---|---|
| Replication | 9 | 27.35 | 3.038 | ||
| Concentration | 3 | 1462.93 | 487.645 | 235.19 | 0.00 |
| Time | 4 | 696.67 | 174.167 | 84.00 | 0.00 |
| Concentration*Time | 12 | 39.89 | 3.324 | 1.60 | 0.94 |
| Error | 171 | 354.55 | 2.073 | ||
| Total | 199 | 2581.39 |
Meanwhile, after the application of PebB1, maximum fecundity was observed at the highest protein concentration and the minimum was observed at the lowest concentration. The fecundity also decreased with time (Fig. 6). Analysis of variance showed that time (F1 = 85, p < 0.00) and concentration (F2 = 235, p < 0.00) produced a significant defence, but the interaction (F3 = 1.06, p < 0.94) was not significant (Table 5).
| S.O.V | DF | SS | MS | F-Value | P-Value |
|---|---|---|---|---|---|
| Replication | 9 | 20.84 | 2.316 | ||
| Concentration | 3 | 2110.7 | 703.565 | 425.94 | 0 |
| Time | 4 | 885.72 | 221.43 | 134.06 | 0 |
| Concentration*Time | 12 | 236.48 | 19.707 | 11.93 | 0 |
| Error | 171 | 282.45 | 1.652 | ||
| Total | 199 | 3536.19 |
3.4 Effect of Hrip 1 on the key associated genes related to JA, SA, and ET pathways
To evaluate the putative role of Hrip 1 in plant resistance, we quantified the expression of key genes that induced the defence mechanism against rice leaf folder after 24, 48, 72, and 96 h of feeding rice leaf folder (Fig. 7a). RT-qPCR showed that all the genes associated with jasmonic acid (JA) pathway were slightly upregulated. Two genes (LOC_Os12g37350.1 and LOC_Os11g39220.1) were down-regulated after 96 h of feeding, while the other genes LOC_Os06g23760.1 and LOC_Os08g39850.1 were up-regulated at each interval of time. Maximum gene expression occurred after 72 h of rice leaf folder feeding (Fig. 7a). All four genes (LOC_Os11g15040.4, LOC_Os01g56380.1, LOC_Os03g53200.1, and LOC_Os05g41210.1) associated with the SA pathway were strongly up-regulated at each interval of time. The maximum up-regulation was observed 48 and 72 h after rice leaf folder feeding (Fig. 7b). Three genes (LOC_Os01g08380.1, LOC_Os03g01130.1, and LOC_Os03g37710.1) associated with the ethylene (ET) pathway were also moderately up-regulated, except for LOC_Os01g10940.1, which was downregulated 24 and 48 h after feeding of rice leaf folder (Fig. 7c).
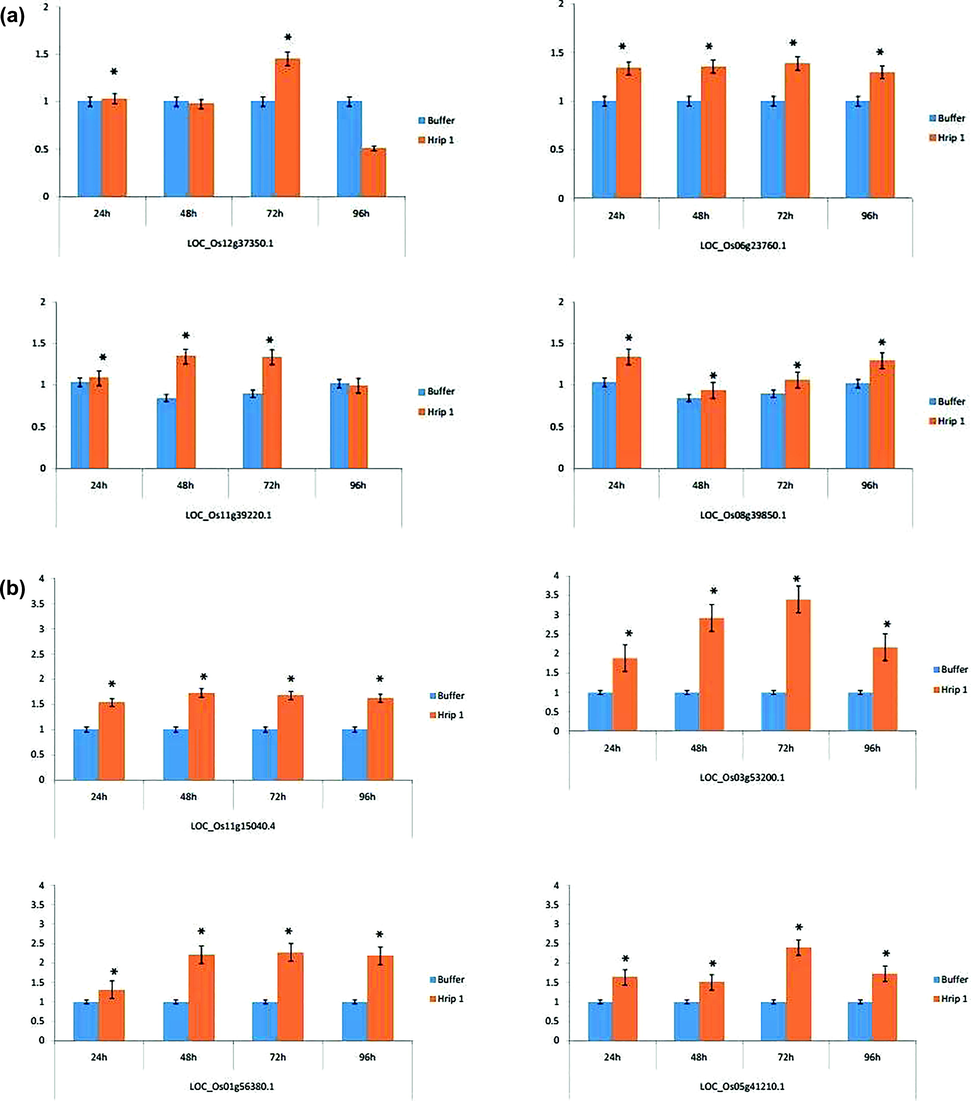
- The expression profile of (a) JA (b) SA (c) ET associated genes after the application of Hrip 1. The blue colour shows the buffer treated sample while the orange colour indicates the elicitor treated sample. An asterisk indicates significance between the treatments using Student’s t-test at p < 0.05.
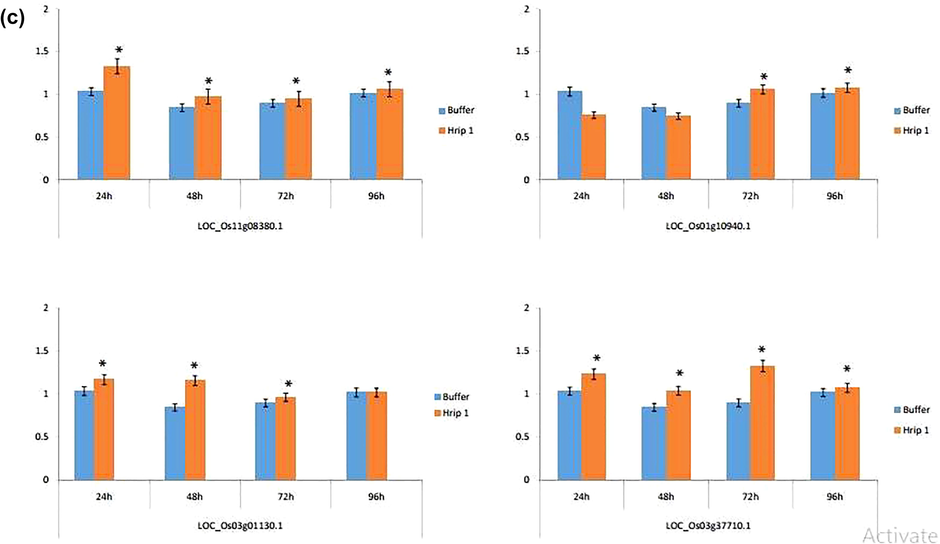
- The expression profile of (a) JA (b) SA (c) ET associated genes after the application of Hrip 1. The blue colour shows the buffer treated sample while the orange colour indicates the elicitor treated sample. An asterisk indicates significance between the treatments using Student’s t-test at p < 0.05.
3.5 Effect of PebB1 on the key associated genes related to the JA, SA and ET pathways
Indistinguishable results were found after the application of PebB1, in the key genes expressions related to the JA, SA, and ET pathways. After the exogenous application of PebB1, the expression of all key associated genes in rice that were related to the JA, SA, and ET pathways were different (Fig. 8a, b, and c). All the genes associated with the JA pathway were slightly up-regulated at each interval of time except LOC_Os08g39850.1, which was downregulated 24 and 96 h after applying PbbB1 to feed the rice leaf folder (Fig. 8a). All four genes associated with the SA pathway were strongly up-regulated (Fig. 8b). A similar trend was found in the ET pathway gene to the JA pathway. All genes associated with JA, SA, and ET pathways were moderately up-regulated at each interval of time of rice leaf folder feeding (Fig. 8c).Fig. 9.
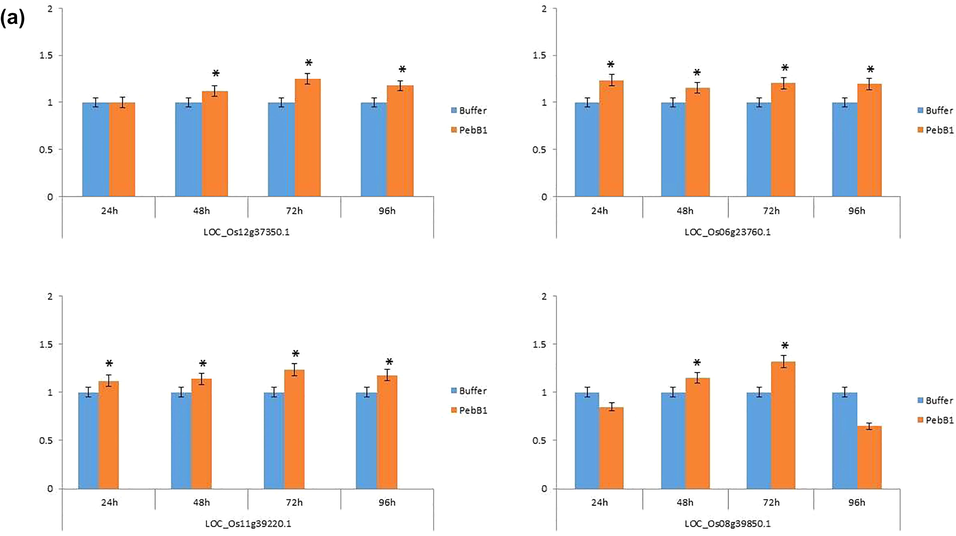
- The expression profile of (a) JA (b) SA (c) ET associated genes after the application of PebB1. The blue colour shows the buffer treated sample while the orange colour indicates the elicitor treated sample. The asterisk indicates significance between the treatments using Student’s t-test at p < 0.05.
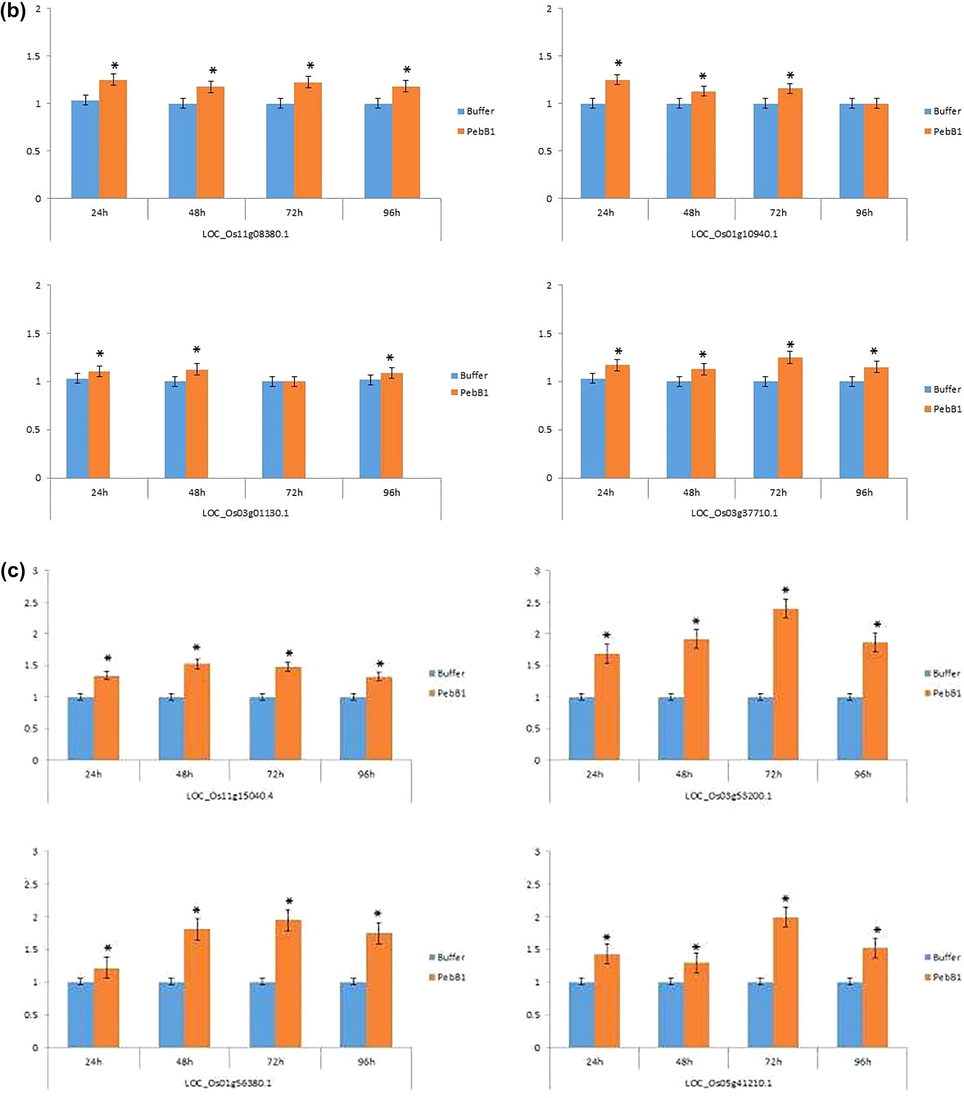
- The expression profile of (a) JA (b) SA (c) ET associated genes after the application of PebB1. The blue colour shows the buffer treated sample while the orange colour indicates the elicitor treated sample. The asterisk indicates significance between the treatments using Student’s t-test at p < 0.05.
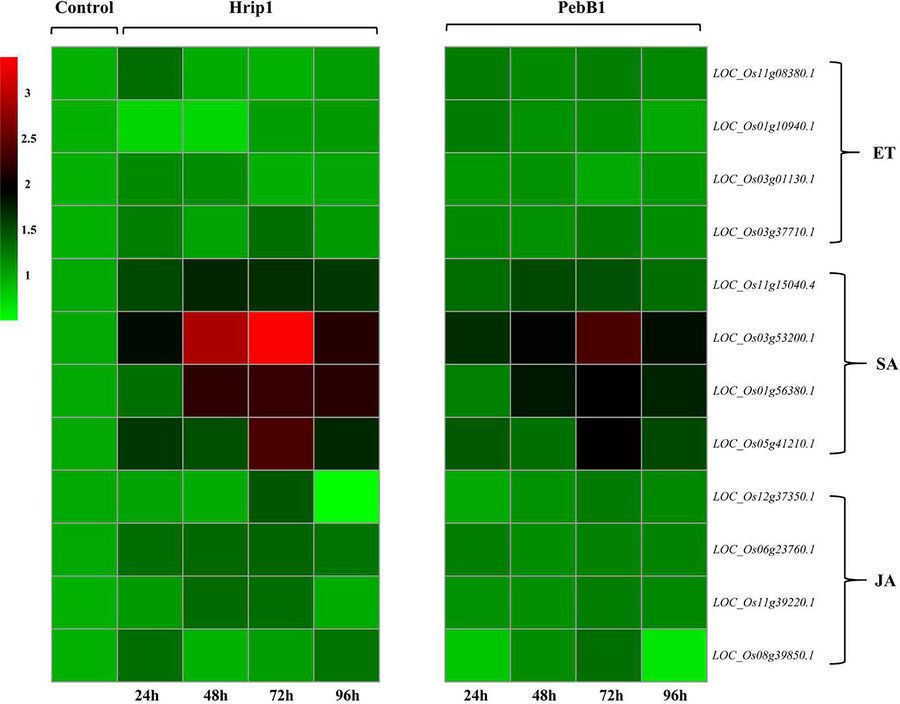
- Heat map of key associate genes involved in the JA, SA and ethylene pathway after the treatment of both protein Hrip 1 and PebB1.
4 Discussion
The protein elicitors obtained from different types of fungi have been evaluated to induce systemic resistance and defence responses in plants against different phytophagous insect pests and many pathogens (Thomma et al., 2011; Zhang et al., 2011; Basit et al., 2019). However, both protein elicitors Hrip 1 (extracted from Alternaria tenuissima) and PebB1 (from Beauveria bassiana) have not been studied, especially in controlling rice leaf folder. The present research program confirmed the positive role of Hrip 1 and PebB1 in controlling rice leaf folder.
Proteins elicitors play a vital role in the plant signalling defence system and have been evaluated as a novel biological tool for the control and management of insect pests. Numerous necrotrophic and biotrophic microorganisms, such as pathogenic fungi, are the major sources of various microbial elicitors, such as MAMPs and PAMPs (Garcia-Brugger et al., 2006). Hrip 1 and PebB1 were purified with the ability to increase the length of the lifecycle and survival rate of rice leaf folder and decreased its fecundity. Furthermore, our two protein elicitors had the capacity for the up-regulation of the key genes associated with JA, SA, and ET pathways infested by rice leaf folder in treated plants. Our results concur with those of previous studies that demonstrated the molecular characterization of the protein elicitors that enhanced the life cycle and decreased the fecundity of sucking insect pests (Basit et al., 2019). Furthermore, the mechanism by which protein elicitors produce systemic resistance in plants against pests is well described (Garcia-Brugger et al., 2006).
The population of rice leaf folder treated with Hrip 1 and PebB1 showed significant differences compared to the control, which was consistent with previous studies (Bostock et al., 2001; Cooper and Goggin, 2005). Some studies have reported that elicitors produce resistance against insect pests by quantifying the expression profile of numerous protein inhibitors (Bostock et al., 2001; Garcia-Brugger et al., 2006; Hamza et al., 2018). Protein inhibitors and polyphenol oxidases have significantly reduced the occurrence of sucking insect pests (Thaler et al., 1996; Cooper and Goggin, 2005).
SA, JA, and ET signalling pathways play a vital role in the induction of resistance against insect pests in plants. Our results are consistent with those of previous studies (Thaler et al., 2012; Basit et al., 2019 Basit et al., 2020) which evaluated different protein elicitors derived from Botrytis cinerea and Bacillus amyloliquefaciens. For example, NC6 strain extracted protein concentration dramatically enhanced the life cycle of plants, decreased the fecundity of the sucking insect pest, and was involved in the up-regulation of downstream plant signalling pathways, suggesting its tremendous impact on plant defence against herbivores (Moran and Thompson, 2001; Thaler et al., 2012). Our findings also showed the local expression profile of the JA, SA, and ET pathway responsive genes in rice leaves, although no systemic changes were observed in the expression of these genes. Hrip 1 and PebB1 produced a significant up-regulation of the expression profile of all JA, SA, and ET pathways related to responsive genes.
5 Conclusion
A series of bioassays was conducted to demonstrated the effect of both protein Hrip 1 and PebB1 protein elicitors, on the survival and developmental time of rice leaf folder was prolonged while fecundity was reduced. The expression profiles of key associated genes involved in JA, SA, and ET pathways were different. Both the protein elicitors (Hrip 1, extracted from Alternaria tenuissima and PebB1, from Beauveria bassiana) can be used as biological control agents against rice leaf folder.
Acknowledgments
This project was funded by the researchers supporting project number (RSP-2021/99) King Saud University, Riyadh, Saudi Arabia. This research is supported by the following projects: National Natural Science Foundation of China (No. 31972222, 31560489), the Program of Introducing Talents of Discipline to Universities of China (111 Program, D20023), National Key Technology Research and Development Program of the Ministry of Science and Technology of China (2014BAD23B03/03), Talent Project of Guizhou Science and Technology Cooperation Platform ([2017]5788-5, [2019]5641 and [2020]5001), and the Guizhou Science, Technology Department International Cooperation Base project ([2018]5806).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- An elicitor of plant volatiles from beet armyworm oral secretion. Science. 1997;276(5314):945-949.
- [Google Scholar]
- Bale, J.S., Masters, G.J., Hodkinson, I.D., Awmack, C., Bezemer, T.M., Brown, V.K., et al. (2002). Herbivory in global climate change research: direct effects of rising temperature on insect herbivores. Global Change Biol. 8, 1–16. https://doi.org/10.1046/j.1365-2486.2002.00451.x.
- Rice Cnaphalocrocis and Marasmia (Lepidoptera: Pyralidae) leaf folder complex in the Philippines: taxonomy, bionomics and control. Philipp. Entomol.. 1991;8:987-1074.
- [Google Scholar]
- Molecular and functional characterization of elicitor PeBC1 extracted from Botrytis cinerea involved in the induction of resistance against green peach aphid (Myzus persicae) in common beans (Phaseolus vulgaris L.) Insects. 2019;10(2):35.
- [CrossRef] [Google Scholar]
- MAMP-triggered resistance induced by elicitor protein PeBA1 derived from Bacillus amyloliquefaciens NC6 in common bean (Phaseolus vulgaris L.) against green peach aphid (Myzus persicae Sulzer) Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2020;48(2):705-715.
- [Google Scholar]
- Signal interactions in induced resistance to pathogens and insect herbivores. Eur. J. Plant Pathol.. 2001;107:103-111.
- [Google Scholar]
- Is photosynthetic transcriptional regulation in Triticum aestivum L. cv. ‘TugelaDN’ a contributing factor for tolerance to Diuraphis noxia (Homoptera: Aphididae)? Plant Cell Rep.. 2006;25:41.
- [Google Scholar]
- Pep-13, a plant defense-inducing pathogen-associated pattern from Phytophthora transglutaminases. EMBO J.. 2002;21:6681-6688.
- [Google Scholar]
- Burges, H. D. (2012). Formulation of microbial biopesticides: beneficial microorganisms, nematodes and seed treatments. Springer Science & Business Media
- Host-microbe interactions: Shaping the evolution of the plant immune response. Cell. 2006;124(4):803-814.
- [Google Scholar]
- Effects of jasmonate-induced defenses in tomato on the potato aphid, Macrosiphum euphorbiae. Entomol. Exp. Appl.. 2005;115(1):107-115.
- [Google Scholar]
- The ethylene biosynthesis-inducing xylanase: Its induction in Trichoderma viride and certain plant pathogens. Phytopathology. 1989;79:1071-1078.
- [Google Scholar]
- Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J.. 1999;18:265-276.
- [Google Scholar]
- AFLP analysis of Russian Alternaria tenuissima populations from wheat kernels and other hosts. Eur. J. Plant Path.. 2007;119(2):175-182.
- [Google Scholar]
- Early signaling events induced by elicitors of plant defenses. Mol. Plant Microbe Interact.. 2006;19(7):711-724.
- [Google Scholar]
- A simulation model for the population dynamics of rice leaf-folders (Lepidoptera: Pyralidae) and their interactions with rice. J. Appl. Ecol.. 1992;29(3):558.
- [CrossRef] [Google Scholar]
- Expression of two barley proteinase inhibitors in tomato promotes endogenous defensive response and enhances resistance to Tuta absoluta. BMC Plant Biol.. 2018;18:24.
- [Google Scholar]
- The impact of biotechnology on hyphomycetous fungal insect biocontrol agents. Biotechnol. Adv.. 1995;13(3):455-490.
- [Google Scholar]
- Age-stage, two-sex life tables of Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae) with a discussion on the problem of applying female age-specific life tables to insect populations. Insect Sci.. 2012;19(2):263-273.
- [Google Scholar]
- Can we use entomopathogenic fungi as endophytes for dual biological control of insect pests and plant pathogens? Biol. Control. 2018;116:36-45.
- [Google Scholar]
- A bibliography of rice leaf folders (Lepidoptera: Pyralidae) Int. J. Trop. Insect Sci.. 1988;9(02):129-174.
- [Google Scholar]
- Purification and expression of a protein elicitor from Alternaria tenuissima and elicitor-mediated defence responses in tobacco. Ann. Appl. Biol.. 2010;156:411-420.
- [CrossRef] [Google Scholar]
- Natural elicitors, effectors and modulators of plant responses. Nat. Prod. Rep.. 2012;29(11):1288.
- [CrossRef] [Google Scholar]
- Moran, P.J., Thompson, G.A. (2001). Molecular responses to aphid feeding in Arabidopsis in relation to plant defense pathways. Plant Physiol. 125, 1074–1085.
- Influence of rice genotypes on folding and spinning behaviour of leaf folder (Cnaphalocrocis medinalis) and its interaction with leaf damage. Rice Sci.. 2013;20(6):442-450.
- [Google Scholar]
- Effects of cultural conditions on fungal biomass, blastospore yields and toxicity of fungal secreted proteins in batch cultures of Metarhizium anisopliae (Ascomycota: Hypocreales) Pest Manag. Sci.. 2010;66(7):725-735.
- [Google Scholar]
- Action on the surface: Entomopathogenic fungi versus the insect cuticle. Insects. 2013;4(3):357-374.
- [Google Scholar]
- Insecticidal and antifeedant activities of proteins secreted by entomopathogenic fungi against Spodoptera littoralis (Lep. Noctuidae) J. Appl. Entomol.. 2006;130(8):442-452.
- [Google Scholar]
- Elicitors and priming agents initiate plant defense responses. Photosyn. Res. 2005;85:149-159.
- [Google Scholar]
- Ruiu, L. (2018). Microbial biopesticides in agroecosystems. Agronomy 8, 235.
- Isolation and characterisation of a protein elicitor from Sclerospora graminicola and elicitor-mediated induction of defence responses in cultured cells of Pennisetum glaucum. Functional Plant Biol.. 2006;33(3):267.
- [CrossRef] [Google Scholar]
- The molecular bases of plant resistance and defense responses to aphid feeding: Current status. Entomol. Exp. Appl.. 2007;122(1):1-16.
- [Google Scholar]
- Exogenous jasmonates simulate insect wounding in tomato plants (Lycopersicon esculentum) in the laboratory and field. J. Chem. Ecol.. 1996;22(10):1767-1781.
- [Google Scholar]
- Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci.. 2012;17(5):260-270.
- [Google Scholar]
- Of PAMPs and effectors: The blurred PTI-ETI dichotomy. Plant Cell. 2011;23(1):4-15.
- [Google Scholar]
- Expression of a harpin-encoding gene in rice confers durable nonspecific resistance to Magnaporthe grisea. Plant Biotech. J.. 2008;6:73-81.
- [Google Scholar]
- Micro-Pathogen Elicitor Hrip1 Protein Isolated from Alternaria tenuissima Induced Disease Resistance against Tomato Yellow Leaf Curl Virus (TYLCV) in Tomato (Solanum lycopersicum) J. Appl. Micob. Res.. 2019;2:8-16.
- [Google Scholar]
- Veit, S., Wörle, J.M., Nuürnberger,T., Koch, W., Seitz ,H.U. (2001) A novel protein elicitor (PaNie) from Pythium aphanidermatum induces dual defense responses in carrot, Arabidopsis. Plant Physiol. 127, 832–841.
- Wraight, S.P., Carruthers, R.I. (1999). Production, delivery, and use of mycoinsecticides for control of insect pests on field crops. In Biopesticides: Use and Delivery; Springer: Berlin/Heidelberg, Germany pp. 233–269.
- Progresses in management technology of rice leaf folders in China. J. Plant Prot.. 2015;42:691-701.
- [Google Scholar]
- High levels of stable resistance in transgenic rice with a cry1Ab gene from Bacillus thuringiensis Berliner to rice leaf folder, Cnaphalocrocis medinalis (Gueneé) under field conditions. Crop Prot.. 2003;22(1):171-178.
- [Google Scholar]
- A draft sequence of the rice genome (Oryza sativa L. ssp indica) Science. 2002;296(5565):79-92.
- [CrossRef] [Google Scholar]
- Elicitor signal transduction leads to the production of plant secondary metabolites. Biotech. Adv.. 2005;23:283-333.
- [Google Scholar]
- PeaT1-induced systemic acquired resistance in tobacco follows the salicylic acid-dependent pathway. Mol. Biol. Rep.. 2011;38:2549-2556.
- [Google Scholar]
- Review on safety of the entomopathogenic fungi Beauveria bassiana and Beauveria brongniartii. Biocontrol. Sci. Technol.. 2007;17(6):553-596.
- [Google Scholar]







