Translate this page into:
Effect of silver nanoparticles alone and in combination with fluconazole on Candida albicans
⁎Corresponding author at: Department of Botany & Microbiology, College of Science (Girls Campus), King Saud University, Riyadh 11421, Saudi Arabia. kperveen@ksu.edu.sa (Kahkashan Perveen)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Silver nanoparticles (SNP) alone and in combination (Combi) with the antibiotic fluconazole (Flu) were explored for their effective role in controlling Candida albicans. The C. albicans isolates used were identified by amplifying the ITS region of rDNA with primers ITS 5 and ITS 4. The MIC of the SNP and Flu alone and in conjunction with each other were assayed by the micro dilution plate method and checkerboard assay respectively. The synergistic effects of SNP and Flu were established by calculating the fractional inhibitory concentration (FIC) index. The effects of these treatments (½ MIC) on budding and on germ tube formation were determined. The identity of all five isolates as C. albicans was confirmed by molecular analysis of ITS rDNA regions and BLAST search. The MIC of SNP alone against C. albicans isolates ranged from 24 to 12 µg/ml and Flu alone was found to be 20 µg/ml. The FIC index of the Combi treatment ranged from 0.125 to 0.5 against C. albicans isolates. The average percent reduction in budding of 5 isolates by SNP, Flu, and Combi was 78.2%, 72.4%, and 92.6% respectively. A notable reduction in germ tube development in C. albicans isolates treated with SNP, Flu, and Combi (95%, 75.6%, and 99.2%. respectively) was recorded. The findings clearly prove that the SNP and Flu have synergistic effects on C. albicans. Further investigation can be undertaken to develop a strategy for utilizing silver nanoparticles with antibiotics to treat Candida infection.
Keywords
Silver nanoparticles
Candida albicans
Fractional inhibitory concentration index
Fluconazole
- CFU
-
Colony forming Unit
- Combi
-
Silver nanoparticles + Fluconazole
- DIW
-
deionised water
- FIC
-
Fractional inhibitory concentration
- Flu
-
Fluconazole
- ITS
-
Internal transcribed spacer
- MIC
-
minimum inhibitory concentration
- PVP
-
Polyvinylpyrrolidone
- SDA
-
Sabouraud Dextrose Agar
- SNP
-
Silver nanoparticles
- YPD
-
Yeast Extract Peptone Dextrose
Abbreviations
1 Introduction
Nanoparticles are garnering a lot of attention as potential antifungal complements to antibiotics because they may bridge the gaps where antifungals typically fall short. Using nanoparticles with antimicrobial capabilities as the foundation for new formulations is seen to be a fresh and creative approach. Moreover, metal nanoparticles could serve as an excellent carrier to support and supplement conventional antifungals (Sharma et al., 2016). Silver nanoparticles have been shown to have adequate antifungal properties (Ishida et al., 2014; Lee and Jun, 2019). Recent research has shown that adding nanoparticles to antifungal medications like fluconazole can increase their effectiveness against certain diseases (Sharma et al., 2016; Sun et al., 2016).
Candida spp. is potential human infection-causing fungi among commensal pathogens (Horn et al., 2009). Numerous virulence characteristics and adaptation traits of C. albicans promote its capacity to infect a variety of host habitats. There are several characteristics such as the morphological change between the yeast and hyphal forms, the secretion of adhesins and invasins on the cell surface, thigmotropism, biofilms formation, and the release of hydrolytic enzymes which are regarded as virulence factors of Candida (Orsi et al., 2014).
The prevalence of candidiasis caused by Candida species has dramatically increased during the past few decades. C. albicans, which makes up about 90% of all instances of severe fungal infection, is the primary pathogen linked to such infections among the many Candida species (Douglas, 2003). It is clear that C. albicans is the most prevalent species responsible for such infections based on several investigations for candidemia carried out at different hospitals in Riyadh (Al-Jasser and Elkhizzi, 2004; Alshaikh and Perveen, 2021).
Unjudicial use of antimicrobials has resulted in the surge of drug resistant strains of pathogens (Ksiezopolska and Gabaldón, 2018). This is a global problem that need a solution to control the microbial infection caused due to drug resistant pathogens. Discovering novel antibiotics is crucial, but there is also a need to figure out how to make the ones that are presently on the shelf more effective. In this study, silver nanoparticles alone and in combination with antibiotic fluconazole were explored for their effective role in controlling C. albicans. The C. albicans isolates were identified by ITS primers 5 and 4.
2 Material and methods
2.1 Silver nanoparticles
The nanoparticles were purchased from US Research Nanomaterials Inc, Houston, USA. Silver (Ag) Nanoparticles (Ag, 99.99%, <20 nm, w/∼0.2% PVP) Polyvinylpyrrolidone coated for water dispersion (Fig. 1).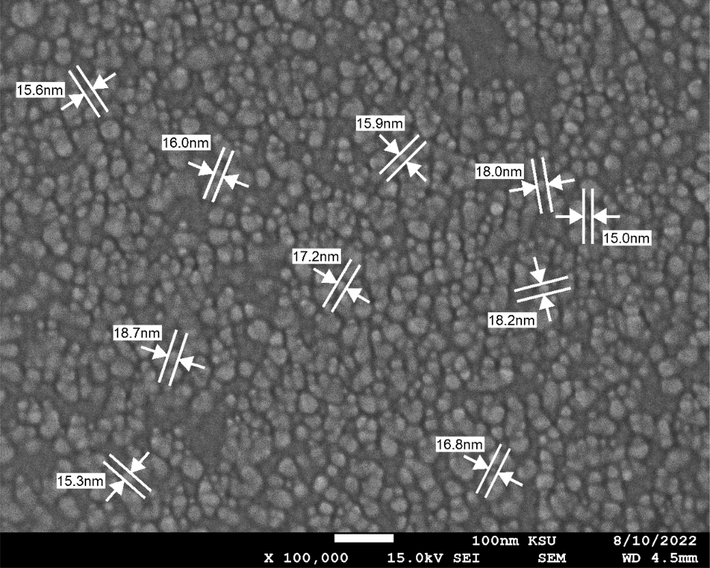
SEM image of Silver nanoparticles (SNP) from US Research Nanomaterials Inc, Houston, USA. The particles were coated (w/∼0.2% PVP), size <20 nm.
2.2 Candida albicans isolates
Five isolates of Candida albicans were obtained from the Ministry of National Guard Health Affairs, Riyadh, Saudi Arabia. For the purification of fugal strains, sub-culturing was performed from single colony on Sabouraud Dextrose Agar (SDA) medium. The C. albicans isolates were initially identified on the basis of cultural, microscopic characteristics, and germ tube formation and later by molecular analysis.
2.3 Molecular identification of Candida albicans
DNA from Candida albicans samples were extracted using standard procedure (Scherer and Stevens, 1987). C. albicans were cultured at 37 °C for one or two days on SDA agar plates. A single colony from plate was picked and subculture in Yeast Extract Peptone Dextrose (YPD) broth. The culture was incubated at 37 °C in shaking incubator. The fungal cells obtained by centrifugation and followed by suspending them in 1 ml of 1000 mM sorbitol-50 mM phosphate buffer (pH 7.5) containing yeast lytic enzyme and 2% of β-mercaptoethanol. After 1 hr of incubation C, the cell suspension was centrifuged and pellet was reconstituted in 0.5 ml EDTA (50 mM, pH 8.0) containing 0.2% SDS. The reaction mixture was heated for 30 min at 70 °C and then potassium acetate (5 M) was added. Finally, the suspension was again incubated at 0 °C for 30 min. The reaction mixture was centrifuged to settle down the debris and supernatant was given RNase treatment. For DNA isolation, equal volume of chloroform and isoamyl alcohol was used and then DNA was precipitated in chilled ethanol. The DNA was dissolved in TE buffer for stored for further studies.
The ITS regions between the small nuclear 18S rDNA and large nuclear 28S rDNA, including 5.8S rDNA, were amplified. ITS5 and ITS4 primers were used in the PCR reactions to amplify the ITS region of the rRNA operon (Table 1).
Primer
Sequence 5′3′
ITS5 (forward)
GGAAGTAAAAGTCGTAACAAGG
ITS4 (reverse)
TCCTCCGCTTATTGATATG
For all PCR mixture contained 10 µL of Red taq ready mix, 0.5 µL of each primer pair, 8 µL of analytical grade sterile water (Sigma–Aldrich) and 5 µL of DNA and total volume was 24 µL. The thermocycling program used was an initial denaturation (94 °C for 5 min), 30 cycles of denaturation (94 °C for 1 min), annealing (65 °C for 1 min) and elongation (72 °C for 1 min), and then a stabilization (72 °C for 5 min) (Alwadai et al., 2022). The molecular analysis was carried out by Macrogen, Korea.
2.4 Silver nanoparticles and fluconazole solution preparation
SNP solution was prepared by dispersing SNP in deionised water (DIW) and sonicating it for 30 min. a stock solution of 30 µg/ml was prepared.
Standard laboratory powder of fluconazole (Flu) was used in this study. The Flu stock solution of 128 µg/ml was prepared in DIW.
3 Determination of minimum inhibitory concentration (MIC) of SNP, Flu and Combi
The anticandidal activity of SNP and Flu was determined by micro broth dilution assay in 96 well plates (Jafri et al., 2020). The solution of SNP (100 µL) and Flu (100 µL) were double fold diluted into the wells. 100 µL C. albicans isolate (∼1.5 × 108 CFU/ml) was taken as inoculum. Control group was not given any treatment and DIW was used as the control. The cultures were incubated for 48 h at 37 °C in incubator. The microbial growth was monitored calorimetrically at 540 nm to determine their antifungal potential. Further, the lowest concentration of SNP and Flu that inhibited the fungal growth (no turbidity) was considered as MIC. For the determination of MIC for Combi, two-dimensional checkerboard assay was carried out in 96 well plates. Fractional inhibitory concentration (FIC) index was calculated to find out the synergistic effects of the combination (Hall et al., 1983) The following equation was used to calculate synergistic effect. where
“FIC” is Fractional inhibitory concentration, “A” refers to the MIC value of SNP in combination with Flu, “MIC A” refers to the MIC of SNP alone, “B” refers to the MIC value of Flu in combination with SNP, “MIC B ” refers to the MIC of Flu alone.
FIC index value, <0.5 indicates synergy, 0.5–4 indifference, and >4 antagonism.
3.1 Effect of SNP, Flu and Combi on budding
The effect of SNP, Flu and Combi on the budding of C. albicans isolates was tested at their respective half inhibitory concentration (½ MIC) (Alshaikh and Perveen, 2017). Briefly, C. albicans isolates were cultured with and without treatments for 24 h at 37 °C. The fungal cells were observed under light microscope (40X) and one hundred cells were counted in each smear for the calculation of percentage of budding cells.
3.2 Effect of SNP, Flu and Combi on germ tubes formation
The effect of SNP, Flu and Combi on germ tubes formation of 5 strains of C. albicans was also tested at their respective ½ MICs. Briefly, the isolates were cultures at ½ MIC of SNP, Flu and their combination for 3 h at 37 °C. Following incubation, 100 cells from each sample were counted using light microscope. The germ tubes were considered positive when germ tubes were seen arising from the cells without a constriction at the place of their origin from the cells (Alshaikh and Perveen, 2021).
4 Results
The obtained isolates of Candida albicans were cultured on SDA. The growth on SDA showed colonies with white to cream in colour, glistening, round, curved, soft and smooth to wrinkled yeast-like in appearance. The molecular identification of all 5C. albicans isolates by PCR amplification and sequencing of ITS regions with primers ITS5 and ITS4 was carried out. The BLAST search identified all isolates as C. albicans with 100–99% similarity with the C. albicans sequences available in NCBI genbank (Table 2).
C. albicans isolates
Percent similarity (%)
Identity
Acession no. in genbank
Ca1
100
C. albicans
MN318604.1
Ca2
99
C. albicans
ON845627.1
Ca3
99
C. albicans
MH718839.1
Ca4
100
C. albicans
OW988780.1
Ca5
100
C. albicans
MN263159.1
The MIC values of SNP, Flu and Combi was determined by micro broth dilution assay in 96 well plates. The MIC values of SNP against C. albicans isolates ranged 24–12 µg/ml and Flu was found to be 20 µg/ml. The FIC index analysis was used to determine the synergistic effects of the Combi. The FIC index range 0.125–0.187 for the combinations of SNP and Flu (Table 3). MIC values of SNP and Flu in the Combi were 0.75–3 µg/ml and 1.25–5 µg/ml. SNP- Silver nanoparticles, Flu-Fluconazole, Combi – combination of SNP + Flu, FICi - Fractional inhibitory concentration index.
C. albicans isolates
SNP (µg/ml)
Flu (µg/ml)
Combi (µg/ml)
FICi of Combi
Ca1
24
20
1.5/2.5
0.187
Ca2
24
20
1.5/2.5
0.187
Ca3
24
20
0.75/1.25
0.125
Ca4
12
20
1.5/2.5
0.187
Ca5
12
20
0.75/1.25
0.125
To find out the effect of SNP and Flu and combi on budding the sub inhibitory concentration (½ MIC) of the treatments were used. Results show that SNP and Flu together inhibited the bud formation considerably better than either of them did alone. The average percent reduction in budding of 5 isolates by SNP, Flu and Combi was 78.2%, 72.4% and 92.6% respectively (Fig. 2). The effects of SNP and Flu and Combi on budding were clearly observed under the microscope. The number of cells with buds was less in the Combi treatment than in the other two treatments. In the case of control, not only was budding high, but along with that, the cells were also observed to be healthy and well formed (Fig. 3).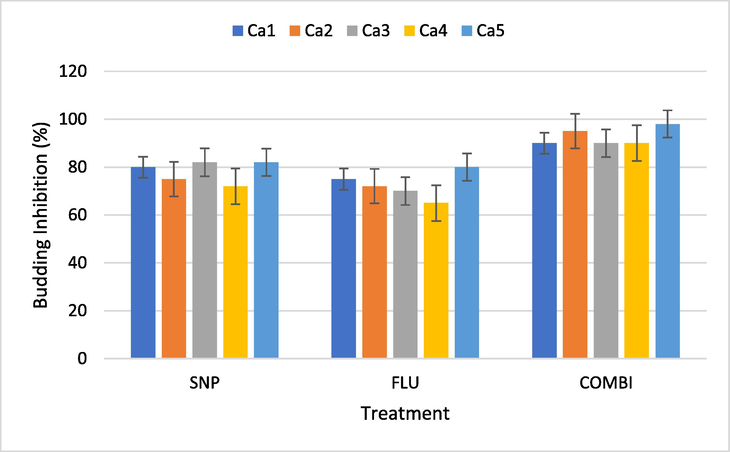
Percent inhibition of budding in C. albicans isolates by SNP, Flu and Combi (½ MIC). SNP - Silver Nanoparticles, FLU- Fluconazole, Combi - (SNP + Flu), Ca – C. albicans Bars represent data mean ± Sd (n = 3).
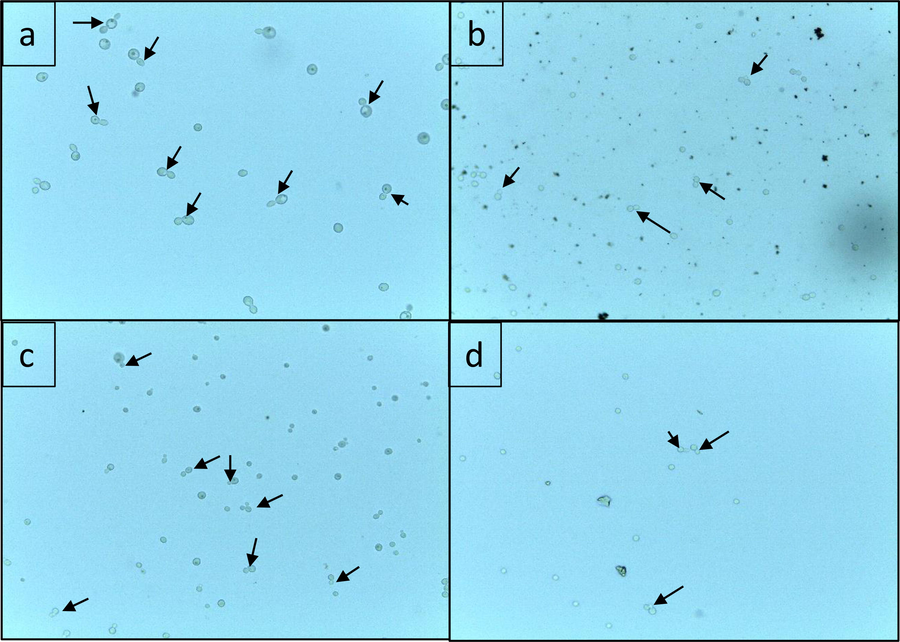
Effect of SNP and Flu and Combi on budding in C. albicans isolates a) untreated C. albicans cells b) C. albicans cells treated with SNP alone (½ MIC) c) C. albicans cells treated with Flu alone (½ MIC) d) C. albicans cells treated with Combi (½ MIC). The arrows indicate the budding cells observed under light microscope (40X).
The effect of SNP, Flu and Combi in inhibiting the germ tube formation in C. albicans was also evaluated (Figs. 4 and 5). All treatments were able to reduce formation of germ tube in all tested C. albicans isolates. An average reduction in germ tube development in isolates treated with only SNP and Flu was found to be 95% and 75.6%, respectively. The germ tube formation in isolates treated with Combi were reduced 99.2%. The results clearly show that germ tube formation was strongly inhibited by Combi at ½ MIC. Overall, it was observed that the Combi treatment had fewer and smaller cells than the control. Moreover, the germ tubes were seldom seen (Fig. 5).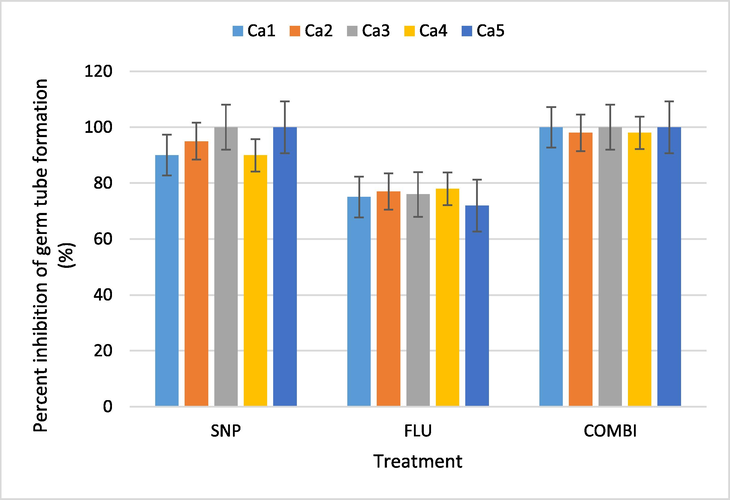
Percent inhibition of germ tube formation of C. albicans isolates by SNP, Flu and Combi (½ MIC). SNP - Silver Nanoparticles, FLU- Fluconazole, Combi - (SNP + Flu), Ca – C. albicans; Bars represent data mean ± Sd (n = 3).
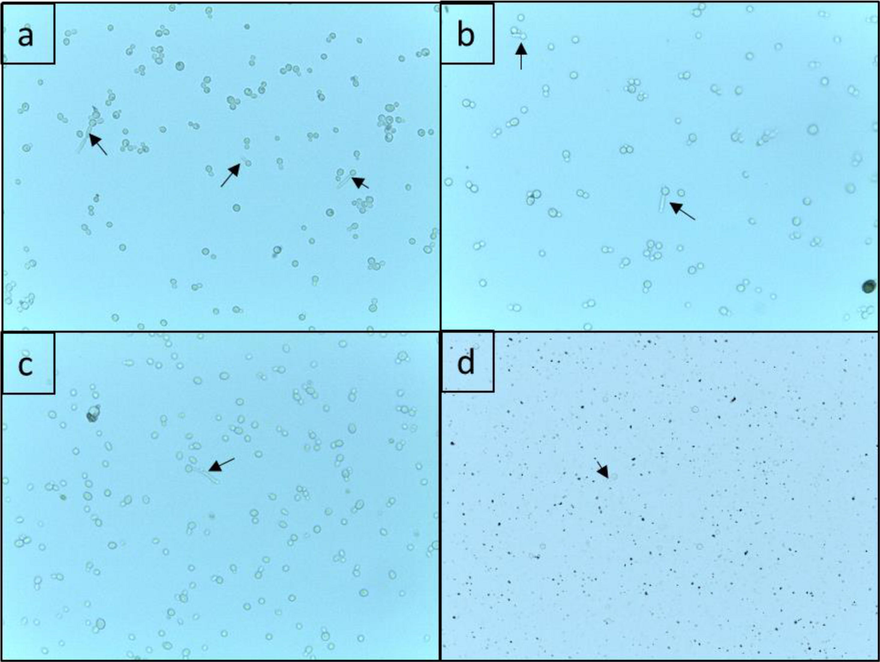
Effect of SNP and Flu and Combi on germ tube formation in C. albicans isolates a) untreated C. albicans cells b) C. albicans cells treated with SNP alone (½ MIC) c) C. albicans cells treated with Flu alone (½ MIC) d) C. albicans cells treated with Combi (½ MIC). The arrows indicate germ tube formation observed under light microscope (40X).
5 Discussion
The application of nanoparticles has proven to be a novel strategy for controlling microorganisms. Nanoparticles of various sizes and shapes have shown tremendous promise in inhibiting pathogens. The morphology, size and surface area-to-mass ratio of nanoparticles provide them with exceptional properties. The antimicrobial properties of nanoparticles have been studied by several workers (Khan and Al-Khedhairy, 2017; Lallo da Silva et al., 2019; Perveen et al., 2021). Due to silver’s well-known antimicrobial properties, SNP have been utilized by many for antimicrobial applications (Al-Otibi et al., 2021; F. Al-Shlawi et al., 2022; Khan and Al-Khedhairy, 2017; Lee and Jun, 2019). In present study the anticandidal activity of silver nanoparticles alone and in synergy with fluconazole were evaluated. C. albicans is found normally in the human microbiota. However, it is an opportunist pathogen that may cause infections in exceptional conditions, such as its infection is prevalent in immunocompromised patients. Candida spp. Are responsible for dermal, superficial mucosal, and bloodstream infections (Gulati et al., 2018.
In the present study-five C. albicans isolates were selected for the investigation. The identity of the isolates was confirmed by molecular analysis of the ITS regions of the rDNA by ITS primers (ITS5 and ITS5). The genomic DNA sequence contains 18S rRNA gene, ITS1, 5.8S rRNA gene, ITS2, 28S rRNA gene. All five isolates confirmed as C. albicans. The use of morphological as well as molecular tools is necessary for the correct identification of microorganisms. Candida species have been successfully identified by amplifying ITS regions, which is considered a fast and accurate analysis. (Asadzadeh et al., 2019).
The present findings clearly indicate that SNP alone and Combi treatment were effective in inhibiting the growth of C. albicans isolates and the Combi treatment showed the synergistic effects. Earlier, the MIC value of 12.5 µg/ml of biogenic SNP (11–15 nm) against C. albicans was reported (Ahamad et al., 2022). Another study found the MIC value as 1080 – 580 µg/ml of green synthesized SNP against C. albicans (Mare et al., 2021. The variation in the MIC value of SNP could be due to a variety of factors, including the size and shape of the nanoparticles or the methodologies used for the determination, or could be due to the variation in the C. albicans phenotypic or genotypic makeup. MIC values of the individual components in combination (SNP + Flu) treatment were found to be much lower than those in solo treatment. “Synergism is defined as a positive interaction created when two agents are combined, and together they cause an inhibitory effect that is greater than the sum of their individual effects” (Roell et al., 2017). A study has shown that using nanoparticles alone or in combination with antifungal agents, may become more effective anticandidal agent against Candida strains. An earlier study found that fluconazole or voriconazole combined with PVP-coated silver nanoparticles was effective in treating drug-resistant C. albicans (Li et al., 2018). Scientists have reported that nanoparticles disrupt the functioning of fungal cells by affecting the morphology of cell wall as well as cell membrane (Artunduaga Bonilla et al., 2017; Khan and Al-Khedhairy, 2017; Lee and Jun, 2019).
SNP alone and in combination with Flu were also found effective in controlling the budding and germ tube formation of C. albicans. The dimorphic shift from the budding form to the filamentous form aids the fungus in penetrating epithelia (Gow, 1997). SNP improves antibiotics' ability to adhere to cell membranes when administered together with other antibiotics and block the budding process. SNP has showed the properties of a biofilm inhibitor (Artunduaga Bonilla et al., 2017; Guisbiers et al., 2017). The nanoparticles’ dimension strongly influences their antimicrobial potential, smaller the size the greater the effect. However, extremely smaller size may be hazardous to mammalian cells, moreover higher concentration can provide a problem to human and environment (Alsuwayyid et al., 2022; Khan and Al-Khedhairy, 2017; Lallo da Silva et al., 2019). It has been noted that the toxicity of SNP against mammalian cells was lowered when combined together with antifungal agent (Parthiban et al., 2019). The precise route of action of SNP is unknown despite extensive research on the antifungal effect of nanoparticles. In this work, the effect of SNP on budding and germ tubes was examined. It illustrates how significant these two elements are in determining the effectiveness of SNP. To ascertain the efficacy and mechanism of action of SNP, additional experiments can be carried out, such as how SNP affects biofilm development and gene expression.
6 Conclusion
The management of infectious diseases has become a difficult task as a result of the global emergence and spread of antimicrobial resistance in pathogens. Testing nanoparticles synergistic effects with the antifungal agents against Candida can be a strategy that could be utilized to overcome the above-mentioned hurdle. SNP alone and together with fluconazole were both successful in effectively suppressing the growth of fungal isolates. These treatments were also successful in preventing budding and germ tube development. Thus, current findings can be explored further to use silver nanoparticles together with antibiotics to develop affective strategy for managing C. albicans infection.
Acknowledgment
The authors extend their sincere appreciation to the Researchers Supporting Project Number (RSP-2021/358), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Antibiofilm activities of biogenic silver nanoparticles against Candida albicans. Front. Microbiol.. 2022;12
- [CrossRef] [Google Scholar]
- Distribution of Candida species among bloodstream isolates. Saudi Med. J.. 2004;25:566-569.
- [Google Scholar]
- Biosynthesis of silver nanoparticles using Malva parviflora and their antifungal activity. Saudi J. Biol. Sci.. 2021;28:2229-2235.
- [CrossRef] [Google Scholar]
- Anti-candidal Activity and Chemical Composition of Essential Oil of Clove (Syzygium aromaticum) J. Essential Oil Bear. Plants. 2017;20:951-958.
- [CrossRef] [Google Scholar]
- Susceptibility of fluconazole-resistant candida albicans to thyme essential oil. Microorganisms. 2021;9(12):2454.
- [Google Scholar]
- Barleria acanthoides Vahl. Mediated Green Synthesis of Silver Nanoparticles and their Antibacterial Activity. Asian J. Adv. Res. Rep. 2022:41-50.
- [CrossRef] [Google Scholar]
- Effect of zinc oxide nanoparticles on Triticum aestivum L. and bioaccumulation assessment using ICP-MS and SEM analysis. J. King Saud. Univ. Sci.. 2022;34(4):101944.
- [Google Scholar]
- The isolation and characterization of antagonist Trichoderma spp. from the Soil of Abha, Saudi Arabia. Molecules. 2022;27(8):2525.
- [Google Scholar]
- Rapid and accurate identification of candida albicans and candida dubliniensis by real-time PCR and melting curve analysis. Med. Principles Practice. 2019;27
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles using maltose and cysteine and their effect on cell wall envelope shapes and microbial growth of Candida spp. J. Nanosci. Nanotechnol.. 2017;17(3):1729-1739.
- [Google Scholar]
- Candida biofilms and their role in infection. Trends Microbiol.. 2003;11:30-36.
- [CrossRef] [Google Scholar]
- Inhibition of Candida albicans biofilm by pure selenium nanoparticles synthesized by pulsed laser ablation in liquids. Nanomedicine. 2017;13(3):1095-1103.
- [Google Scholar]
- In vitro culturing and screening of Candida albicans biofilms. Curr. Protoc. Microbiol.. 2018;50
- [CrossRef] [Google Scholar]
- The fractional inhibitory concentration (FIC) index as a measure of synergy. J. Antimicrob. Chemother.. 1983;11(5):427-433.
- [Google Scholar]
- Epidemiology and outcomes of Candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin. Infect. Dis.. 2009;48:1695-1703.
- [CrossRef] [Google Scholar]
- Silver nanoparticle production by the fungus Fusarium oxysporum: Nanoparticle characterisation and analysis of antifungal activity against pathogenic yeasts. Mem. Inst. Oswaldo Cruz. 2014;109(2):220-228.
- [Google Scholar]
- Synergistic interaction of eugenol and antimicrobial drugs in eradication of single and mixed biofilms of Candida albicans and Streptococcus mutans. AMB Express. 2020;10:185.
- [CrossRef] [Google Scholar]
- Metals and metal oxides: important nanomaterials with antimicrobial activity. In: Antimicrobial Nanoarchitectonics: From Synthesis to Applications. Elsevier; 2017. p. :195-222.
- [Google Scholar]
- Evolutionary emergence of drug resistance in candida opportunistic pathogens. Genes (Basel). 2018;9(9):461.
- [Google Scholar]
- Increased antibacterial activity of ZnO nanoparticles: Influence of size and surface modification. Colloids Surf B Biointerfaces. 2019;177:440-447.
- [Google Scholar]
- Silver nanoparticles: Synthesis and application for nanomedicine. Int. J. Mol. Sci.. 2019;20(4):865.
- [Google Scholar]
- Synergistic effect between silver nanoparticles and antifungal agents on Candida albicans revealed by dynamic surface-enhanced Raman spectroscopy. Nanotoxicology. 2018;12(10):1230-1240.
- [Google Scholar]
- In vitro antifungal activity of silver nanoparticles biosynthesized with beech bark extract. Plants. 2021;10(10):2153.
- [Google Scholar]
- Impact of Candida albicans hyphal wall protein 1 (HWP1) genotype on biofilm production and fungal susceptibility to microglial cells. Microb. Pathog.. 2014;69-70:20-27.
- [Google Scholar]
- Green synthesis of silver-nanoparticles from Annona reticulata leaves aqueous extract and its mosquito larvicidal and anti-microbial activity on human pathogens. Biotechnol. Rep,. 2019;21:e00297.
- [Google Scholar]
- Microwave-assisted rapid green synthesis of gold nanoparticles using seed extract of trachyspermum ammi: Ros mediated biofilm inhibition and anticancer activity. Biomolecules. 2021;11:1-16.
- [CrossRef] [Google Scholar]
- An introduction to terminology and methodology of chemical synergy-perspectives from across disciplines. Front. Pharmacol. 2017
- [CrossRef] [Google Scholar]
- Application of DNA typing methods to epidemiology and taxonomy of Candida species. J. Clin. Microbiol.. 1987;25(4):675-679.
- [Google Scholar]
- Synergistic activity of doped zinc oxide nanoparticles with antibiotics: Ciprofloxacin, ampicillin, fluconazole and amphotericin B against pathogenic microorganisms. An. Acad. Bras Cienc. 2016;88(3 suppl):1689-1698.
- [Google Scholar]
- Synergy between polyvinylpyrrolidone-coated silver nanoparticles and azole antifungal against drug-resistant Candida albicans. J. Nanosci. Nanotechnol.. 2016;16
- [CrossRef] [Google Scholar]







