Translate this page into:
Effect of phenological stages on essential oil composition of Cytisus triflorus L’Her
⁎Corresponding author at: Laboratoire de Chimie Agro-industrielle (LCA), Université de Toulouse, INRA, INPT, Toulouse, France. othmane.merah@ensiacet.fr (Othmane Merah)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Essential oil is widely used in perfumery, cosmetic, pharmaceutic and food industries. The economics of essential oil production depend on both parameters; the cultivation of aromatic herbs and the extraction of oils from these herbs. This study investigated for the first time the variation in essential oil and composition of Cytisus triflorus L'Her plant at different growth times (vegetative, flowering and fruiting stages). Essential oil was extracted by hydro-distillation. The flowering stage show the highest essential oil yield (0.61 ± 0.02 mL/100 g) compared to those of vegetative (0.41 ± 0.01 mL/100 g) and fruiting (0.17 ± 0.01 mL/100 g) stages. For all growth stages, Thirty-eight compounds were identified, representing 93.21% – 95.47% of the oil, where the main components were β-linalool (28.28 to 38.04%), Geraniol (19.82 to 27.73%), Retinal (4.33 to 9.26%), p-Mentha-1,4-dien-8-ol (2.09 to 4.08%), γ-Terpinene (1.27 to 4.86%) and Eugenol (2.07 to 2.28%). Monoterpene hydrocarbons were detected at higher level at flowering compared to vegetative and fruiting stages. In contrast, aldehydes were noted at higher percentage at vegetative stage. This study highlighted difference in content and composition of essential oil in Cytisus triflorus L'Her during growth stages. Flowering was the interesting stage for harvesting and with more specific composition in essential oil.
Keywords
Cytisus triflorus L’Her
Phenological stage
Essential oil
Chemical composition
1 Introduction
The Algerian flora is characterized by its floral diversity: Mediterranean, Saharan and paleotropical. In Algeria, there are about 53 genes and 339 species. The Fabaceae family is the most important family among dicotyledonous which provided the greatest number of species useful to man, be they food, industrial or medicinal.
The genus Cytisus is a Fabaceae, which comprises about 60 species, particularly disseminated in the Mediterranean basin, of which eight species grow in northern Algeria (Quezel and Santa, 1963). The traditional uses attributed to these genus are diversified and related to their bioactive properties including antioxidant, antifungal, cardiotonic, cathartic, emetic, vasoconstrictive, depurative, diuretic, hepatoprotective, anti-hemorrhagic, lithotriptic, hypnotic and anxiolytic activities (Pinela et al., 2011; González et al., 2013; Sundararajan and Koduru, 2014; Larit et al., 2018). The genus Cytisus as customary in plants and due to their inability to move, they have developed a metabolic diversity in order to defend themselves against the attacks of herbivores and microorganisms (Verma et al., 2010). These brooms contain more than 70 phyto-constituents belonging to highly diversified groups fitted with pharmacological and toxicological properties, including alkaloids, carotenoids, vitamins, sugars, fatty acids, phenolic compounds and terpenoïds (Sundararajan and Koduru, 2014; Larit et al., 2018). Among these molecules, essential oils retained attention as potential compounds for different uses in cosmetics but also for biocidal effects (Barragan Ferrer et al., 2016; Sayed Ahmad et al., 2017). The valorization of essential oils requires knowledge of their chemical composition as well as their toxicological profile dependent on genetic variations and the influence of the phenological stage (Verma et al., 2010; Padalia et al., 2011; Toncer et al., 2017). These intrinsic factors inaugurate a quantitative and qualitative paradigm in terms of yield and accumulation of bioactive substances (Barragan Ferrer et al., 2018).
Cytisus triflorus L’Her, locally known by several names as “Gikio”, “bouharis”, “el lougui”, or “tellouguit”, is the most widespread species among the eight species growing naturally in the North of Algeria (Quezel and Santa, 1963). Locally known for its medicinal properties, C. triflorus was used in particular to treat abdominal pain, wound healing and jointly as antifungal. A very few literature data (Aourahoum et al 2014, 2015; Benabderrahmane et al., 2018) exist concerning the Cytisus triflorus L’Her species. To our knowledge, only one previous study describes the essential oil composition of C. triflorus (Aourahoum et al., 2013). Studies on variation of essential oil yield and composition were undertaken in several aromatic species (Verma et al., 2010; Padalia et al., 2011; Toncer et al., 2017). However, there no reports on variation of essential oil yield and composition during C. triflorus growth cycle.
The aim of this study was to investigate for the first time the effect of phenological stages on the essential oil yield and composition of C. triflorus from northern Algeria.
2 Materials and methods
2.1 Plant material
The aerial parts of C. triflorus were collected in 2015 in the Chrea National Park of Blida region (Altitude of 800 m; Latitude of 36°21′(N); Longitude of 2°45′(E)) at 50 km south of Algiers, Algeria. Harvesting was took place at the beginning of every phenological stage of the year: vegetative stage (V’st) (01 April), flowering stage (FL’st) (28 May), and fruiting stage (FR’st) (30 June). Voucher specimen was stored in the herbarium of the Department of Botany, National High School for Agronomy (ENSA), Algiers and already identified at the herbarium of university of Constantine 1 (CTA 125/03/11).
2.2 Essential oil extraction
The C. triflorus aerial parts collected were dried at ambient temperature (24 ± 1 °C). Then, the biomass was crushed with a ball mill (Retsch model) into fine powders and stored carefully in a dry place. Crushed biomass was subjected to hydrodistillation for 3 h in a Clevenger-type apparatus according to the method recommended in the European Pharmacopoeia (2004). Essential oil was stored at 4 °C protected from light for further analysis.
2.3 GC–MS and GC-FID analysis
The chemical analysis of the essential oils was performed by gas chromatography coupled to mass spectrometry (GC/MS Thermo Electron, QP7890A) and gas chromatography coupled to flame ionization detection (GC/FID). The essential oil was diluted in acetone in 3.5% and 1 µl was injected. Analyses were performed on a fused silica capillary column ZP-5 MS (5% Phenyl-Arylene and 95% Dimethylpolysiloxane, 30 m × 0.25 mm id; 0.25 µm film thickness), helium was used as carrier gas at a flow rate of 1 mL min−1, the injector temperature was at 280 °C and flame ionization detector temperatures were 280 and 300 °C, respectively. Moreover, temperature of GC/FID was programmed initially kept at 50 °C for 5 min, then gradually increased to 250 °C at 3 °C min−1 and held for 10 min. Ion source and MS quadrupole temperatures were 230 °C and 180 °C, respectively. Mass spectrometer operating at 70 eV and electron multiplier was set at 2200 V. Essential oil constituents were identified by comparing the mass spectra with those in the data system library (NIST version 2.0) and by comparing the mass spectra and calculated linear retention indices (RI) with values in the literature (Adams, 2007).
2.4 Statistical analyses
The tests were performed in three replicates and data were statistically analyzed through the ANOVA-Tukey HSD (honestly significant difference) test, comparing the impact of Cytisus triflorus L’Herr phenological stages (vegetative, flowering and fruiting stages) on essential oil yield and composition using Duncan’s Multiple Range, Kruskal-wallis tests at p ≤ 0.01 level (Past, Hammer et al. (2001)).
3 Results
According to the results presented in Fig. 1, the phenological index of C. triflorus had significant impact at p < 0.01 on the essential oil yield. This impact was revealed by the highest oil yield (Fig. 1) obtained from the flowering stage, followed by fruiting and vegetative stages (0.17% in V’st < 0.41% in FR'st < 0.61% in FL'st).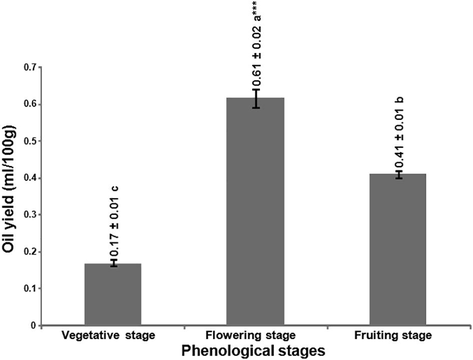
Effect of the phenological stages on the essentials oil yield of C. triflorus. Means ± standard deviation followed by the letters are significantly different, as indicated by Duncan’s multiple range test, at P < 0.01.
The qualitative results of C. triflorus essential oil collected during the three phenological stages are presented in Table 1. Thirty-eight compounds were determined and identified which representing 93.21%, 94.96% and 95.47% of the oil obtained at the vegetative, flowering, and fruiting stage respectively. Results show that essential oil contain a complex mixture consisting mainly of oxygenated monoterpenes (56.27 to 61.04%), monoterpenes hydrocarbons (12.95 to 26.20%), aldehydes (5.83 to 11.17%) and hydrocarbons (4.83 to 7.30%) (Fig. 2A). The main components founded in oil at different growth stages were β-linalool (28.28 to 38.04%), Geraniol (19.82 to 27.73%), Retinal (4.33 to 9.26%), p-Mentha-1,4-dien-8-ol (2.09 to 4.08%), γ -Terpinene (1.27 to 4.86%) and Eugenol (2.07 to 2.28%). The percentage of these main components varies according the growth stage (Fig. 2B). Indeed, difference of percentages of the components was observed (Table 1, Fig. 2). Twelve compounds were higher at vegetative stage compared to the two other stages (Table 1 and Fig. 2). At flowering stage, sixteen compounds exhibited higher level than at the two other stages. In contrast, fruiting stage was characterized by the predominance of nine and the absence of three compounds. For example, Carvacrol, which was present at low level in both vegetative and fruiting stages increased noticeably at flowering stage (Table1). Similarly, even present at low level, Thymol was detected only at flowering stage. As summarized in Fig. 2, at flowering stage, monoterpene hydrocarbon, esters, alcohols and phenolics were detected at higher level than at the other stages. In contrast, aldehydes, ketones and oxynated sesquiterpes were noted at higher percentage at fruiting stage. Oxygenated monoterpenes and hydrocarbons were higher at vegetative stage (Table 1). RI: Retention indices. ND: Compounds not detected.
Compounds
RI
GC area %
V’st
FL'st
FR'st
β-linalool
1082
38.04
28.28
37.91
Eucalyptol
1059
0.33
0.26
0.09
Geraniol
1252
22.67
27.73
19.82
γ-Terpinene
998
3.53
4.86
1.27
o-Cymene
1128
0.30
0.84
0.13
Isolongifolene epoxide
1305
0.29
0.50
0.76
Nonanal
1104
0.20
0.26
0.18
Benzaldehyde
982
0.12
0.13
0.05
Undecanal
1303
0.08
0.94
0.66
Retinal
2184
4.33
5.30
9.26
p-Menth-1-en-9-al
1217
1.10
1.20
1.02
4-Methyl-2-pentyl acetate
856
0.32
0.52
0.17
Benzyl Benzoate
1738
0.60
0.55
0.84
Oleic acid.methyl ester
2080
0.07
0.84
0.32
E-9-tetradecenol
1664
0.28
0.49
0.03
1-Hexanol
860
0.10
0.60
0.02
4-Terpineol
1160
0.46
0.87
N.D
Z-9-hexadecen-1-ol
1863
0.07
1.20
1.60
1-Octen-3-ol
979
1.69
1.24
1.09
Oleyl Alcohol
2061
1.41
2.30
2.94
Retinol
2453
0.84
0.07
0.12
2.6-di-tert-butyl-p-Benzoquinone
1462
1.00
1.01
1.58
2-Heptanone
853
0.73
0.17
0.20
p-Mentha-1.4-dien-8-ol
1083
4.08
2.09
2.86
Heneicosane
2109
0.07
1.12
0.02
Heptadecane
1700
0.50
0.23
0.13
Hexacosane
26000
1.02
1.03
1.44
Octacosane
2804
1.62
0.31
1.39
Styrene
883
0.01
0.05
0.31
Myristic acid
1754
1.00
0.53
2.10
Hexadecanoic acid
1973
0.82
1.35
2.75
Ricinoleic acid
1991
1.15
1.42
0.27
Nonanoic acid
1277
0.48
0.40
0.36
Cresol
1014
0.10
0.04
N.D
Carvacrol
1304
0.50
3.15
0.88
Thymol
1283
N.D
0.19
N.D
Eugenol
1364
2.28
2.09
2.07
Gibberellic acid
2653
1.02
0.80
0.83
Total identified compounds
93.21
94.96
95.47
Oxygenated monoterpenes
61.04
56.27
57.82
Monoterpenes hydrocarbons
7.92
10.06
12.95
Oxygenated sesquiterpenes
0.29
0.50
0.76
Phenolics
3.90
4.04
3.78
Fatty acids
3.45
3.70
5.48
Aldehydes
5.83
7.83
11.17
Esters
0.99
1.93
1.33
Alcohols
4.85
6.77
6.10
Ketone
1.73
1.18
1.78
Hydrocarbons
3.21
2.69
2.98
93.21
94.96
95.47
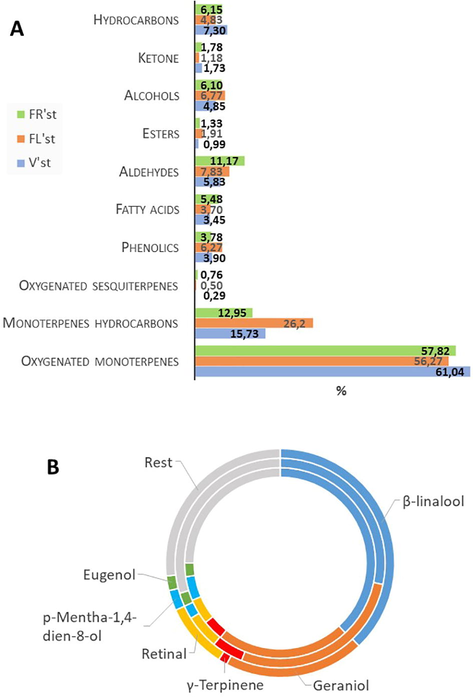
(A) Graph of the distribution (%) of chemical classes identified in the essential oil of C. triflorus plant. (B) Graph of the distribution (%) of main components identified in the essential oil of C. triflorus plant at different phenological stages. From inside to outside: vegetative (V’st), flowering (FL’st) and fruiting (FR’st) stages. Rest = 32 from the total (38 compounds).
4 Discussion
The chemical composition of the essential oil of C. triflorus was described for the first time at different phenological stages. Comparing our results to those reported by the only existing study in the literature (Aourahoum et al., 2014), the composition of the oil is very different. Indeed, Aourahoum et al. (2013) have studied the essential oil composition of C. triflorus from Azazga region (North Algeria) at March 2011 period. They founded 61 volatile compounds in C. triflorus oil, where Linalool (20.9%), α-terpineol (6.4%), Nerol (1.2%), Geraniol (6.5%), 4-vinyl-2-ethoxy-phenol (3.5%), Eugenol (1.7%), 3-dimethoxy-styrene (4.3%), 6,10,14-trimethyl-2-pentadecanone (7.6%), Tetradecanoic acid (2.5%), Geranyl acetone (1.6%), Heneicosane (1.9%), Tricosane, (2.1%), Hexadecanoic acid (5.6%) and musk ambrette (2.3%) are the main components (Aourahoum et al., 2014). This difference in oil composition with that founded by Aourahoum et al (2013, 2015) could be related to the geographical region (Azazga region, North Algeria) and the harvest time (March 2011). Intriguingly, Carvacrol, which presented a low concertation during vegetative stages, increased markedly at flowering stage and returned to its initial level after this stage (Table 1). This fact was not reported in literature. A tentative explanation of this fact is due to its biocidal effect in order to protect the reproductive stages of plants (Fonseca et al., 2019; Sun et al., 2020) and/or to attract insect for pollination (Sayed Ahmad et al., 2017).
The variation of essential oil yield at different phenological stages may be due to genetic factors, developmental stages, plant origin, harvesting, drying and storage methods, extraction and analysis methods (Moghaddam et al., 2015; Barragan Ferrer et al., 2016, 2018; Sayed Ahmad et al., 2018). The phenological stages could affect significantly the essential oils yield of medicinal and aromatic plants, and therefore the previous rapports synchronizing the preferential essential oils’ accumulations indicated by the high levels in flowering stage (Verma et al., 2010; Padalia et al., 2011; El-Kalamouni et al., 2017; Toncer et al., 2017; Aldarkazali et al., 2019) where they hastily decreased thereafter (Sangwan et al., 2001), whereas other research restore that the lowest essential oil yield was marked in the vegetative stage. The low amount of volatile compounds’ biosynthesis during the vegetative stage may be due to partial inactivation of the enzymes necessary for the biosynthesis of some compounds (Sangwan et al., 2001; Hamrouni-Sellami et al., 2009; Sayed Ahmad et al., 2017), while the highest rate in the flowering stage is strongly linked to the high production of biomass and oil content at this stage (Toncer and Kizil, 2005; Toncer et al., 2017). Essential oil content is reduced during developmental periods due to the accumulation of photosynthetic products in the endosperm (Ozcan and Chalchat, 2006).
The phenological variations on the chemical composition of essential oils from C. triflorus have been renovated in a striking way, whereby it is conceivable that these changes are accompanied by the modifications of the secondary metabolism. The hypothesis put forward corroborates with some study in which it was reported that the effect of different phenological stages on the essential oil and its composition may be due to its influence on the enzymatic activity and metabolism of the essential oil production (Sangwan et al., 2001; Hamrouni-Sellami et al., 2009; Sayed Ahmad et al., 2017). However, plant ontogenesis has a very close relation with the secondary metabolites accumulation in plants, which has a significant influence on the essential oils’ composition (Ozcan and Chalchat, 2006; Padalia et al., 2011; Barragan Ferrer et al., 2016, Roche et al., 2019).
It seems that the accumulative aptitudes of bioactive molecules in plants provide marginality due to the synchronization of a phenological stage to another incriminating certain organs in relation to others. Indeed, each phenological stage accumulates different quantities of bioactive molecules in relation to the intrinsic capacities of the organ migration. This hypothesis of bioactive molecules has been strongly documented where it has been stipulated that the plant age is a factor determining the bioactive molecules’ composition in qualitative and quantitative terms (Ozcan and Chalchat, 2006; Padalia et al., 2011; Sarmoum et al., 2019). Nevertheless, environmental factors such as soil fertility, abiotic stresses, pests’ injuries or plant growth regulators have also significant effects on amounts accumulation and can affect their distribution amongst the different plant organs (Zebib et al., 2015, Aldarkazali et al., 2019; Roche et al., 2019. Sarmoum et al., 2019).
5 Conclusion
Large variations of essential oil yield and composition were observed between the three growth stages (vegetative, flowering and fruiting stages) of C. triflorus. Higher yield was noticed at flowering stage. The main oil components of C. triflorus at different phenological stages were β-linalool, Geraniol, Retinal, p-Mentha-1,4-dien-8-ol, γ-Terpinene and Eugenol as the major oil components. Moreover, composition varied greatly between flowering and both other stages. Some components (carvacrol and thymol) were detected at higher concentration or only at flowering stage. This period of growth was characterized by the predominant of monoterpene hydrocarbon, whereas, vegetative stage was mostly represented by aldehydes. Therefore, by considering the yield of essential oil, flowering stage seems to be the most interesting stage for harvesting plant material. This work is the first report studying variation of essential oil during growth cycle of C. triflorus. Further investigations are necessary to examine the valorization of this essential oil for different uses.
CRediT authorship contribution statement
Selma Daghbouche: Conceptualization, Methodology, Formal analysis, Investigation, Writing - original draft. Imene Ammar: Conceptualization, Methodology, Formal analysis, Investigation. Dorsaf Moalla Rekik: Conceptualization, Formal analysis, Investigation. Zahr-Eddine Djazouli: Conceptualization, Formal analysis, Investigation, Writing - original draft, Writing - review & editing. Bachar Zebib: Methodology, Writing - original draft, Writing - review & editing. Othmane Merah: Conceptualization, Methodology, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Review of identification of essential oil components by gas chromatography/mass spectrometry. J. Am. Soc. Mass Spectrom.. 2007;18:803-806.
- [Google Scholar]
- The growth and development of sweet basil (Ocimum basilicum) and bush basil (Ocimum minimum) grown under three light regimes in a controlled environment. Argon. 2019;9:743.
- [CrossRef] [Google Scholar]
- Pharmacological potential of Cytisus triflorus l’Hérit. Extracts as antioxidant and anti-inflammatory agent. Pharm. Lett.. 2015;7:104-110.
- [Google Scholar]
- The synthetic antioxidant butylated hydroxytoluene, a naturally occurring constituent of the broom Cytisus triflorus L’Hérit. J. Nat. Prod.. 2014;7:58-64.
- [Google Scholar]
- Identification and in vitro activity of bioactive compounds extracted from Tussilago farfara (L.) plant grown in Lithuania and France. Free Rad. Antiox.. 2018;8:40-47.
- [CrossRef] [Google Scholar]
- Interest of Tussilago farfara (L.) whole plant of Lithuanian and French origins for essential oil extraction. Am. J. Essent. Oil Nat. Prod.. 2016;4:12-15.
- [Google Scholar]
- Chemical constituents, in vitro antioxidant and antimicrobial properties of ethyl acetate extract obtained from Cytisus triflorus l’Her. Nat. Prod. Res.. 2018;1–5
- [CrossRef] [Google Scholar]
- Chemical composition, antioxidant, antibacterial, and antifungal activities of the essential oil of wild Achillea millefolium L. plant grown in France. Medicines. 2017;4:30.
- [CrossRef] [Google Scholar]
- Council of Europe, Strasbourg (5th ed.). 2004. p. :217-218.
- Development of antimicrobial and antioxidant electrospun soluble potato starch nanofibers loaded with carvacrol. Int. J. Biol. Macromol.. 2019;19:1182-1190.
- [CrossRef] [Google Scholar]
- Potential use of Cytisus scoparius extracts in topical applications for skin protection against oxidative damage. J. Photochem. Photobio.. 2013;125:83-89.
- [CrossRef] [Google Scholar]
- PAST: paleontological statistics software package for education and data analysis. Palaeontol. Electron.. 2001;4:1-9.
- [Google Scholar]
- Effect of growth stage on the content and composition of the essential oil and phenolic fraction of sweet marjoram (Origanum majorana L.) Ind. Crop. Prod.. 2009;30:395-402.
- [CrossRef] [Google Scholar]
- Secondary metabolites from the aerial parts of Cytisus villosus Pourr. Phytochem. Lett.. 2018;24:1-5.
- [CrossRef] [Google Scholar]
- Changes in composition and essential oil yield of Ocimum ciliatum at different phenological stages. Eur. Food Res. Technol.. 2015;240:199-204.
- [CrossRef] [Google Scholar]
- Effect of collection time on chemical time on chemical composition of the essential oil of Foeniculum vulgare subsp. piperitum growing wild in Turkey. Eur. Food Res. Technol.. 2006;224:279-281.
- [CrossRef] [Google Scholar]
- Variation in the volatile constituents of Artemisia annua var. CIM-Arogya during plant ontogeny. Nat. Prod. Comm.. 2011;6:239-242.
- [Google Scholar]
- Influence of the drying method in the antioxidant potential and chemical composition of four shrubby flowering plants from the tribe Genisteae (Fabaceae) Food Chem. Toxic.. 2011;49:2983-2989.
- [CrossRef] [Google Scholar]
- Quezel, P., Santa, S., 1963. Nouvelle flore de l’Algérie et des régions désertiques méridionales, CNRS ed., Paris.
- Effect of sowing date on fatty acid and phytosterols patterns of Carthamus tinctoria L. Appl. Sci.. 2019;9:2839.
- [CrossRef] [Google Scholar]
- Regulation of essential oil production in plants. Plant Growth Regul.. 2001;34:3-21.
- [CrossRef] [Google Scholar]
- Effect of salinity and water stress on the essential oil Rosemary essential oil composition (Rosmarinus officinalis) Argonomy. 2019;9:214.
- [CrossRef] [Google Scholar]
- Fennel seed oil and by-products characterization and their potential applications. Ind. Crop. Prod.. 2018;111:92-98.
- [CrossRef] [Google Scholar]
- The Apiaceae: ethnomedicinal family as source for industrial uses. Ind. Crop. Prod.. 2017;109:661-671.
- [CrossRef] [Google Scholar]
- Effect of spray-drying temperature on physicochemical, antioxidant and antimicrobial properties of pectin/sodium alginate microencapsulated carvacrol. Food Hydrocol.. 2020;100:105420
- [CrossRef] [Google Scholar]
- Cytisus scoparius: a review of ethnomedical, phytochemical and pharmacological information. Indo. Am. J. Pharm. Res.. 2014;4:2151-2169.
- [Google Scholar]
- Essential oil composition of Ocimum basilicum L. at different phenological stages in semi-arid environmental conditions Fres. Environ. Bull.. 2017;26:5441-5446.
- [Google Scholar]
- Determination of yield and yield components in wild Thyme (Thymbra spicata L. var. spicata) as influenced by development stages. Hort. Sci.. 2005;32:100-103.
- [CrossRef] [Google Scholar]
- Changes in the essential oil content and composition of Origanum vulgare L.: during annual growth from Kumaon Himalaya. Curr. Sci.. 2010;98:1010-1012.
- [Google Scholar]
- Chemical composition of the essential oil of Satureja myrtifolia (Boiss. & Hohen.) from Lebanon. J. Essent. Oil Bear. Plant.. 2015;18:248-254.
- [CrossRef] [Google Scholar]







