Translate this page into:
Effect of montmorillonite content in natural Saudi Arabian clay on its adsorptive performance for single aqueous uptake of Cu(II) and Ni(II)
⁎Corresponding author. nmdalhat@iau.edu.sa (Nuhu Dalhat Mu'azu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The effect of montmorillonite content on adsorptive performance of natural Saudi Arabian clay for aqueous uptake of Cu(II) and Ni(II) was investigated under kinetic and equilibrium batch studies. The clay and bentonite used were characterized using scanning electron microscopy (SEM), Brunauer-Emmet-Teller (BET), cationic exchange capacity (CE), energy dispersive X-ray (EDX) and X-ray diffraction (XRD) techniques. The clay was composed of kaolinite and muscovite with lower quartz and calcite contents, while the bentonite was mainly montmorillonite mineral possessing surface area of 20.83 and 44.27 m2/g, respectively. Increase in montmorillonite content in the clay significantly, resulted in decrease in Cu(II) adsorption capacity while increasing the capacity for Ni(II) adsorption. Equilibrium time was found to increase considerably as the montmorillonite content was increased and was higher for the clay compared to the bentonite with 16.38 and 8.66 mg/g maximum adsorption capacities for the metal ions, respectively. For both metal ions and the different montmorillonite content, the experimental data predominantly, fitted pseudo-second-order model (R2 = 0.96–0.999) while both Langmuir and Freundlich isotherm models described well the two ions removal mechanisms (R2 = 0.91–0.99). The two metal ions exhibited divergent behaviors for having distinctive affinities towards the clay and bentonite surfaces, respectively. This suggests that proportion of montmorillonite in clay adsorbents is susceptible to influence different metal ions removal from water in different manner.
Keywords
Montmorillonite content in clay
Mineralogical composition
Adsorption capacity
Copper and nickel ions uptake
Adsorption kinetics and isotherms
1 Introduction
Industrial activities result in discharge of heavy metals contaminated water which is liable to cause serious environmental degradation and impaired human and animals health problems (Nagajyoti et al., 2010; Alves et al., 2014; Lakherwal, 2014). As consequence, several physical-chemical technologies such as ion exchange, chemical precipitation, membrane filtration, adsorption, solvent extraction, electrochemical have been developed for the removal of heavy metals from contaminated water prior to discharge into the ecosystem. However, each technology has its limitations with some relatively expensive (Babel and Kurniawan, 2003; Lakherwal, 2014). Among the existing technologies, adsorption becomes an attractive alternative technique in treatment heavy metals wastewater contaminated due to its documented cost effectiveness (Lakherwal, 2014). Recently, different adsorbents such as activated carbon, sand, waste tea leaves, eggshell, zeolites, olive stones, clay, wood sawdust (Lakherwal, 2014), chitosan, fly ash, coal and peat moss (Babel and Kurniawan, 2003) were used to remove heavy metals from aqueous solution. Due to their excellent properties such as high surface area, ion exchange capability, present of negative surface charge and their universal abundant in nature, natural clays minerals are considered as cost-effective alternative to the widely used expensive activated carbon for the removal of heavy metal ions from wastewater (Bhattacharyya and Gupta, 2008a; Lukman et al., 2013; Padilla-Ortega et al., 2013; Wongkoblap et al., 2013). Bhattacharyya and Gupta (2006), Gupta and Bhattacharyya (2006) worked on adsorption of Ni and Cu on different types of clays minerals. They observed that the Ni adsorption capacity to increase according in the order montmorillonite > ZrO-montmorillonite>, TBA-montmorillonite > kaolinite > ZrO-kaolinite>, and TBA-kaolinite (Gupta and Bhattacharyya, 2006). However, the affinity towards Cu adsorption to follow the order: montmorillonite > TBA-derivative > ZrO-derivative > kaolinite > TBA-derivative >> ZrO-derivative (Bhattacharyya and Gupta, 2006). On the other hand, during electrokinetic removal (EKR), it was reported that the process efficacy for different soil types followed the order sand < silt < kaolinite < illite < montmorillonite (bentonite) due to variability of charge density which greatly influence the sorption in the EKR chamber (Acar et al., 1995; Virkutyte et al., 2002). Moreover, during EKR of several heavy metals from soils of different mineralogy, Lageman (1993) observed that kaolinite and clayey sand yielded better performances compared to clay that is predominantly montmorillonite in nature. Additionally, Reddy et al. (1997) reported heavy metals EKR removal order from Na-montmorillonite < kaolin < glacial till, where they underscored the relevance of soil mineralogical composition during EKR. Similarly, Lukman (2013) reported that the EKR for removal of heavy metals from which bentonite soil (100% montmorillonite) showed significant improvement compared to other non-montmorillonite clay soils. Recently, Mu'azu et al. (2016) reported EKR removal of heavy metals from different blends of bentonite and clay, hence, having different mineralogical composition proportion. They observed that an increase in the montmorillonite content significantly, increased the EKR removal efficiency of heavy metals. They attributed such observations to higher surface area, humic substances as well as the CEC of the bentonite (mainly montmorillonite) which demonstrated high EKR removal capacity. They suggested that to it might be as results of lower sorption yield compared to other non-montmorillonite clay soils (Lageman, 1993; Mu'azu et al., 2016). Inferably, it was expected that proportion of mineral components in different clayey minerals to have influence on the adsorptive removals of heavy metals from water. Hence, the main objective of this study was to investigate the influence of different montmorillonite content in a natural clay mineral on the adsorptive behavior of removal of Cu(II) and Ni(II) from water. To achieve that, five different mixtures ratios of bentonite in Saudi Arabian clay were considered in batch mode experiments conducted at different operating conditions of contact time, mass of adsorbent, pH, and initial metal concentration.
2 Material and methods
2.1 Preparation and characterization of clay and bentonite
Natural Saudi Arabian clay was obtained from Al-Hassa city near Jabal Al Qarah caves of Hofuf. This area is known for its rich in mineral deposits (Al-Safarjalani, 2004; Hussain et al., 2006). Meanwhile the bentonite was supplied by Riyadh Geotechnical and Foundations Company. Prior to using the two adsorbents for the adsorption experiments, the minerals were powdered and sieved through mesh to obtain particles size less than 200 mesh (i.e. 63 µm) and mildly washed with 0.1 N HNO3, then followed by deionized water to remove any attached impurity. Afterwards, samples surface state and structure of the sample were characterized using a SM-6610LV JEOL with an energy dispersive X-ray (EDX) detector (Oxford INCA Penta FETx3) attached to enable determination of the samples elemental compositions. In order to minimize charging effect, the samples were gold (thin layer) for the SEM analyses. An accelerating voltage of 5 kV was used at a short working distance of 2 mm undertaken under magnifications ranging from x500 to x40,000. The accelerating voltage of the SEM was maintained at 20 kV during microchemical analysis. For determining the phase constitution of the samples, a Rigaku Ultima IV MPD X-ray diffractometer (XRD) fitted with a monochromator was employed. The samples diffraction spectra peaks were indexed using Rigaku PDXL software which were generated under Cu Kα radiation (λ = 1.54184 Å) source operating at 40 mA and 40 kV. The scanning was undertaken for 2theta diffraction angles (2θ) between 5 to 80 degrees at a rate of 1 degree per minute. The area and average diameter were measured using the Brunauer-Emmet-Teller (BET) method that is depended on N2 gas adsorption technique using ASAP 2020, Micromeritics. The cation exchange capacity (CEC) was determined by EPA 9081 method (USEPA, 1986).
2.2 Chemical reagents
All chemicals used were of analytical grade from which standard solution (1000 mg/L; pH ≤ 2) of Cu(II) and Ni(II) were prepared separately from Cu (NO3)·3H2O and Ni (NO3)·6H2O respectively, using deionized water prepared from a Millipore Ultrapure instrument. The different pH of stock solutions were adjusted to target values by using 0.1 N HNO3 or 0.1 N NaOH.
2.3 Batch adsorption experiments
Batch adsorption experiments in 125 mL glass bottles containing a constant volume of 50 mL were conducted on shaker under constant stirring at 200 rpm at room temperature 22 ± 3 °C. The experiments were conducted in four different sets to investigate the effect of contact time (5, 10, 15, 30, 45, 60, 120, 240, 360, and 480 min), bentonite to clay mass ratio, B/C, (which represents the montmorillonite content) of 0, 10, 30, 50, 100%, adsorbate concentration (20, 40, 60, 80, and 100 mg/L), total mass of adsorbent (0.2. 04, 0.6, 0.8, and 1 g), and the solution pH (3.5, 5, 6.5 and 8). The removal efficiency (R), adsorption capacity at any time t (qt) and equilibrium (or maximum) adsorption capacity (qe) all in mg/g were calculated using Eqs. (1), (2) and (3), respectively (Lukman et al., 2013).
2.4 Kinetics study
Based on the batch experiments, the kinetics study was undertaken at constant conditions of pH 5, amount of adsorbent 0.2 g, and initial concentration 100 mg/L considering different equilibrium time of 240 min and 360 min for Cu(II) and Ni(II), respectively. Pseudo first order and pseudo second order kinetic models were employed for estimating the kinetic coefficients using Eq. (4) (Plazinski et al., 2009; Sen Gupta and Bhattacharyya, 2011) and Eq. (5), respectively.
2.5 Isotherms study
The adsorption isotherms for copper and nickel were studied at their respective equilibrium time, pH 5, amount of adsorbent 0.2 g and at different initial concentrations (0–100 mg/L). The Langmuir and Freundlich isotherms given in Eqs. (6) and (7) (Malamis and Katsou, 2013; Zubair et al., 2017), respectively, were used for calculating different equilibrium isotherms parameters.
2.6 Heavy metals analysis
Immediately, after any of the above adsorption experiments, all the samples were filtered through a 0.45 μm membrane filter using a syringe filter. The filtrates were quantitatively analyzed for concentration of Cu(II) and Ni(II) using well calibrated inductively coupled plasma mass spectrometry instrument (Agilent 710 ICP-OES).
3 Results and discussions
The results obtained from the batch, kinetic and equilibrium experiments are presented and discussed in detail below. These include characterization of the clay minerals and adsorption studies for Cu(II) and Ni(II) which included effects of operating conditions on the adsorption process, isotherms study and kinetics study.
3.1 Characterization of clay and bentonite
Table 1 and Figs. 1–2 show some characterization results obtained from numerous analyses techniques as discussed earlier. The XRD pattern in Fig. 1 shows that the clay was mainly composed of kaolinite (47.27%), muscovite (23.53%), 7.63% quartz and11.57% calcite. Muscovite is one of clay mineral which belongs to mica group while quartz can be present in clay materials in lower proportion (Rowse and Jepson, 1972; Sayin and Jackson, 1979). Meanwhile the bentonite was dominantly composed of montmorillonite mineral (87.32%). Similarly, the microgram scan and surface structure of clay and bentonite captured by the SEM analysis shown in Fig. 2A shows marked differences in surface structure and morphology between clay and bentonite. Apparently, the chemical compositions breakdown of the two studied minerals obtained from EDX analysis provided in Fig. 2B shows the main dominant elements in the clay and bentonite were oxygen, silica, aluminum and iron. Thus, the clay SEM picture (Fig. 2A) suggests an entirely amorphous material with high presence silicon and oxygen as supported by the EDX chemical composition (Musić et al., 2011; Ogunmodede et al., 2015). Also, the bentonite possessed a discrete and rough pattern indicating the presence of higher iron oxide (Fig. 2B) (Oliveira et al., 2003; Ogunmodede et al., 2015). Interestingly, the clay sample elemental composition indicated presence of gold (Au) which agrees with earlier documented study (Al-Safarjalani, 2004). This indicated a slightly higher Si/Al (2.9%) for the bentonite compared to that of the clay (2.3%). As shown in Table 1, both the bentonite BET surface area (44.278 m2/g) and CEC (101.3 meq/100 g) were high, about 2 two folds and 5 folds than that of the clay, respectively. These values of the CEC are within the typical values reported in literature (Rollins and Pool, 1968, 1999; Dohrmann et al., 2012). Meanwhile, the mean particles sizes in clay were almost twice that of the bentonite. These characteristics indicate that both the two minerals have sorption potentials for heavy metals removal from water (Bhattacharyya and Gupta, 2008b,a). *Cationic exchange capacity; *BET – Branaur-Emmett-Teller.
Property
Clay
Bentonite
Specific gravity (ASTM D 854)
2.65
2.77
BET Specific surface area, m2/g
20.83
44.28
Average Diameter nm
112.82
61.55
Passing through sieving No.
200
200
CEC meq/100 g (EPA 9081)
20.9
101.3
pH (ASTM D 4972)
9.53
8.3
Organic matter, %
4.3
2.59
![XRD Results showing mineralogical compositions for (A) Clay and (B) Bentonite [Mu'azu, 2018].](/content/185/2020/32/1/img/10.1016_j.jksus.2018.06.003-fig1.png)
XRD Results showing mineralogical compositions for (A) Clay and (B) Bentonite [Mu'azu, 2018].
![Clay and bentonite (A) high resolution SEM showing microgram structure and (B) EDX elemental compositions analysis [Mu'azu, 2018].](/content/185/2020/32/1/img/10.1016_j.jksus.2018.06.003-fig2.png)
Clay and bentonite (A) high resolution SEM showing microgram structure and (B) EDX elemental compositions analysis [Mu'azu, 2018].
3.2 Effect of operating conditions on adsorption of copper and nickel
3.2.1 Effect of contact time
For the different bentonite-clay blends, Fig. 3A and Table 2 show the effect of contact time on adsorptive capacity and removal efficiency for adsorption of Cu(II) and Ni(II) at fixed 100 mg/L initial concentration and solution pH 5. Both the trends for Cu(II) and Ni(II) depicted in Fig. 4A indicate that as the montmorillonite content was increased, the time for attaining equilibrium decreased. Respectively, for 100% clay, the equilibrium time was established at 240 min and 360 min which reduced (rapidly for Cu) to 30 min and 45 min when the adsorbent used was 100% bentonite. As given in Table 2, both the Cu(II) removal efficiency (64 to 34.85%) as well as adsorption capacity (16.38 to 8.7 mg/g) was higher – almost doubling that of Ni(II)- and they decreased continuously as the montmorillonite content was increased from 0 to 100%. Conversely, Ni sorption capacity increases as the bentonite ratio increase and also its adsorption process was much slower (Fig. 3A) yielding lower equilibrium adsorption capacity and removal efficiency (Table 2) which slightly improved within narrow range as the montmorillonite content was increased. Moreover, the highest Ni adsorptive capacity was observed when montmorillonite content was 50% as results of the decline of capacity at longer contact time when the adsorbent was 100% bentonite. Comparable and divergent behaviors during adsorption of metal ions on different clay minerals have been reported elsewhere (Bhattacharyya and Gupta, 2006, 2008c; Jiang et al., 2010; Liu and Zhou, 2010; Aljlil and Alsewailem, 2014). Increase in agitation time is expected to induce decrease in the adsorption sites as they are gradually filled up by metal ions and the adsorption kinetics become dependent on the transport of the metal ions from the bulk liquid phase to the actual adsorption sites (Bhattacharyya and Gupta, 2006, 2008b; Jiang et al., 2010).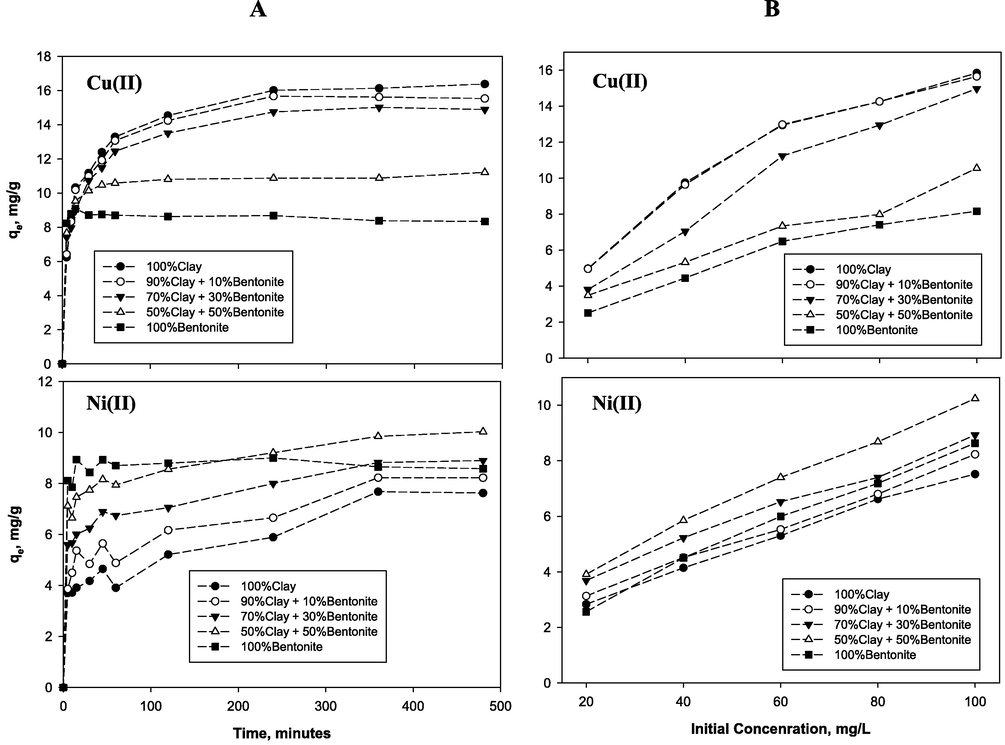
The effect of (A) contact time and (B) initial concentration on adsorption capacity for copper and nickel for the different adsorbents.
Mixture ratio of adsorbent
Cu(II)
Ni(II)
te (min)
qe (mg/g)
Removal efficiency %
te (min)
qe (mg/g)
Removal efficiency %
100% clay
240
16
64
360
7.7
30.7
90% clay + 10% bentonite
240
15.6
62.6
360
8.2
32.9
70% clay + 30% bentonite
240
14.7
59
360
8.8
35.3
50% clay + 50% bentonite
120
10.8
43.2
360
9.8
39.3
100% bentonite
30
8.7
34.85
45
8.9
35.7
Cu(II)
Ni(II)
C0, mg/L
qe (mg/g)
Removal efficiency %
qe (mg/g)
Removal efficiency %
100% clay
20
4.97
99.47
2.83
56.6
100
15.84
63.38
7.51
30
90% clay + 10% bentonite
20
4.96
99.21
3.1
62.6
100
15.65
62.6
8.2
32.9
70% clay + 30% bentonite
20
3.8
76.1
3.68
73.7
100
14.97
59.9
8.9
35.7
50% clay + 50% bentonite
20
3.5
69.9
3.9
78.2
100
10.5
42.2
10.2
40.9
100% bentonite
20
2.5
50.2
2.56
51.2
100
8.2
32.7
8.63
34.5
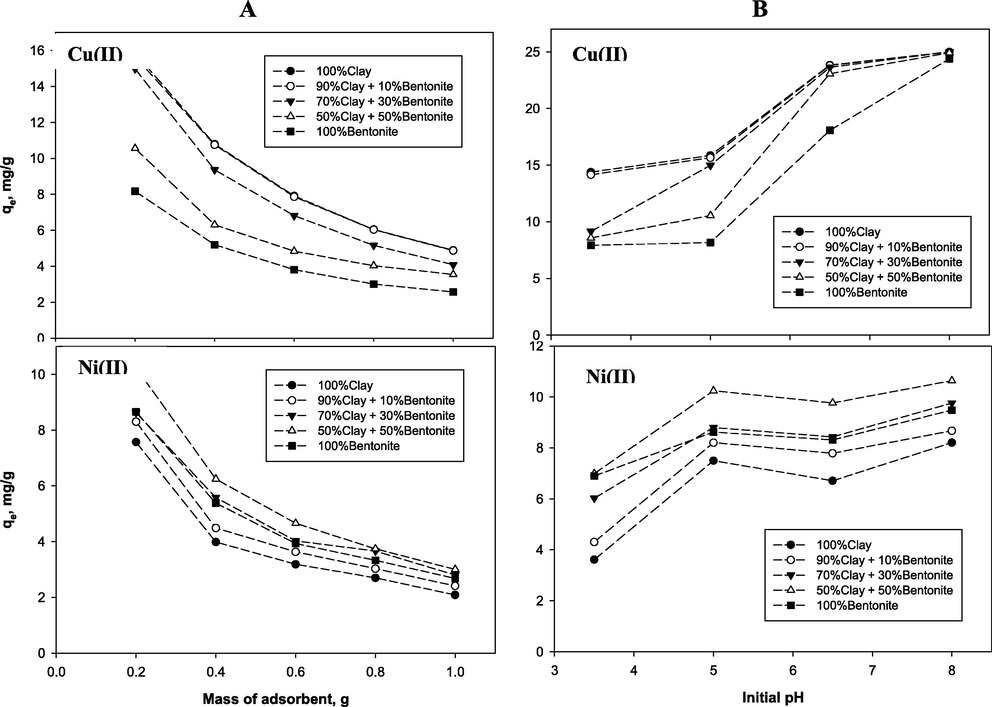
The effect of (A) mass of adsorbent and (B) initial pH on adsorption capacity for copper and nickel for the different adsorbents.
3.2.2 Effect of initial concentration
As presented in Fig. 3B and Table 2, the adsorption capacity for both metal ions increases proportionally, with increase in the initial concentration for all the different adsorbents. Thus, the observed increased in the maximum equilibrium adsorption capacity as given in Table 2. However, the removal efficiency decreased with an increasing in the initial concentration of both the metals ions for all the different adsorbents. The maximum removal efficiency at equilibrium time shows significant decreased as results of increased in the concentration from 20 to 100 mg/L. These divergent behaviors between equilibrium and adsorption capacity corroborate authors’ findings (Jiang et al., 2010; Vieira et al., 2010; Tan and Ting, 2014) which was attributed to the fact that, at high metal ions concentration, the number of molecules of the metal ions reaching the adsorbent sites increased resulting in higher chance of interaction between metal ions and soil particles, thereby increasing the adsorption capacity. On the other hand, it was expected that there would be an increase in the ratio of the number metal ions in solution to the available number of adsorption sites as the initial metal ions increase in solution. Consequently, this created a competitive environment which was prone to reducing the overall removal efficiency.
3.2.3 Effect of adsorbent mass
Under constant conditions initial concentration of 100 mg/L, solutions pH at 5, temperature of (22 ± 3 °C) at contact time of 240 min and 360 min (at the equilibrium) for copper and nickel respectively, the effect of the amount of adsorbents (0.2, 0.4, 0.6, 0.8, and 1 g) was assessed. The results are provided in Table 3 and Fig. 4A which show that, at equilibrium, the adsorption capacity decreased with increasing in the amount of dosage for the different adsorbents. Earlier studies (Sen Gupta and Bhattacharyya, 2008; Ding et al., 2009; Vieira et al., 2010; Zhang et al., 2012; Anna et al., 2015) reported similar behaviors during metal ions adsorption which they presumably attributed to two reasons. First, higher adsorbent amount forms particle aggregation, leading to decrease in the total surface area and increase in the diffusion path length both which led to decrease in the adsorption capacity. Second, increase in the adsorbent dosage leads to decrease in the unsaturation of the adsorption sites and therefore, the number of available sites per unit mass reduces resulting in comparatively less adsorption at higher adsorbent dosage. However, Table 3 shows the maximum removal efficiency and adsorption capacity of Cu(II) and Ni(II) with different adsorbent mass for all adsorbents at equilibrium time. At a fixed initial concentration, an increase in exchangeable sites and surface area which could receive more metal ions onto their surfaces was expected as the adsorbent amounts was increased (Zhang et al., 2012; Anna et al., 2015).
Mixture ratio of adsorbent
Adsorbent mass (g)
Cu(II)
Ni(II)
qe (mg/g)
Removal efficiency %
qe (mg/g)
Removal efficiency %
100% clay
0.2
15.8
63.38
7.65
30.2
1
4.88
97.73
2.08
41.7
90% clay + 10% bentonite
0.2
15.6
62.6
8.29
33.2
1
4.86
97.36
2.4
48.2
70% clay + 30% bentonite
0.2
14.97
59.99
8.63
34.5
1
4.08
81.7
2.8
55.9
50% clay + 50% bentonite
0.2
10.55
42.2
10.23
40.9
1
3.5
70.9
2.99
59.98
100% bentonite
0.2
8.16
32.67
8.65
34.62
1
2.57
51.42
2.67
53.5
pH
Cu(II)
Ni(II)
qe (mg/g)
Removal efficiency %
qe (mg/g)
Removal efficiency %
100% clay
3.5
14.3
57.5
3.6
14.46
8
24.99
99.96
8.2
32.8
90% clay + 10% bentonite
3.5
14.1
56.6
4.3
17.2
8
24.99
99.96
8.6
34.67
70% clay + 30% bentonite
3.5
9.15
36.6
6
24.1
8
24.9
99.9
9.7
39
50% clay + 50% bentonite
3.5
8.57
34.3
7
27.9
8
24.9
99.9
10.6
42.5
100% bentonite
3.5
7.9
31.7
6.9
27.6
8
24.38
97
9.48
37.9
3.2.4 Effect of pH
The solution pH is one of the major factors affecting the adsorption process using natural clay minerals. As such, the influence of initial solution pH on the removal of Cu and Ni ions from aqueous solution was carried out under different pH conditions (3.5, 5, 6.5 and 8) initial concentration of 100 mg/L, mass of adsorbent of 0.2 g, and temperature of (22 ± 3 °C) and at contact time of 240 min and 360 min for Cu(II) and Ni(II) respectively. An increase in adsorption capacity (Fig. 4B) and removal efficiency (Table 3) was observed as the initial solution pH increases from 3.5 to 8. This observation has also been reported by other authors during adsorption of Cu(II) and Ni(II) as well as other heavy metals on clay minerals (Gupta and Bhattacharyya, 2006; Bhattacharyya and Gupta, 2008b; Mu'azu et al., 2017). This can be attributes to decrease in the H+ ions as pH the was increased from acidic to basic condition thereby decreasing the strong competition between H+ ions and metal ions for active adsorption sites (Bhattacharyya and Gupta, 2008b). Moreover, the active sites on clay surface were demonstrated to be weakly acidic in nature (Padmavathy et al., 2003; Boonamnuayvitaya et al., 2004) from which they gradually get more deprotonated as the pH increases thereby resulting in the observed higher sorption of the two metal ions (Bhattacharyya and Gupta, 2008b). The higher adsorption capacity observed at pH 8, especially for Cu which is 24.99 mg/g for all the different montmorillonite content could be as result of precipitation of the metal hydroxides (Bhattacharyya and Gupta, 2008b; Sen Gupta and Bhattacharyya, 2008; Ding et al., 2009). Bhattacharyya and Gupta (2008a,b) out rightly attributed a sharp rise in Ni(II) uptake on natural and modified kaolinite and montmorillonite above pH 8 to start of precipitation of the Ni(II). Generally, depending on solution pH, clay minerals are known to possess two types of surface charges; a net permanent surface charge and a variable charge which can be, positive, or zero at the point of zero charge (Polati et al., 2006; Sponza et al., 2015; Mu'azu et al., 2017). Other studies suggest that the clay sorbents can be good adsorbent for metal ions regardless of pH (Hunter and James, 1992; Zadaka et al., 2010). Consequently, lower pH doesn't always favor sorption of heavy metals onto clay minerals due to disassociation of more anions, higher attraction forces and precipitation of metal hydroxides at higher pH (Ismail et al., 2009). Thus, other pH dependent mechanisms such as complexation reactions, speciation, redox as well as co-pollutant effects can play significant influence (Fiol and Villaescusa, 2009; Nurchi and Villaescusa, 2011; Mu’azu et al., 2017).
3.3 Kinetics study
Due to its successful in industrial applications for the adsorption reactors design, the kinetics of adsorption of Cu(II) and Ni(II) were investigated using the different blends of the clay minerals. The kinetic study experimental data obtained were fitted to the linearized forms of pseudo-first-order and pseudo-second-order models (Eqs. (4) and (5)) and presented in Fig. 5A and 5B, respectively. The kinetic parameters as well as the model’s predicted equilibrium adsorption capacity obtained from the slope and intercept of the plots are given in Table 4. Apparently, that the pseudo-second-order model parameters are well in agreement with the experimental data for all the different adsorbents tested (Fig. 6B). The R2 ranged from 0.995–1 and with from 0.96–0.999 for Cu(II) and Ni(II) respectively. Moreover, the equilibrium adsorption capacity qe obtained from pseudo-second-order model were in good agreed with the experimental data with very low percent error ranging between 0.07 to 4.47%. The pseudo-second order rate constant, k2, varying from 0.102 to 3.558 to g-mg−1 min−1 and from 0.0452 to 0.885 g-mg−1 min−1 as the montmorillonite content was increased for Cu(II) and Ni(II), respectively (Table 4). Inferably, the bentonite catalyzed the adsorption process to reached equilibrium faster for both the two adsorbents, though dwindling the equilibrium uptake capacity (i.e. qe) in case of Cu(II). This is clearly, manifested in the steeper slope in the plots in Fig. 5B as the montmorillonite content increases for Cu(II) and vice-verse for Ni. This implies that the kinetic behavior of adsorption of both ions was similar which was likely to be controlled by chemical process such as ion exchange between adsorbent and adsorbate (Ho and McKay, 1999; Plazinski et al., 2009). Similarly, other researchers (Bhattacharyya and Gupta, 2008b; Liu and Zhou, 2010; Vieira et al., 2010; Koyuncu and Kul, 2014) reported the suitability of the pseudo-second-order model compared with pseudo-first-order model for adsorption of Cu(II) and Ni(II) onto different types of clays.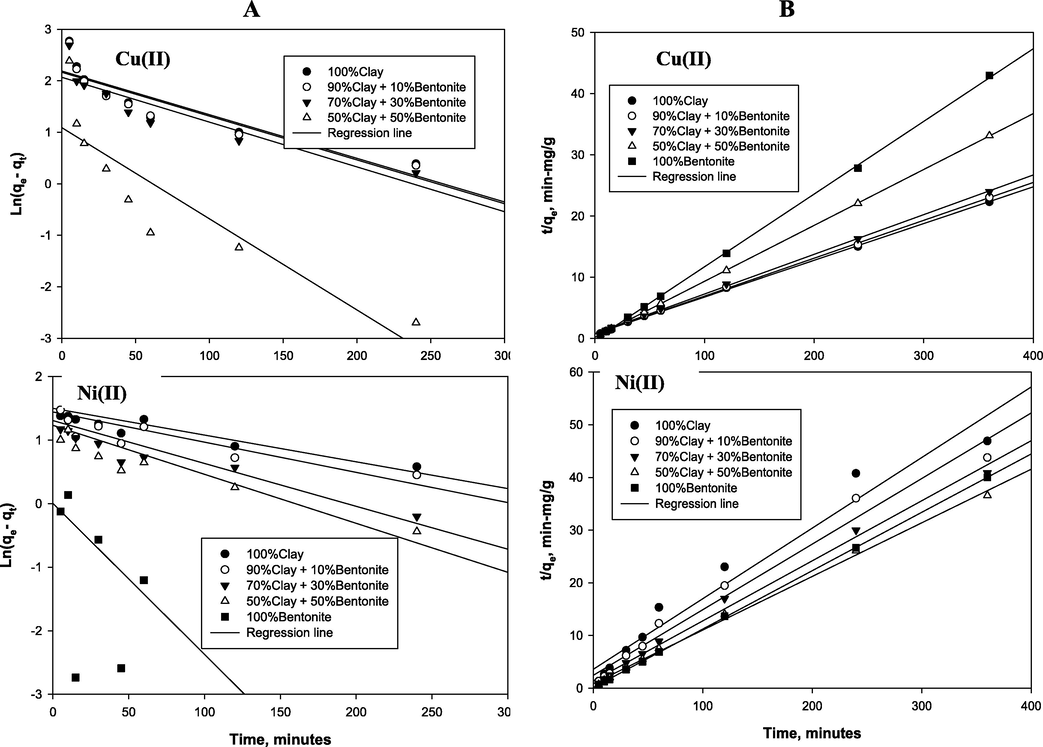
(A) Pseudo-first order and (B) Pseudo-second order for the adsorption of copper and nickel onto the different adsorbents.
Type of adsorbents
Kinetic model
Cu(II)
Ni(II)
Experimental qe (mg/g)
R2
K
Predicted qe (mg/g)
R2
K
Predicted qe (mg/g)
100% Clay
1st
0.796
0.010
7.75
0.851
0.0042
4.47
7.67
2nd
0.996
0.102
16.34
0.959
0.0452
7.33
90% Clay + 10% Bentonite
1st
0.791
0.0101
7.51
0.763
0.0042
4.13
8.22
2nd
0.996
0.103
15.97
0.977
0.061
7.9
70% Clay + 30% Bentonite
1st
0.758
0.0098
6.70
0.640
0.0044
3.36
8.81
2nd
0.996
0.112
15.01
0.993
0.0992
8.70
50% Clay + 50% Bentonite
1st
0.188
0.0083
0.42
0.538
0.0045
3.03
9.84
2nd
0.999
0.529
10.95
0.997
0.14
9.7
100% Bentonite
1st
0.0225
0.0016
1.25
0.104
0.006
2.05
9
2nd
1
3.558
8.62
0.999
0.885
9.01
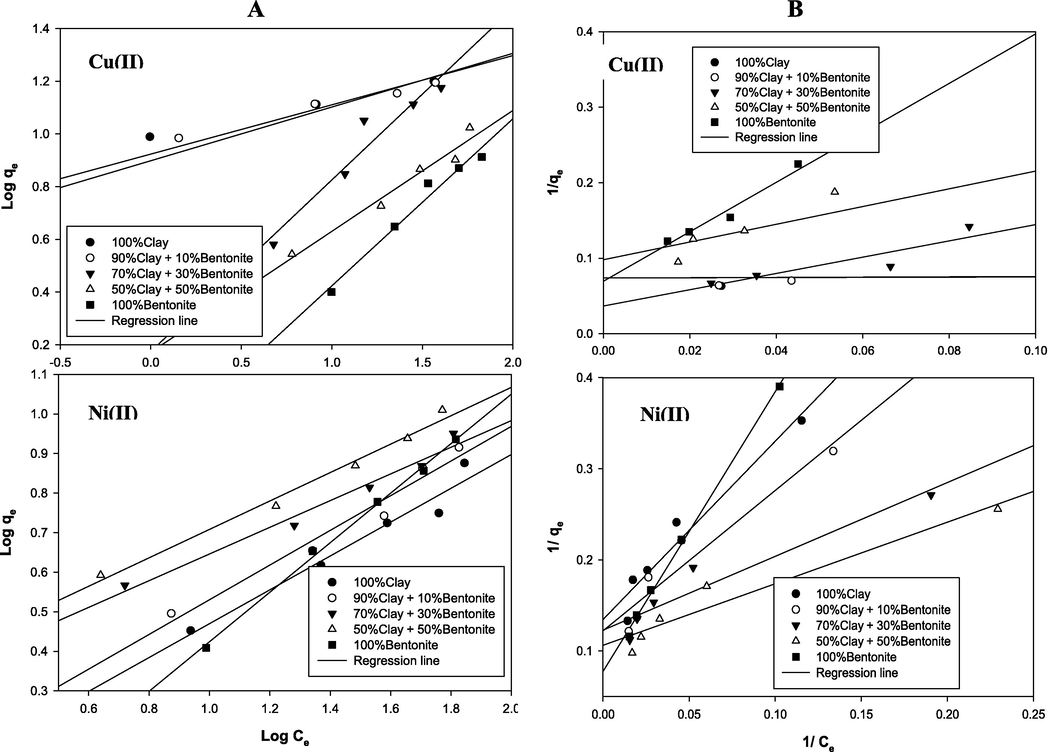
(A) Freundlich isotherm and (B) Langmuir isotherm plots for the adsorption of copper and nickel onto the different adsorbents.
3.4 Isotherm study
Adsorption isotherms are usually employed to evaluate the applicability of adsorption processes as a unit operation and to find the relation between the distributed adsorbate within the liquid phase and the solid phase at equilibrium. To study the adsorption isotherm of Cu and Ni ions on studied adsorbents, the isotherm experimental data for both ions were fitted to the linearized form of Langmuir and Freundlich models (Eqs. (6) and (7)) with the later model’s plot provided in Fig. 6A and B, respectively. According to Table 5, both the adsorption data of the Cu(II) and Ni(II) fitted both the Langmuir and Freundlich isotherm models for all the different adsorbents with high R2 of 0.941–0.996 and 0.93–0.974 for Cu(II) and of 0.957–0.997 and 0.914–0.997 for Ni(II) respectively. This could be attributed to the fact that adsorption sites can be naturally characterized by both monolayer and hetero-layer as reported by Malamis and Katsou (2013). From Table 5, the Freundlich isotherm coefficients nf ranged between 1.53–5.34 and 1.6–2.96 for Cu(II) and Ni(II), respectively. These nf values which are larger than unity indicate a decreasing in affinities with increasing in adsorption density i.e. conformity of the data to multilayer formation at the adsorbent surface (Sawyer and Parkin, 2003). Meanwhile, it can be observed that the Freundlich adsorption capacity coefficients kf has values of 1.48–8.38 and of 0.6–2.23 for Cu(II) and Ni(II), respectively. Higher kf values was obtainable for Cu(II) are than the corresponding values for Ni with the highest value (8.38 mg/g) associated with clay alone which drastically, decreased as the montmorillonite content was increased. However, in the case of Ni, increasing in the montmorillonite content slightly changed lower recorded kf values. On the other hand, the Langmuir equilibrium coefficients kL has values of 0.022–5.466 and 0.025–0.157 for Cu(II) and Ni(II), respectively. According to Vieira et al. (2010) and Gupta and Bhattacharyya (2006), this parameter determine the direction of movement the equilibrium interaction; clay (adsorbent) + metal (adsorbate) = clay-metal with higher values indicate that the equilibrium shifts to the right side resulting formation of the adsorbate–adsorbent complex. Accordingly, the kL obtained here suggest that the interaction between and the adsorbents was greater when the montmorillonite content is small with highest interaction obtainable when the absorbent was 100% clay (5.466). However, when the montmorillonite content increased above 10% in case of Cu(II) and also for all the five different adsorbents for Ni, there appeared to be very low interactions between adsorbate–adsorbent complexes as indicated by the lower values of kL. Furthermore, the separation factor, RL, for Langmuir isotherm ranged from 0.0036–0.485 and from 0.113–0.442 for Cu(II) and Ni(II), respectively. As the values of the RL were less than one, the adsorption of Cu(II) and Ni(II) on the different blends of the clay bentonite were favorable (Vieira et al., 2010).
Ni(II)
Type of adsorbents
Freundlich Isotherm
Langmuir Isotherm
R2
nf
kf
R2
qmax
kL
RL
100% Clay
0.95
5.34
8.38
0.97
13.55
5.466
0.0036
90% Clay + 10% Bentonite
0.96
4.90
7.91
0.97
13.56
3.630
0.0054
70% Clay + 30% Bentonite
0.93
1.53
1.49
0.97
27.24
0.034
0.37
50% Clay + 50% Bentonite
0.96
2.17
1.48
0.94
10.21
0.083
0.193
100% Bentonite
0.97
1.57
1.62
0.99
14.40
0.022
0.4855
Cu(II)
100% Clay
0.95
2.33
1.10
0.94
7.44
0.068
0.225
90% Clay + 10% Bentonite
0.96
2.28
1.23
0.91
8.19
0.079
0.201
70% Clay + 30% Bentonite
0.97
2.96
2.03
0.91
8.14
0.151
0.116
50% Clay + 50% Bentonite
0.98
2.78
2.23
0.93
9.42
0.157
0.113
100% Bentonite
0.99
1.59
0.62
0.99
12.9
0.025
0.442
Jarrah (2010) has shown that decrease in Si/Al ratio resulted in increase in clay minerals CEC of clays which is expected to result in higher metal ions uptake. Considering the clay possessed much lower CEC compared to the bentonite (Table 1), however, the slightly lower Si/Al ratio was attributed to the bentonite as observed earlier (Fig. 2). Thus, in this case the higher CEC of the bentonite could be attributed to its higher organic matter which was susceptible to surpass the mineralogical compositions contribution to the CEC value (Parfitt et al., 1995; Soares and Alleoni, 2008). Additionally, CEC is also known to be highly influenced by other factors such as the organic matter-clay particles interactions and physico-chemical besides the Al/Si ratio (Ma and Eggleton, 1999).
Generally, the uptake of the Cu ions was by far higher than that of Ni when the proportion of the bentonite (BET surface area of 42.13 m2/g) was low despite the more than 4 folds’ lower clay BET surface area (9.07 m2/g). Conversely, at higher bentonite ratio, all the obtained results consistently suggest slight improvement of Ni(II) uptake compared to that of Cu(II). This clearly demonstrated that the Cu ions had more attracted to the active sites of the clay rather than onto the bentonite. This observed phenomenon of Cu ions having tendency towards higher uptake onto natural clay compared Ni have been reported by many other authors (Malandrino et al., 2006; Mishra and Patel, 2009; Lukman et al., 2013; Sis and Uysal, 2014). Bhattacharyya and Gupta (2006), Gupta and Bhattacharyya (2006) observed that different types of montmorillonite (natural and modified) had better affinity towards both Cu(II) and Ni(II) compared to natural and modified kaolinite clay minerals. Thus, these results presented here show that Cu(II) and Ni(II) exhibited divergent behaviors with distinctive affinity towards the clay and bentonite surfaces, respectively. Consequently, the distinctive behaviors of uptake of Cu(II) and Ni(II) onto different adsorbents could be attributed to the differences in the dominant adsorption mechanisms as well as the characteristics and mineralogical composition of the two types of clay investigated. This suggests that proportion of different mineralogical components in clay adsorbents would differently, affect heavy metal ions removal from water (as well as other processes such as electro-kinetic remediation of soil and groundwater).
4 Conclusion
This work investigated the influence of montmorillonite content in natural Saudi Arabian clay on the adsorption of Cu(II) and Ni(II) under batch mode experiments at different initial pH, clay to bentonite ratio, initial metal ions concentration and sorbent dosage. The naturally occurring clay and bentonite minerals employed were characterized using SEM, BET, CEC, EDX and XRD techniques. The clay was composed of kaolinite and muscovite with lower quartz and calcite contents, while the bentonite was mainly montmorillonite mineral with BET surface area of 20.83 and 44.27 m2/g, respectively. An increase in the montmorillonite content in the clay resulted in decrease in Cu(II) adsorption capacity while increasing the adsorption capacity of Ni(II). The time for attainment of equilibrium increases as the montmorillonite content increases and was significantly higher for the clay compared to that of the bentonite. The maximum bentonite adsorption capacities for Cu(II) and Ni(II) were 8.33 mg/g and 8.66 mg/g, while for the clay was 16.38 mg/g and 8.21 mg/g respectively. For both metals ions and different montmorillonite content, the experimental data well fitted pseudo-second-order model (R2 of 0.96–0.999) while both Langmuir and Freundlich isotherm models described the adsorption mechanisms (R2=0.91–0.99). These results show that the two metal ions exhibited divergent behaviors for having distinctive affinities towards the clay and bentonite surfaces, respectively. This suggests that proportion of different mineralogical components used in clay adsorbents is susceptible to influence heavy metal ions removal from water (as well as other processes such as electrokinetic remediation of soil and groundwater) in different manner.
Acknowledgement
The authors acknowledge the facilities and supports provided by Civil and Environmental Engineering Department at King Fahd University of Petroleum & Minerals (KFUPM).
References
- Electrokinetic remediation: basics and technology status. J. Hazard. Mater.. 1995;40:117-137.
- [Google Scholar]
- Placer gold deposits in the Hofuf Formation The Eastern Province of Saudi Arabia. King Faisal University Faculty of Agriculture, Department of Soil and Water; 2004.
- Adsorption of Cu & Ni on bentonite clay from waste water. Athens J. Natural Formal Sci.. 2014;1:21-30.
- [Google Scholar]
- Metal concentrations in surface water and sediments from Pardo River, Brazil: human health risks. Environ. Res.. 2014;133:149-155.
- [Google Scholar]
- Adsorption of Cd(II), Cu(II), Ni(II) and Pb(II) onto natural bentonite: study in mono- and multi-metal systems. Environ. Earth Sci.. 2015;73:5435-5444.
- [Google Scholar]
- Low-cost adsorbents for heavy metals uptake from contaminated water: a review. J. Hazard. Mater.. 2003;97:219-243.
- [Google Scholar]
- Kaolinite, montmorillonite, and their modified derivatives as adsorbents for removal of Cu(II) from aqueous solution. Sep. Purif. Technol.. 2006;50:388-397.
- [Google Scholar]
- Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: a review. Adv. Colloid Interface Sci.. 2008;140:114-131.
- [Google Scholar]
- Influence of acid activation on adsorption of Ni(II) and Cu(II) on kaolinite and montmorillonite: kinetic and thermodynamic study. Chem. Eng. J.. 2008;136:1-13.
- [Google Scholar]
- Kaolinite and montmorillonite as adsorbents for Fe(III), Co(II) and Ni(II) in aqueous medium. Appl. Clay Sci.. 2008;41:1-9.
- [Google Scholar]
- Removal of heavy metals by adsorbent prepared from pyrolyzed coffee residues and clay. Sep. Purif. Technol.. 2004;35:11-22.
- [Google Scholar]
- Removal of copper from aqueous solutions by bentonites and the factors affecting it. Min. Sci. Technol. (China). 2009;19:489-492.
- [Google Scholar]
- Interlaboratory CEC and exchangeable cation study of bentonite buffer materials: I. Cu(II)-triethylenetetramine method. Clays Clay Miner.. 2012;60:162-175.
- [Google Scholar]
- Determination of sorbent point zero charge: usefulness in sorption studies. Environ. Chem. Lett.. 2009;7:79-84.
- [Google Scholar]
- Pseudo-second order model for sorption processes. Process Biochem.. 1999;34:451-465.
- [Google Scholar]
- Charge reversal of kaolinite by hydrolyzable metal ions: an electroacoustic study. Clays Clay Miner.. 1992;40:644-649.
- [Google Scholar]
- The Jabal Al Qarah Caves of the Hofuf Area, Northeastern Saudi Arabia: a geological investigation. J. Cave Karst Stud.. 2006;68:12-21.
- [Google Scholar]
- Adsorption kinetics of cadmium ions onto powdered corn cobs. Can. J. Chem. Eng.. 2009;87:896-909.
- [Google Scholar]
- Adsorption of Cu (II) and Pb (II) from aqueous solution using Jordanian natural zeolite based on factorial design methodology. Desalin. Water Treat.. 2010;16:320-328.
- [Google Scholar]
- Adsorption of Pb(II), Cd(II), Ni(II) and Cu(II) onto natural kaolinite clay. Desalination. 2010;252:33-39.
- [Google Scholar]
- An investigation of Cu(II) adsorption by native and activated bentonite: kinetic, equilibrium and thermodynamic study. J. Environ. Chem. Eng.. 2014;2:1722-1730.
- [Google Scholar]
- Electroreclamation. Applications in the Netherlands. Environ. Sci. Technol.. 1993;27:2648-2650.
- [Google Scholar]
- Adsorption of copper and nickel on Na-bentonite. Process Saf. Environ. Prot.. 2010;88:62-66.
- [Google Scholar]
- Study on Integrated Electrokinetics-Adsorption Remediation Technique for Simultaneous Removal of Heavy Metals and Organics from Saline-Sodic Soil: Effects of Operating Parameters. Saudi Arabia: King Fahd University of Petroleum and Minerals; 2013. p. :237.
- Adsorption and desorption of heavy metals onto natural clay material: influence of initial pH. J. Environ. Sci. Technol.. 2013;6:1.
- [Google Scholar]
- A review on zinc and nickel adsorption on natural and modified zeolite, bentonite and vermiculite: examination of process parameters, kinetics and isotherms. J. Hazard. Mater.. 2013;252–253:428-461.
- [Google Scholar]
- Adsorption of heavy metals on vermiculite: influence of pH and organic ligands. J. Colloid Interface Sci.. 2006;299:537-546.
- [Google Scholar]
- Removal of lead and zinc ions from water by low cost adsorbents. J. Hazard. Mater.. 2009;168:319-325.
- [Google Scholar]
- Evaluation of the influence of clay montmorillonite content on the aqueous uptake of lead and zinc. Water Environ. Res.. 2018;90
- [CrossRef] [Google Scholar]
- Augmenting granular activated carbon with natural clay for multicomponent sorption of heavy metals from aqueous solutions. Water Sci. Technol.. 2017;76(8):2213-2221.
- [Google Scholar]
- Pulsed electrokinetic removal of chromium, mercury and cadmium from contaminated mixed clay soils. Soil Sediment Contam.: An Int. J.. 2016;25:757-775.
- [Google Scholar]
- Removal of phenolic compounds from water using sewage sludge-based activated carbon adsorption: a review. Int. J. Environ. Res. Public Health. 2017;14:1094.
- [Google Scholar]
- Precipitation of amorphous SiO2 particles and their properties. Braz. J. Chem. Eng.. 2011;28:89-94.
- [Google Scholar]
- Heavy metals, occurrence and toxicity for plants: a review. Environ. Chem. Lett.. 2010;8:199-216.
- [Google Scholar]
- The Chemistry Behind the Use of Agricultural Biomass as Sorbent for Toxic Metal Ions: pH Influence, Binding Groups, and Complexation Equilibria. INTECH Open Access Publisher; 2011.
- Adsorptive removal of anionic dye from aqueous solutions by mixture of Kaolin and Bentonite clay: Characteristics, isotherm, kinetic and thermodynamic studies. Iranica J. Energy Environ.. 2015;6:147-153.
- [Google Scholar]
- Clay–iron oxide magnetic composites for the adsorption of contaminants in water. Appl. Clay Sci.. 2003;22:169-177.
- [Google Scholar]
- Binary adsorption of heavy metals from aqueous solution onto natural clays. Chem. Eng. J.. 2013;225:535-546.
- [Google Scholar]
- Biosorption of nickel(II) ions on Baker's yeast. Process Biochem.. 2003;38:1389-1395.
- [Google Scholar]
- Contribution of organic matter and clay minerals to the cation exchange capacity of soils. Commun. Soil Sci. Plant Anal.. 1995;26:1343-1355.
- [Google Scholar]
- Theoretical models of sorption kinetics including a surface reaction mechanism: a review. Adv. Colloid Interface Sci.. 2009;152:2-13.
- [Google Scholar]
- Sorption of pesticides on kaolinite and montmorillonite as a function of hydrophilicity. J. Environ. Sci. Health, Part B. 2006;41:333-344.
- [Google Scholar]
- Effects of soil composition on the removal of chromium by electrokinetics. J. Hazard. Mater. Electrochem. Decontamination Soil Water. 1997;55:135-158.
- [Google Scholar]
- Measurement of exchangeable cations in bentonites. Clays Clay Miner.. 1968;16:165-172.
- [Google Scholar]
- Determination of the Cation Exchange Capacity (CEC) of clay minerals using the complexes of Copper(II) ion with triethylenetetramine and tetraethylenepentamine. Clays Clay Miner.. 1999;47:386-388.
- [Google Scholar]
- Chemistry for Environmental Engineering and Science. New York: McGraw-Hill; 2003.
- Size and shape of fine quartz in the clay fraction of soils and geological materials. Zeitschrift für Pflanzenernährung und Bodenkunde. 1979;142:865-873.
- [Google Scholar]
- Immobilization of Pb(II), Cd(II) and Ni(II) ions on kaolinite and montmorillonite surfaces from aqueous medium. J. Environ. Manage.. 2008;87:46-58.
- [Google Scholar]
- Kinetics of adsorption of metal ions on inorganic materials: a review. Adv. Colloid Interface Sci.. 2011;162:39-58.
- [Google Scholar]
- Removal of heavy metal ions from aqueous medium using Kuluncak (Malatya) vermiculites and effect of precipitation on removal. Appl. Clay Sci.. 2014;95:1-8.
- [Google Scholar]
- Contribution of soil organic carbon to the ion exchange capacity of tropical soils. J. Sustainable Agric.. 2008;32:439-462.
- [Google Scholar]
- Comparative sorption of methylene blue onto hydrophobic clays. Environments. 2015;2:388-398.
- [Google Scholar]
- Alginate-immobilized bentonite clay: adsorption efficacy and reusability for Cu(II) removal from aqueous solution. Bioresour. Technol.. 2014;160:115-118.
- [Google Scholar]
- USEPA, 1986. Cations-Exchange Capacity of Soils (Sodium Acetate). 7-10.
- Sorption kinetics and equilibrium for the removal of nickel ions from aqueous phase on calcined Bofe bentonite clay. J. Hazard. Mater.. 2010;177:362-371.
- [Google Scholar]
- Electrokinetic soil remediation – critical overview. Sci. Total Environ.. 2002;289:97-121.
- [Google Scholar]
- Wongkoblap, A., Ngernyen, Y., Budsaereechai, S., Charoenbood, A., 2013. Heavy metal removal from aqueous solution by using bentonite clay and activated carbon. Chemeca 2013: Challenging Tomorrow, 689.
- Applying zeta potential measurements to characterize the adsorption on montmorillonite of organic cations as monomers, micelles, or polymers. J. Colloid Interface Sci.. 2010;352:171-177.
- [Google Scholar]
- Adsorption of Pb (II) from aqueous solution using natural clay. Ier-Institute.Org. 2012;6:55-60.
- [Google Scholar]
- Adsorption of eriochrome black T from aqueous phase on MgAl-, CoAl- and NiFe- calcined layered double hydroxides: Kinetic, equilibrium and thermodynamic studies. J. Mol. Liq.. 2017;230:344-352.
- [Google Scholar]







