Translate this page into:
Effect of method and time of extraction on total phenolic content in comparison with antioxidant activities in different parts of Achyranthes aspera
*Corresponding author. Tel.: +91 831 2475477/78; fax: +91 831 2475479 drpaisr@gmail.com (Sandeep R. Pai)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 25 April 2015
Peer review under responsibility of King Saud University.
Abstract
The main purpose of this study was to evaluate the effect of extraction method with respect to time of exposure on total phenolic content and antioxidant potential of methanolic extracts (95%) from Achyranthes aspera leaves, stem and roots. Total phenolic content (TPC) was determined using the Folin–Ciocalteu method and antioxidant potential was tested using DPPH radical scavenging and FRAP assays. Plant populations aged 60 and 120 days were considered during the study. Overall, highest TPC was evident in mature plants (120 days) compared to younger (60 days). Similarly, leaves accumulated higher phenolics followed by roots and stem. Results showed the MAE technique to be efficient over USE and CSE methods. Same was evident in both the antioxidant assays tested. A similar trend was observed in both antioxidant assays as that of TPC, indicating phenolics to be the major contributor in the antioxidant potential of the plant. In conclusion it can be said that the yield of phenolic compounds depends on parameters viz. age of plant, part used for extraction, method of extraction and time required for the same.
Keywords
Achyranthes aspera
Continuous shaking
Ultrasonic
Microwave
Total phenolics
Antioxidant
- TAE
-
tannic acid equivalent
- CAE
-
caffeic acid equivalent
- TPC
-
total phenolic content
- AEAC
-
ascorbic acid equivalent antioxidant capacity
- TEAE
-
trolox equivalent antioxidant capacity
- CSE
-
continuous shaking extraction
- MAE
-
microwave assisted extraction
- USE
-
ultra sonic extraction
Abbreviations
1 Introduction
Achyranthes aspera L. commonly known as devils horse whip, is a weed belonging to family Amaranthaceae, widely distributed throughout tropical and warmer parts of the world (Hooker, 1885). It has spread in many countries of Asia, Africa, America, Europe and Australia (Prain, 1963; deLange et al., 2004). It has been listed as a noxious weed in many countries including South Africa (Henderson, 2001). Although listed as a weed in India too, it has been identified among the 46 high volume traded medicinal species by Ved and Goraya (2007).
The plant individually and in combination is widely used in traditional medicine all over the world (Pai et al., 2010, 2015; Fikru et al., 2012; Shibeshi et al., 2006). It is used as an anti-inflammatory and in anti-arthritic treatment (Girach and Khan, 1992; Gokhale et al., 2002); as an abortifacient (Pakrashi and Bhattacharya, 1977). It has been tested as an antifertility agent (Prakash, 1986; Varshney et al., 1986; Wadhwa et al., 1986), and also for its anti-cancer activity (Chakraborty et al., 2002). Barua and co-workers (2012) have examined it for healing wounds and burns, while its antimicrobial potential is reported by many others (Sharma et al., 2011; Raman et al., 1996). It has also been investigated for energy production (Subramanian and Sampathrajan, 1999). The plant has also been reported to enhance the immunity of fish in aquaculture (Kaleeswaran et al., 2012).
The medicinal properties of the plants are mainly attributed to their phytochemical constituents such as polyphenols, saponins, alkaloids etc. (Dinda et al., 2007a,b; Francis et al., 2002; Podsędek, 2007). These phytochemicals are responsible for the magnitude of biological effects, including antioxidant, antimicrobial and anti-cancer activities. Extraction is an important rate limiting step to attain optimum yield of any compound. Two major parameters affecting content yield of analytes, includes method of extraction and time required for it. There are a number of methods identified for extraction of plant based activities and metabolites (Pai et al., 2011a,b, 2015; Murugan and Parimelazhagan, 2014; Nimbalkar et al., 2012; Patil et al., 2012; Pawar et al., 2011).
As there are no such studies carried out in A. aspera and owing to its medicinal importance the present work was undertaken. Thus the study herein evaluates total phenolic content with antioxidant potential of A. aspera on the basis of age of plant; parts (leaves, stem roots) and different extraction methods with various times of exposures.
2 Materials and methods
2.1 Chemicals
Trolox, caffeic acid (3-(3,4-dihydroxyphenyl)-2-propenoic acid) were procured from Sigma Aldrich, India. Folin and Ciocalteu phenol reagent, sodium acetate trihydrate, sodium hydrogen carbonate, tannic acid, ascorbic acid, 2,2-diphenyl-1-picryl hydrazyl (DPPH), 2,4,6-Tris(2-pyridyl)-s-triazine (TPTZ), and ferric chloride were obtained from Hi media, India. Solvents like methanol, glacial acetic acid, and hydrochloric acid were from Qualigens, India. All chemicals used in the study were of analytical grade.
2.2 Sample preparation
Leaves, stem and roots of A. aspera were obtained from a single population from the north central corridor of the Western Ghats from Belgaum district in Karnataka state, India (GPS: N 15.88°; E 74.52°, 801 M above MSL). Plants were collected at 60 days and 120 days interval after germination. A specimen was authenticated and deposited at the Herbarium, Regional Medical Research Centre, Belgaum (Voucher Number: RMRC 1250). The material obtained was dried separately at 40 ± 5 °C for 48 h, finely powdered and sieved through a 20 μm stainless sieve and used for extraction.
2.3 Efficiency of extraction method
The extraction methods earlier described by Pai et al. (2011a) for the extraction of betulinic acid from Ancistrocladus heyneanus, with minor modifications were employed during the study.
2.3.1 Continuous shaking extraction (CSE)
Continuous shaking extractions were carried out by subjecting 5 g of fresh plant material (leaves, stem and roots separately) into a 150 mL conical flask. The flask was subjected with 100 mL of 95% methanol and placed on an orbital shaker (Rivotek, Riviera, India). A constant stirring of 110 ± 2 rpm was maintained for the suspensions at a controlled temperature (25 ± 5 °C). Plant materials were extracted with 95% MeOH for 30, 180 and 360 min separately.
2.3.2 Ultrasonic extraction (USE)
Ultrasonic extractions were performed by subjecting 1 g of plant material with 20 mL 95% methanol in a 150 mL beaker on an ultrasonic bath (Soncis vibracell) at a working amplitude of 60 Hz. Samples were prepared as above and were exposed to sonication for 5, 15 and 30 min at room temperature.
2.3.3 Microwave assisted extraction (MAE)
Leaves, stem and roots (1 g) were individually put into 150 mL Erlenmeyer flasks and were added with 20 mL 95% methanol. All flasks with suspension were exposed for 1, 3 and 5 min in a microwave oven (Godrej, GMX 30GAI SIM, India) at 180 W. The suspensions were cooled at regular intervals to avoid bumping of the solvent out of the flask. The above steps were repeated in order to complete the required time of microwave irradiation. The extracts were then filtered and the volume was made up to 20 mL with the solvent.
All extracts were filtered through filter paper (Whatman No. 1) re-volumised (to obtain 5% concentration) and the filtrates were used for further analysis.
2.4 Total phenolic content (TPC)
Total phenolic content was quantified using the modified Folin–Ciocalteu method previously described by Wolfe et al. (2003). The absorbance of blue color was read at 760 nm using distilled water instead of standards in the reaction mixture as blank on a double beam spectrophotometer (Thermo Scientific, multiskan Go 1510, USA). Similarly, extracts prepared (5%) were also quantified and the results were compared to the standard curves and expressed as mg/tannic or caffeic acid equivalent per gram dry powder for the samples.
2.5 Antioxidant activities
2.5.1 DPPH radical scavenging assay
Antioxidant activities were determined for the plant extracts as a measure of radical scavenging, using the DPPH assay determined by Brand-Williams et al. (1995). Various concentrations of ascorbic acid and/or Trolox were used instead of the plant extract as reference standard during the experiment. The inhibition percentage of DPPH (% DPPH) was calculated and the results were expressed as % RSA as described by Pawar et al. (2011).
2.5.2 Ferric Reducing Antioxidant Power (FRAP) assay
Antioxidant activity assays were performed by the method described by Benzie and Strain (1996). In the FRAP assay, reductants (antioxidants) in the sample reduce Fe3+/tripyridyltriazine with an increase in absorbance at 593 nm. Results were expressed as μM ascorbic acid and or Trolox equivalent antioxidant capacity (AEAC/TEAC).
2.6 Statistical analysis
All results were expressed as mean ± SD for three replications. Statistical analysis of data was performed by analysis of variance and tested for significance by the Duncan test, which allowed a multiple comparison among the data to individualize the significant differences. Differences were considered significant if p < 0.05. At least three independent analyses were carried out per sample to compute it on IBM, SPSS Statistics, Ver. 22 software.
3 Results and discussion
Preliminary experiments were set to assess the effect of age of plant, plant part, method of extraction and time required for extraction on productivity of total phenolics and correspondingly antioxidant activity. In all, 18 samples were analysed during the study. Fig. 1, represents TPC (mg/g) obtained using 3 plant parts extracted by 3 extraction methods and 3 different exposure times for each method. The amount of total phenolics varied in the samples and ranged from 0.39 ± 0.02 to 5.26 ± 0.26 mg/g for TAE and from 0.28 ± 0.02 to 4.06 ± 0.20 mg/g for CAE.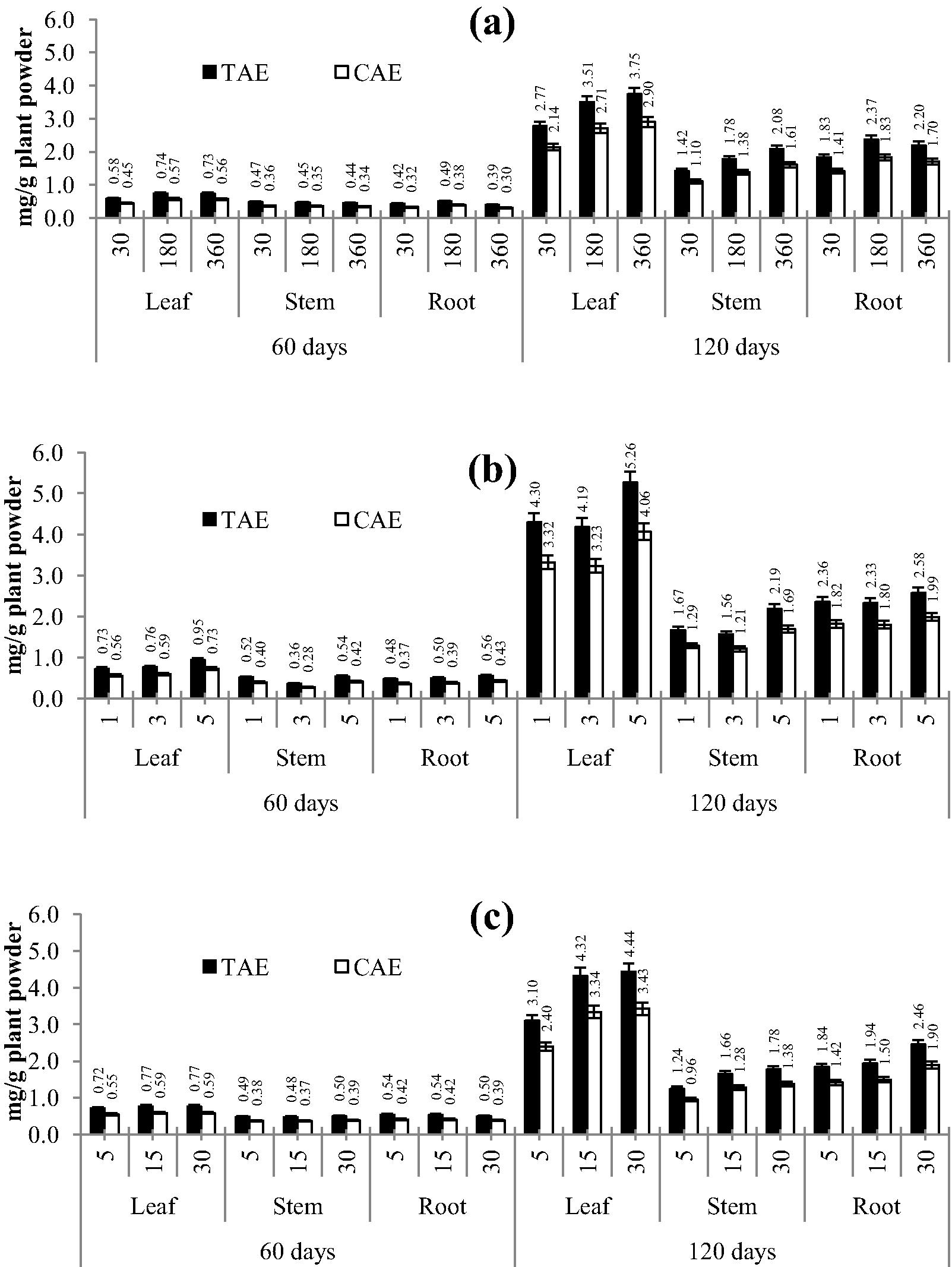
Effect of various time periods with respect to extraction methods on the yield of total phenolics in leaves, stem and roots of Achyranthes aspera; a: CSE; b: MAE; c: USE, figures on x axis are time in minutes.
The extraction conditions certainly affected extraction productivity of TPC. Though specifically it was observed that, age of the plant followed by plant part and method of extraction were important factors in optimization, time of extraction least affected variation of yield (Fig. 1). A relation between time of extraction and TPC was established, wherein increase in time of exposure showed highest TPC. Leaves of a 120 day old plant extracted using the MAE method for 5 min yielded the highest (5.26 ± 0.26 mg TAE/g and 4.06 ± 0.21 mg CAE/g) TPC compared to any other method and parts in the study (Fig. 1). There was not much difference in the content of TPC in 60 day old plants. It is evident from the present investigation that leaves are a better source of phenolics as compared to stem and roots.
Tannic and caffeic acids were used as reference standards during the study for TPC. Calibration curves for tannic and caffeic acids yielded equations with coefficient of determination (R2) not below 0.960 (Table 1). The slope obtained for the tannic acid curve was higher than caffeic acid and this may be due to the higher reducing power corresponding to the higher absorbance obtained at similar concentrations. Thus in all methods tested, the TAE yield was higher than CAE and the percent difference between both (TAE and CAE) were the same. The absorbance could be related to the differences in chemical structure of the two acids, where tannic acid is a hydrolysable tannin while caffeic acid is a hydroxycinnamic acid (Mujica et al., 2009). where y is the absorbance at 760 nm for TPC and 593 nm for the FRAP assay, and x is the respective concentration of tannic acid, caffeic acid, ascorbic acid and Trolox used during the study.
Parameter
Total phenolic content
FRAP antioxidant assay
Tannic acid
Caffeic acid
Ascorbic acid
Trolox
Concentrations
10–800 μg/mL
10–800 μg/mL
10–1000 μM
10–1000 μM
Calibration points
8
8
10
10
Linearity equation
y = 0.003x + 0.115
y = 0.004x + 0.092
y = 0.0004x + 0.1144
y = 0.0005x + 0.1521
R2
0.968
0.960
0.975
0.919
Figs. 2 and 3 depict DPPH and FRAP antioxidant activity for different extraction methods with different time intervals using leaves, stem and roots of A. aspera. Methanolic extracts of leaves and roots depicted better activity than stem extracts. Higher scavenging activity of the DPPH radical was observed for leaf material, extracted using the MAE method. It was evident that time of exposure of any method enhanced the extraction efficiency. The same observation was obtained for the extracts towards the FRAP assay, where the methanolic extracts of leaves and roots of 120 day old plants had higher activity. Furthermore, it was interesting to note that 60 day old plants in the FRAP assay had higher activity in comparison to that of the DPPH assay. Also the DPPH radical scavenging and FRAP response of extracts were comparable to those of standards/controls (ascorbic acid and Trolox). These findings were in agreement with the previous results demonstrating the antioxidant activity of phenolic compounds (Patil et al., 2012; Upadhya et al., 2013; Subramanya et al., 2015).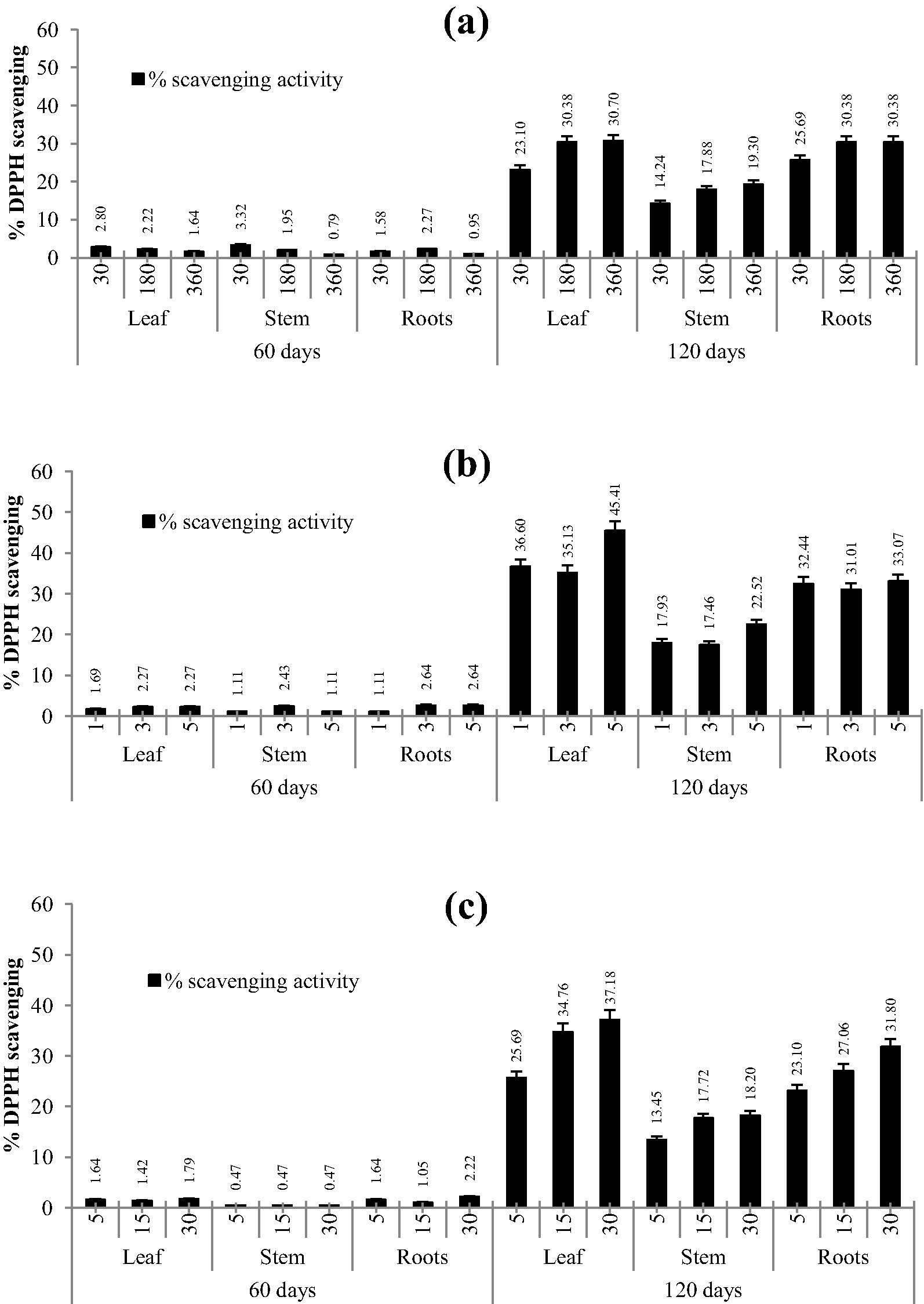
Effect of various time periods with respect to extraction methods on antioxidant activity determined using the DPPH radical scavenging assay in leaves, stem and roots of Achyranthes aspera; a: CSE; b: MAE; c: USE, figures on x axis are time in minutes.
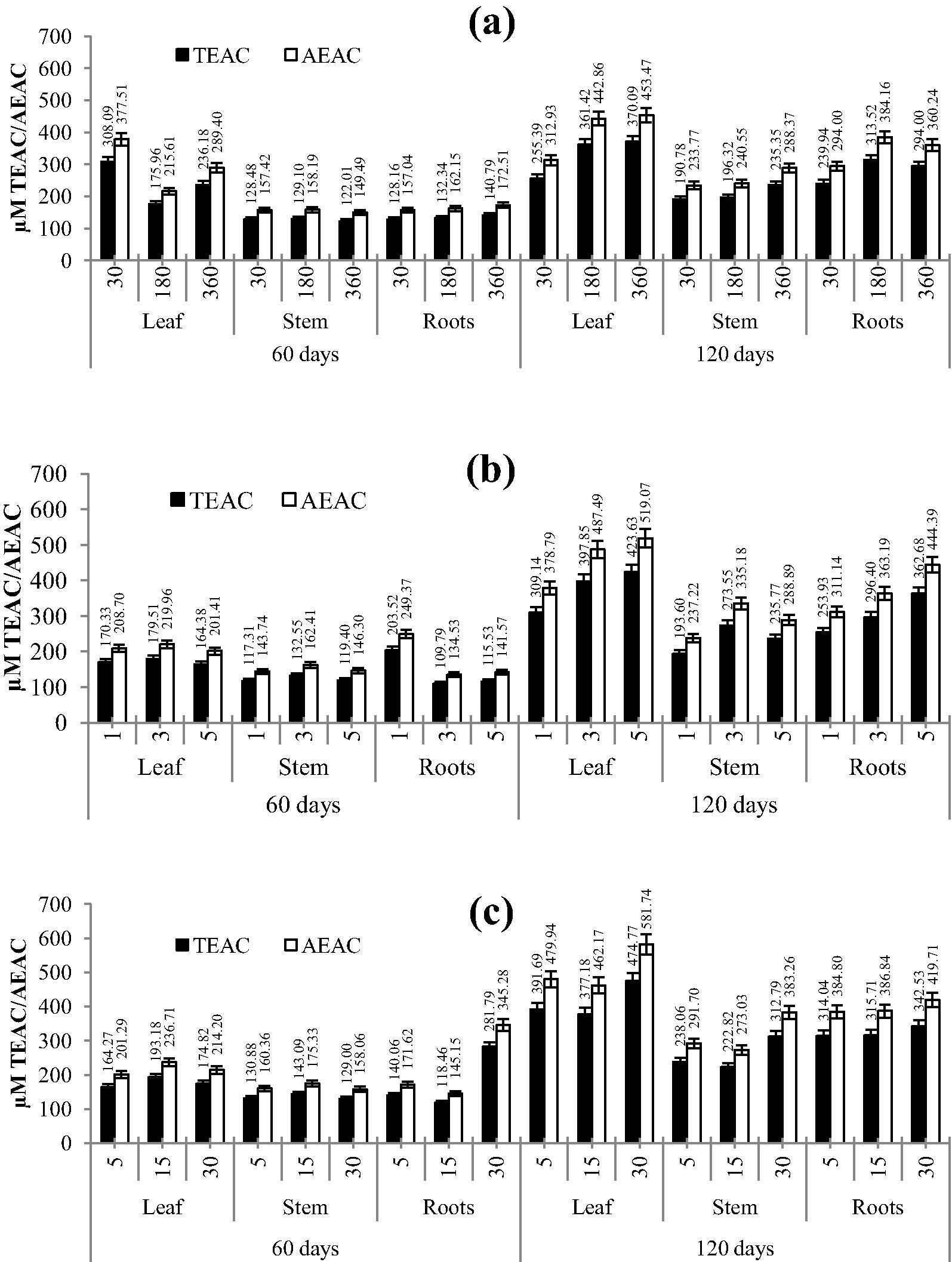
Effect of various time periods with respect to extraction methods on antioxidant activity determined using the FRAP assay in leaves, stem and roots of Achyranthes aspera; a: CSE; b: MAE; c: USE, figures on x axis are time in minutes.
Thus the study highlights the optimization of the extraction method along with the age and plant parts to be used for obtaining a higher total phenolic content with higher antioxidant activity in A. aspera.
4 Conclusions
Conclusively, yield of phenolic compounds depends on all the parameters viz. age of plant, part of plant used for extraction, method of extraction and time required for the same. However, the order of magnitude in the yield may vary with respect to even a slight change in these parameters. Accordingly it is essential to regulate these factors and in turn to understand the correct method to attain greater accuracy in the results. Results of the present investigation depict that 5 min of CSE with 120 day old plant leaves, were found to be optimum for TPC and antioxidant activities. The result also opens an avenue for further research to study such extraction based activities in the plant.
Acknowledgements
All the authors are indebted to the Director-in-Charge, Regional Medical Research Centre, Belgaum and the Indian Council of Medical Research (ICMR), New Delhi. S.R.P. is thankful to the ICMR, New Delhi, India for providing ICMR-PDF grants during the study (Grant No. 3/1/3/PDF-2010/HRD-01) and also to SERB, DST, New Delhi, India, for further financial assistance (Grant No. SB/YS/LS-71/2013).
References
- In vivo wound-healing efficacy and antioxidant activity of Achyranthes aspera in experimental burns. Pharm. Biol.. 2012;50:892-899.
- [Google Scholar]
- The ferric reducing ability of plasma (FRAP) as a measure of Antioxidant Power’: The FRAP assay. Ann. Biochem.. 1996;239:70-76.
- [Google Scholar]
- Use of a free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol.. 1995;28:25-30.
- [Google Scholar]
- Cancer chemopreventive activity of Achyranthes aspera leaves on Epstein-Barr virus activation and two-stage mouse skin carcinogenesis. Cancer Lett.. 2002;177:1-5.
- [Google Scholar]
- Achyranthes aspera (Amaranthaceae), a new indigenous addition to the flora of the Kermadec Islands group. N. Z. J. Bot.. 2004;42:167-173.
- [Google Scholar]
- Naturally occurring iridoids. A review, part 1. Chem. Pharm. Bull.. 2007;55:159-222.
- [Google Scholar]
- Naturally occurring secoiridoids and bioactivity of naturally occurring iridoids and secoiridoids. A review, part 2. Chem. Pharm. Bull.. 2007;55:689-728.
- [Google Scholar]
- Evaluation of in vivo wound healing activity of methanol extract of Achyranthes aspera L. J. Ethnopharmacol.. 2012;143:469-474.
- [Google Scholar]
- The biological action of saponins in animal systems: a review. Br. J. Nutr.. 2002;88:587-605.
- [Google Scholar]
- Ethnomedicinal uses of Achyranthes aspera L. in Orissa, India. Int. J. Pharmacogn.. 1992;30:113-115.
- [Google Scholar]
- Preliminary evaluation of anti-inflammatory and anti-arthritic activity of S. lappa, A. speciosa and A. aspera. Phytomedicine. 2002;9:433-437.
- [Google Scholar]
- Henderson, L., 2001. Alien Weeds and Invasive Plants. A Complete Guide to Declared Weeds and Invaders in South Africa. Plant Protection Research Institute Handbook No. 12, 300pp. PPR, ARC South Africa.
- The Flora of British India. L. Reeve & Co. Ltd, Kent; 1885. p. :713-730.
- Changes in biochemical, histological and specific immune parameters in Catla catla (Ham.) by Cynodon dactylon (L.) J. King Saud Univ. Sci.. 2012;24:139-152.
- [Google Scholar]
- Importance of the extraction method in the quantification of total phenolic compounds in Phaseolus vulgaris L. Interciencia. 2009;34:650-654.
- [Google Scholar]
- Comparative evaluation of different extraction methods for antioxidant and anti-inflammatory properties from Osbeckia parvifolia Arn. – an in vitro approach. J. King Saud Univ. Sci.. 2014;26:267-275.
- [Google Scholar]
- Free amino acid profiling in grain amaranth using LC–MS/MS. Food Chem.. 2012;134:2565-2569.
- [Google Scholar]
- Optimization of extraction techniques and quantification of betulinic acid (BA) by RP-HPLC method from Ancistrocladus heyneanus Wall. Ex Grah. Ind. Crops Prod.. 2011;34:1458-1464.
- [Google Scholar]
- Seasonal discrepancy in phenolic content and antioxidant properties from bark of Nothapodytes nimmoniana (Grah.) Mabb. Int. J. Pharm. Biol. Sci.. 2010;1:1-17.
- [Google Scholar]
- Pai, S.R., Upadhya, V., Hegde, H.V., Joshi, R.K., Kholkute, S.D., 2015. Determination of betulinic acid, oleanolic acid and ursolic acid from Achyranthes aspera using RP-UFLC-DAD analysis and evaluation of various parameters for their optimum yield. Indian J. Exp. Biol., in press.
- Achyranthes coynei Santapau (Amaranthaceae) – an addition of endemic taxon to Flora of Karnataka, India. J. Threatened Taxa. 2011;3:1875-1879.
- [Google Scholar]
- Abortifacient principle of Achyranthes aspera Linn. Indian J. Exp. Biol.. 1977;15:856-858.
- [Google Scholar]
- Chemical characterization, mineral analysis, and antioxidant potential of two underutilized berries (Carissa carandus and Eleagnus conferta) from the Western ghats of India. Crit. Rev. Food Sci. Nutr.. 2012;52:312-320.
- [Google Scholar]
- RP-HPLC analysis of phenolic antioxidant compound 6-gingerol from different ginger cultivars. Food Chem.. 2011;126:1330-1336.
- [Google Scholar]
- Natural antioxidants and antioxidant activity of Brassica vegetables: a review. LWT – Food Sci. Technol.. 2007;40:1-11.
- [Google Scholar]
- Bengal Plants. Calcutta: Botanical Survey of India; 1963. p. :646-655.
- Potentialities of some indigenous plants for antifertility activity. Int. J. Crude Drug Res.. 1986;24:19-24.
- [Google Scholar]
- Studies on the antibacterial properties of Achyranthes aspera stems. Fitoterapia. 1996;67:92-93.
- [Google Scholar]
- Antifungal potential of Achyranthes aspera Linn. collected from Himachal Pradesh, Punjab and Haryana region. J. Pure Appl. Microbiol.. 2011;5:971-976.
- [Google Scholar]
- Effect of Achyranthes aspera L. on fetal abortion, uterine and pituitary weights, serum lipids and hormones. Afr. Health Sci.. 2006;6:108-112.
- [Google Scholar]
- Physical and chemical characterisation of selected weed species for energy production. Biores. Technol.. 1999;70:51-54.
- [Google Scholar]
- Total polyphenolic contents and in vitro antioxidant activities of eight Sida species from Western Ghats, India. J. Ayurveda Integr. Med.. 2015;6:24-28.
- [Google Scholar]
- Phenolic contents and antioxidant properties from aerial parts of Achyranthes coynei Sant. Indian J. Pharm. Sci.. 2013;75:385-500.
- [Google Scholar]
- Antifertility screening of plants Part II. Effect of ten indigenous plants on early and late pregnancy in albino rats. Comp. Physiol. Ecol.. 1986;11:183-189.
- [Google Scholar]
- Demand and Supply of Medicinal Plants in India. Bangalore, India: NMPB, New Delhi & FRLHT; 2007.
- Contraceptive and hormonal properties of Achyranthes aspera in rats and hamsters. Planta Med.. 1986;3:231-233.
- [Google Scholar]







