Translate this page into:
Effect of hemodynamic characteristic changes of the carotid artery on 6-OHDA-induced Parkinson’s disease model rats treated by Gut-acupuncture
⁎Corresponding author. 13656632162@163.com (Li-hong Li)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Background
Based on “gut-brain axis” theory, we employed a rat 6-OHDA lesion model to examine the impact of gut-acupuncture on motor symptoms, the abundance of DA neurons in the SN, the hemodynamic characteristics of the carotid artery and SNH.
Methods
6-OHDA-induced PD rats were treated with gut-acupuncture daily, starting day 15 after surgery. Mobility of the limbs was evaluated, abundance of TH + DA neurons in the SN detected, The diameter of carotid arteries and hemodynamic parameters measured by cervical vascular ultrasound CVUS, and TCS utilized to examine SNH.
Results
Our data show that gut-acupuncture correlates with significantly increased abundance of TH, a marker of DA neurons compared to untreated rats. Furthermore the area of SNH in the injured side of the acupuncture group was significantly reduced. PSV of LCCA, LICA, RICA showed that were significantly improved i.e. decreased in the acupuncture group, while the diameter of LICA and RCCA in acupuncture group was narrower. We also found that the PSV was significantly increased and the vascular diameter narrowed in LCCA and LICA during treatment, whereas after removing the acupuncture needle the PSV decreased and the vascular diameter widened.
Conclusions
Gut-acupuncture can reduce SNH and influence TH abundance in the SN which correlated with changes of hemodynamic characteristics of the lesioned side. We suggest a regulatory mechanism which may affect the vagus nerve through the ENS and cause the change of cervical hemodynamics. It further induces low oxygen tension microenvironment conducive to the proliferation of neural stem cells, which leads to the enrichment of TH in PD model rats of acupuncture group.
Keywords
Parkinson’s disease (PD)
Gut-acupuncture
Substantia nigra hyperechogenicity (SNH)
Cervical vascular ultrasound (CVUS)
Hemodynamics
- TH
Tyrosine hydroxylase
- IHC
immunohistochemistry
- SN
substantia nigra
- SNc
substantia nigral pars compacta
- DA
dopamine, MFB, medial forebrain bundle
- GI
gastrointestinal
- TCS
transcranial sonography
- PSV
peak systolic velocity
- VD
vascular diameter
- CNS
central nervous system
- ENS
enteric nervous system
- SNH
substantia nigra hyperechogenicity
Abbreviations
1 Introduction
Parkinson’s Disease (PD), is the most common motor related disorders up to date (Poewe et al., 2017). Globally affecting 2990 per 100,000 individuals over the age of 70, its prevalence increasing with age (Pringsheimet al., 2014). The typical pathological process is the progressive loss of TH+ DA neurons in the SN region of the midbrain (Goswami et al., 2017). Since the discovery of levodopa half a century ago, which may cause a “honeymoon period” as well as side effects, there has been no further progress in treatment. Recent studies have reported that acupuncture is the most commonly used complementary and alternative therapy (CAM) for a large number of PD patients besides standard treatment (Zeng and Zhao, 2016). Acupuncture can increase the amount of DA in the striatum and the expression of TH, a key enzyme in dopamine synthesis (Kim et al., 2014).
The bidirectional endocrine communication and immunological regulation system between the CNS and ENS is designated the “gut-brain”axis (Mulak and Bonaz, 2015). ENS is a comprehensive network of gastrointestinal parietal neurons and a major participant in the “gut-brain “axis (Dinan and Cryan, 2015). In recent years, the development of PD research has been focused on the gastrointestinal tract and the related ENS (Clairembault et al., 2014). Combining findings from gut-brain axis studies about early warning gastrointestinal (GI) symptoms in PD and the course of the “stomach meridian” in Huangdi Neijing, we employed a 6-OHDA rat model, to elucidate the mechanisms of acupuncture – mediated amelioration of PD symptoms.
2 Material and methods
2.1 Rats
Male Sprague-Dawley (SD) rats were provided by the Zhejiang Medical Laboratory Animal Center, and were fed under standard environmental conditions (temperature 22 ± 1° C, humidity 60 ± 5%, 12 h light: 12 h dark cycle), with free access to water and food. Animals were subjected to experiments after 7 days of adaption. All experimental procedures strictly complied with the regulations of Zhejiang University of traditional Chinese Medicine on the management and protection of experimental animals.
2.2 Experimental animal groups
According to the success rate (80%) of the 6-OHDA lesion PD model, 40 8–10 week old rats (240 ± 20 g) were divided the following way: normal group (n = 8), sham group (n = 8) and 6-OHDA-group (n = 24). In 19 out of 24 rats the 6-OHDA PD model was successfully established and those rats were further sub-divided into model group (n = 8) and acupuncture group (n = 8). Rats were distributed into groups according to random number tables and even distribution of similar average body weight in each group.
2.3 6-OHDA lesion PD model
6-OHDA lesions were applied as described elsewhere [Include citation of Fabrizius et al]. Briefly, 8–10 weeks old rats with a weight of 220–260 g were starved overnight and anesthetized by injection of 0.3% pentobarbital sodium (Shanghai Rongbai Biotechnology Co., Ltd. Cargo number P3761) (i.p.) at 150 mg/kg. During surgery rats were placed on a 36–37 °C heating pad in a stereoscopic locator (below the horizontal coordinate sinus calibrator) then 4 µg (Concentration 4ug/ul) of 6-OHDA (Sigma-Aldrich-DK, added with 0.02% ascorbic acid solution) was injected at a rate of 1 μl/min into each of the following two points, according to the rat brain in stereotaxic coordinates (Paxinos and Watson): first the left MFB: TB, 3.4 mm; AP,-4.0 mm; ML, 0.8 mm; DV, 8.0 mm, second the left SNc: TB,-2.3 mm; AP,-5.2 mm; ML,2.1 mm; DV,-7.8 mm. All animals were closely monitored during surgery. The sham group was injected with 0.9% NaCl and otherwise treated the same. To test the onset of the 6-OHDA lesion model, rotation behavior was observed on the 15th day after surgery. Animals with cycle numbers higher than 7 were regarded as successfully treated. Weight was measured weekly starting day 15 after surgery. Rats were sacrificed on day 45 post-surgery. After behavioral observation, the rats were fasted for 24 h, then anesthetized with pentobarbital sodium and sacrificed. Brain tissue was isolated and fixed with 4% paraformaldehyde.
2.4 Acupuncture
Lesioned animals were either left untreated (sham and model group) or treated with acupuncture. Rats were randomly distributed in these groups (n = 8), except for weight, so that the average weight of each group was similar.
Acupuncture was performed for 30 days, 15 min daily starting at day 15 post-surgery with an acupuncture needle of Φ0.18 × 25 mm (Maanshan Bond Medical Instruments Co., Ltd. China), using the following points: Zhongwan (CV12), XiaWan (CV10), Tianshu (ST25), Qihai (CV6), Guanyuan (CV4). In the course of acupuncture treatment, the rats were drilled into a black cloth bag and fixed on the back on a operating table with adhesive tape. After 15 min of acupuncture treatment, rats were released. Rats were not anesthetized during the whole process.
Control groups were left untreated but were given the same binding force, 15 min daily starting at day 15 post -surgery.
2.5 Limb function test
2.5.1 Rotation test
On day 15 and 45 post-surgery apomorphine hydrochloride (sigma–aldrich, DK) was injected (0.05 mg/kg, i.p.) and after 3 min rats were placed in a plastic basin (50 cm in diameter) and the number of rotations per minute was counted for each rat.
2.5.2 Suspension test
Stainless steel wire of 60 cm length and 0.3 cm diameter was hung on a foam pad 80-cm-above the ground. The rats’ forepaws were placed on the rope and time was recorded during which the rats were able to suspend the rope. Rats dropping the rope or catching the rope with only one claw for less than 3 s were considered failure. Scores were assigned according to literature (Kuribara et al., 1997):1 = 0–4 s; 1 = 5–9 s; 2 = 10–14 s; 3 = 15–19 s; 4 = 20–24 s; 5 = 25–29 s; 6 = 25–29 s; 6 ≥ 30 s, and the average value was taken to evaluate the limb function of each rat. Each rat was tested three times on each occasion and the average value was taken to evaluate the limb function of the test rats. Suspension tests were performed on day 15 and day 45 post-surgery.
2.6 Immunohistochmistry (IHC)
Animals were deeply anesthetized with pentobarbital and euthanized via transcardial perfusion with heparinized saline and paraformaldehyde-lysine-periodate fixative. Brains were removed, fixed with 4% paraformaldehyde and embedded in parafin. Paraffin sections (20 µm) of the SN were sliced, starting 4.8 mm from the front fontanelle. Immunostaining was performed with a anti-TH antibody (1:100, sigma–aldrich) for 72 h, washed and treated with anti-rabbit IgG (1:200). Subsequently, sections were treated with Avidin-HRP (1:500), developed through a DAB reaction, mounted and coverslipped before microscopic evaluation and photography. Stereological counts using optical fractionator was used to estimate TH+ SNc neurons. Three slices of the same site were selected for each animal. The injured sites of the nigral area of the contralateral SNc were counted with the image analysis software IPP6.0 under 40 × objective lens. TH positive areas were counted, by IPP6.0 (unit: pixel), and the average number of positive cells calculated.
2.7 Transcranial sonography (TCS)
To observe SNH, rats were anesthetized (0.3% sodium pentobarbital, 150 mg/kg, i.p.), shaved on the head and ultrasound coupling agent was applied to isolate air interference. Images were scanned and stored at all levels of the SN region of the midbrain. After freezing the image, semi-quantitative evaluation of the echo signal in the SN region was performed: the detected SNH flake echo signal was manually depicted with a cursor, and then the respective area calculated. Abnormal flaky hyperechoic signals were defined by area size, and the SNH area of each group compared. Two doctors with more than 5 years experience in ultrasound diagnosis were selected to collect images and analyze data. Both doctors were randomly double-blind. Ultrasound detection was detected on day 15 and 45 after surgery.
2.8 Cervical vascular ultrasound (CVUS)
The diameter of carotid artery and hemodynamic parameters were measured by CVUS. The vascular diameter, the PSV and spectrum of LCCA, LICA, RCCA and RICA were recorded. In the acupuncture group the probe was held in one position during acupuncture and for the following 10 min after which PSV, vessel diameter and spectrum were measured again at the same position. Due to the long performance time, the data of 4 rats were obtained, and image analyses were recorded for qualitative analysis, without statistics.
2.9 Statistical analysis
The statistical analysis was carried out by SPSS13.0. The data were expressed as mean ± SD. One-way ANOVA analysis was used for multi-group comparison,Dunnett and Bonferroni were used for post-test correction. Paired T-test was used for two-group comparison. P < 0.05 was considered statistical significant.
3 Results
To investigate the mechanism of gut-acupuncture mediated influence on PD development, we applied the 6-OHDA lesion model to rats, which mimics the physiological symptoms of PD. Starting day 15 after surgery the acupuncture group was treated with gut-acupuncture daily for 30 days (Fig. 1A).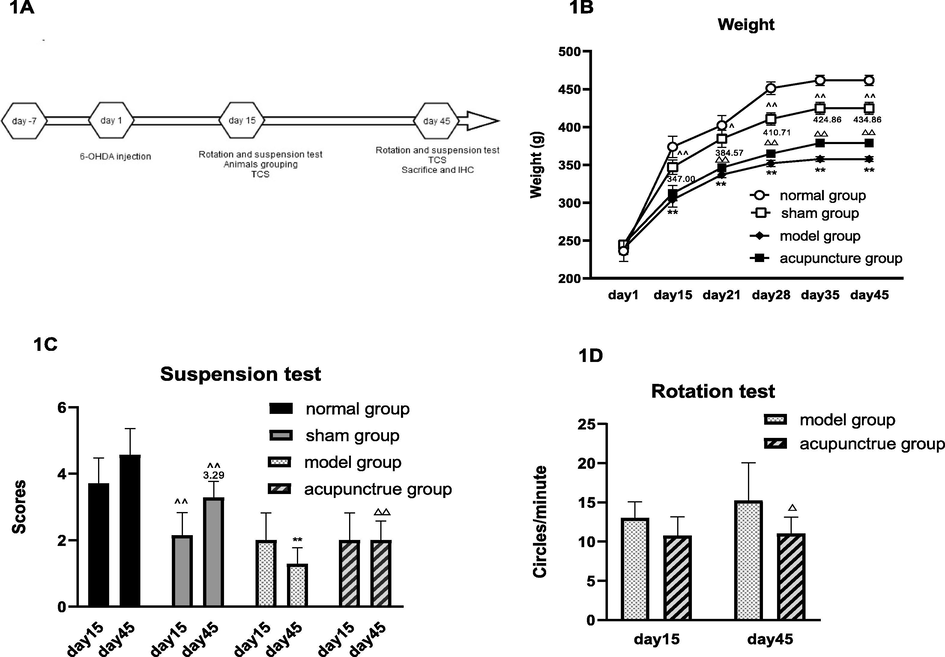
Impact of acupuncture on weight and motor characteristics in 6-OHDA lesioned.
Of note, the 6-OHDA lesioned rats showed significant loss of weight compared to the sham group and the acupuncture group tended to recover faster from weight loss than the model group (Fig. 1B).
Regarding limb function we could observe on day 45 significantly prolonged suspension time (1C) and significantly less rotation (1D) in the acupuncture group compared to the model group.
Legend 1A: 6-OHDA model treatment scheme. Rats were injected with 6-OHDA after 7 days of adaptation. The onset of of the model was confirmed by rotation tests on day15, followed by CVUS of the arteries and suspension tests. All three experiments were performed again on the day 45 before mice were sacrificed and brain tissue was isolated for IHC.
1B: Body weight was measured weekly starting day 1, in each of the 3 control groups and in acupuncture group. Motor symptoms were tested in all groups by suspension tests (1C) and in model group and acupuncture group by rotation tests (1D) on day 15 and day 45, respectively.
Data are expressed as mean ± SD, n = 8, vs normal group, ^ P < 0.05, ^^ P < 0.01; vs sham group, * P < 0.05, ** P < 0.01; vs model group, △ P < 0.05, △△ P < 0.01.
On histological level we found that in the SN DA neurons and TH abundance was significantly increased in the acupuncture group compared with the model group (Fig. 2A, B). These histological characteristics correlated with decreased hypoechogenic areas in the midbrain on day 45, which was examined by transcranial sonography. While no abnormal echo could be detected in the normal and the sham group (data not shown), similar SNH was observed in the model and acupuncture group on day 15, however on day 45 significantly less areas of hyperechogenicity could be detected in the acupuncture group compared with the model group (Fig. 2C, D).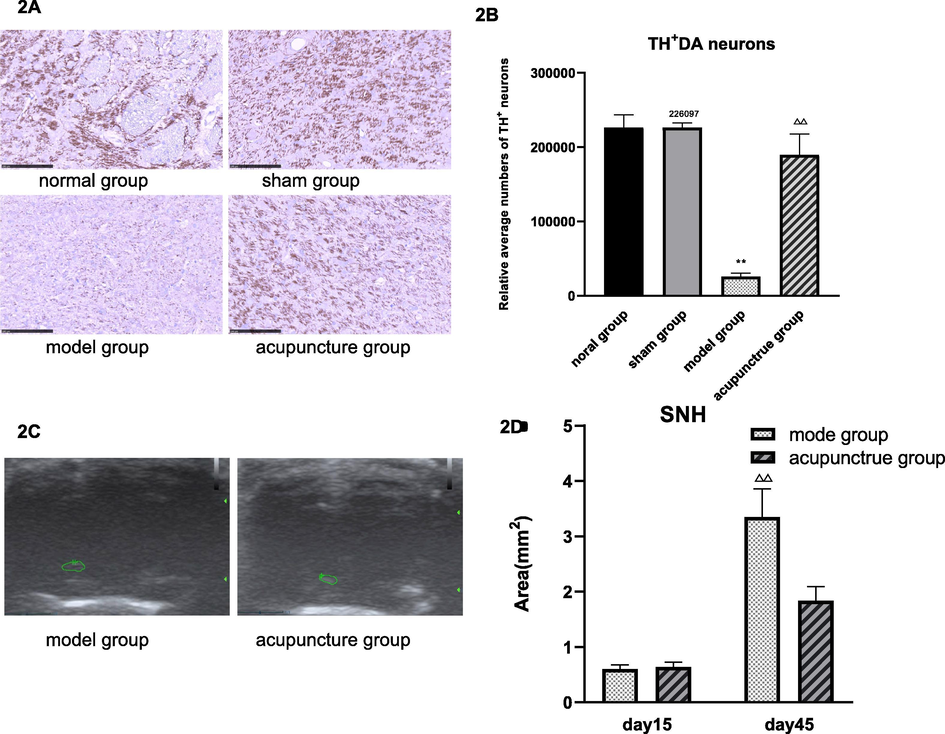
Acupuncture leads to higher abundance of TH+DA neurons and decreased areas of SNH in the SN.
Legend 2A: IHC: Representative brain sections of the SN stained with an anti- TH antibody (dark purple). 2B: Average numbers of TH+ DA neurons in control groups and acupuncture group. Numbers were obtained by counting 3 TH-stained slices of the SN area per animal. 2C: Representative images of SNH flake echo signal in the midbrain (green circles) measured by transcranial sonography. 2D: Quantification of SNH areas in rats of the model group and the acupuncture group on day 15 and day 45. Data are expressed as mean ± SD,n = 8, vs normal group, ^ P < 0.05, ^^ P < 0.01; vs sham group, * P < 0.05, ** P < 0.01; vs model group, △ P < 0.05, △△ P < 0.01.
Taken together we observed typical symptoms of PD in OHDA model rats which could be ameliorated by gut-acupuncture
To elucidate a putative mechanism of gut-acupuncture mediated improvement of PD symptoms, we measured the PSV and the vascular diameter of the LCCA, LICA, RCCA and RICA by CVUS. We found that on day 45, the PSV of LCCA, LICA and RICA was significantly improved, i.e. decreased in the acupuncture group after treatment compared with the model group (Fig. 3A, 3C, 3G), while the diameter of the LICA in the model group and the acupuncture group was significantly narrowed after surgery on day 15 compared with the normal group and sham group (Fig. 3D). Furthermore, the diameter of the LICA in acupuncture group after treatment was significantly wider than that of the model group on the day 45, whereas RCCA was narrower than that of the model group on the day 45 (Fig. 3D, 3F)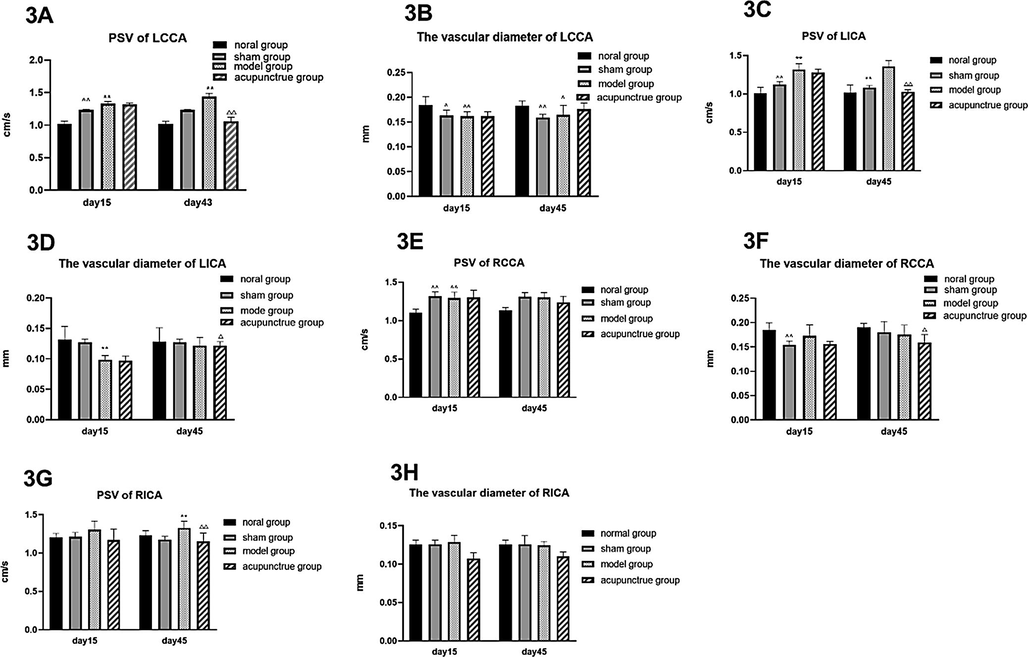
Peak systolic velocity of LCCA, LICA and RICA is decreased by acupuncture.
Legend 3: The PSV and vascular diameter of LCCA (3A, 3B), LICA (3C, 3D), RCCA (3E, 3F) and RICA (3G, 3H) were measured in each rat by CVUS on the indicated days. Diagrams show average values of the PSV and of the vascular diameter for the control groups and the acupuncture group on day 15 and day 45. Data are expressed as mean ± SD,n = 8, vs normal group, ^ P < 0.05, ^^ P < 0.01; vs sham group, * P < 0.05, ** P < 0.01; vs model group, △ P < 0.05, △△ P < 0.01.
Furthermore, we could show that the number of TH± DA neurons was negatively correlated with the area of SNH (Fig. 4A), as well as a positively correlated with the PSV of LCCA and LICA, respectively (Fig. 4B, C).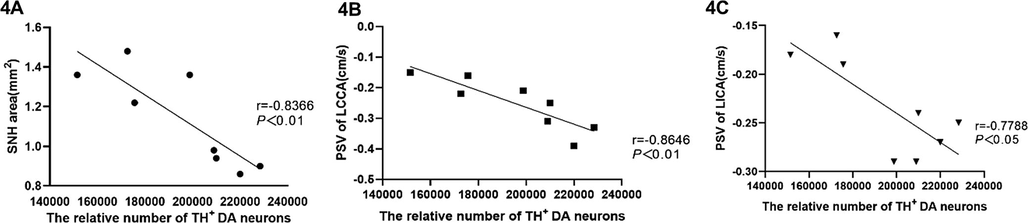
Frequency of TH + DA neurons in acupuncture treated rats correlates with SNH and hemodynamics.
Legend 4: Diagrams show the average numbers of TH + DA neurons in each rat of the acupuncture group (x-axes) plotted against SNH (4A), the PSV of the LCCA (4B) and the PSV of the LICA (4C), respectively. Each dot represents values obtained from an individual rat.
To examine the observed effects of acupuncture on hemodynamic changes in more detail, we measured the PSV and the vascular diameter of the LCCA and LICA via CVUS before, during and after acupuncture treatment in four rats of the acupuncture group. As shown in Fig. 5, we found that the vascular diameter narrowed and PSV slowed down during the treatment. But vascular diameter became wider and PSV increased after treatment (Fig. 5).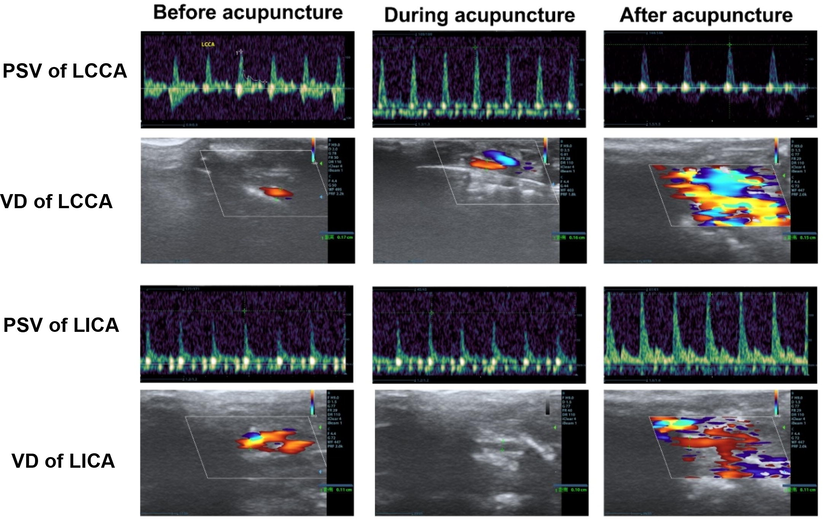
Real-time effects of acupuncture on hemodynamics.
Legend 5: In four rats of the acupuncture group, the PSV and the vascular diameter of LCCA and LICA were measured xxx minutes before, during and 10 min after acupuncture in the same position. Representative images of the frequency spectrum (upper panel) and ultrasound measurements of the arteries (lower panel) of one rat are shown.
4 Discussion
Parkinson’s disease is a rapidly growing health problem in aging generations. Environmental factors may be the cause of PD in most patients (Ritz et al.,2016) and also may have an effect on the occurrence and progression of PD through the GI tract (Kieburtz and Wunderle, 2012). The physiological function and movement of the GI tract are affected by signals of the central nervous system as wells as of the enteric nervous system and the inner intestine where neurotransmitter and immune signals are produced. GI-produced horrmones and neuropeptides may in turn affect the brain (Selkrig et al., 2014). Along this line almost half of the dopamine is produced in the gastrointestinal tract (Wall et al., 2014). Therefore, deregulated GI functions may affect dopamine loss in PD via the “gut-brain” axis. In order to study whether due to these effects, gut-acupuncture could ameliorate PD symptoms, we treated 6-OHDA-induced PD model rats with gut-acupuncture and analyzed putative therapeutic effects by examining the abundance of TH+ DA neurons.
TH is a key enzyme in dopamine synthesis and a marker for DA neurons (Khan et al., 2012). Shahnawaz et al could show that in a MFB model the striatum completely lost its dopamine content within 3 weeks after unilateral MFB injury, while the number of DA neurons gradually decreased. About 5 weeks later, neurons were nearly completely lost (Sarre et al., 2004). This is perfectly in line with our observations, as we detected a significantly higher abundance of TH+ DA neurons in acupuncture treated rats on day 45 after model initiation, while the acupuncture untreated model group showed a severe loss of TH+ DA neurons. In addition, this study revealed a tendency for gut-acupuncture to possibly improve the limb function.
Regarding the impact of gut-acupuncture on the neuronal changes, we could show that SNH was affected in the SN after acupuncture treatment. Clinical studies confirmed the presence of SNH in PD patients (Walter et al., 2007). The cause of SNH formation in PD has not been fully elucidated. Both autopsy and animal studies have found that SNH formation may be associated with an increase in iron content, and iron accumulation in SN is an early feature of PD (Belaidi and Bush, 2016), which is associated with the severity of the motor symptoms in PD patients (Guan et al.,2017). Along this line, in our model, the numbers of TH± DA neurons show a negative correlation with the area of SNH, which may be due to the decreased iron accumulation, however, we did not further investigate iron accumulation in the SN.
Analyses of hemodynamics revealed that the PSV and the vascular diameter of the LCCA and the LICA on the lesioned side was changed in the acupuncture group and restored to normal level, while it had little effect on the PSV and the vascular diameter on the healthy side. However, the CVUS before, during and after a single acupuncture treatment showed that during the gut-acupuncture treatment not only the PSV increased, but also the vascular diameter narrowed. However after acupuncture we found the PSV lower and the vascular diameter wider than before the treatment. Furthermore, we observed a positive correlation between the number of TH± DA neurons and the increase of the PSV of the LCCA and the LICA.
This regulation of cervical hemodynamics may be caused by the ENS of gut-brain axis by gut-acupuncture, and the changes of cervical hemodynamics, the decreased PSV of LCCA and LICA, may induce the proliferation of neural stem cells through a low oxygen tension micro- environment, a low oxygen tension (<1–6%) is beneficial to the proliferation of neural stem cells (Prozorovski et al., 2015). thus mediating the reduction of SNH and the increase of TH in SNc. It has been proved that acupuncture can promote the proliferation of neural stem cells in substantia nigra compact region of PD mouses (Yin et al., 2008), This may be a mechanism that acupuncture promotes the proliferation of neural stem cells and induces the increase of TH.
5 Conclusions
As shown above, gut-acupuncture had an impact on reducing the loss of DA neurons and the area of SNH, changed the internal blood flow velocity of PD rats, and the correspondingly improved limb function. Since we did not detect DA in striatum, we do not know whether TH± DA neurons secret DA in the SN. We suggest that acupuncture contributes to a balanced body state by stimulation of the vagus nerve through the ENS which might lead to a negative feedback effect thereby affecting the blood flow state and vessel diameter of the arteries on the lesioned side. However, the mechanism by which ENS acts on the CNS remains to be analyzed in future studies.
Acknowledgements
The study was approved by Ethics Committee of Zhejiang Provincial People’s Hospital (SYXK(Zhe)-2018-0012). All participants received written informed consent. The authors have stated that no competing interests exist. This paper was written and published by Dr. Lihong Li. Jing Wang, Lisong Zhu collected rats data, Jun Lu and Huiqian Liu were responsible for the experiments and Xiaoqing Jin guided the writing of the manuscript. The research was supported by the Zhejiang Youth Funding of Traditional Chinese Medicine (NO. 2018ZQ005; 2018ZQ006; 2018ZA004; 2019ZA004), the Zhejiang Medical and Health Project (NO. 2019309786) and the Zhejiang Provincial Natural Science Founding of China (NO. S19H090006).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Iron neurochemistry in Alzheimer’s disease and Parkinson’s disease: targets for therapeutics. Neurochem.. 2016;139(Suppl 1):179-197.
- [Google Scholar]
- Enteric glial cells: new players in Parkinson’s disease? Movement Disorders. 2014;30(4):494-498.
- [Google Scholar]
- The impact of gut microbiota on brain and behaviour. Current Opinion Clinical Nutrition Metabolic Care. 2015;18(6):552-558.
- [Google Scholar]
- Neurodegenerative signaling factors and mechanisms in Parkinson’s pathology. Toxicology in Vitro. 2017;43:104-112.
- [Google Scholar]
- Regionally progressive accumulation of iron in Parkinson’s disease as measured by quantitative susceptibility mapping. NMR Biomed.. 2017;30:3489.
- [Google Scholar]
- Electroacupuncture can increase the number of Nestin positive cells, a specific marker of NSCs, in substantia nigra compact area of mice. J. Chengdu. Univ. Tradit. Chin. Med.. 2008;2:21-23.
- [Google Scholar]
- Effects of central depressants on rota-rod and traction performances in mice. Jpn. J. Pharmacol.. 1997;27(1):121.
- [Google Scholar]
- Combined treatment with acupuncture reduces effective dose and alleviates adverse effect of L-dopa by normalizing Parkinson’s disease-induced neurochemical imbalance. Brain Res.. 2014;1544:33-44.
- [Google Scholar]
- Parkinson’s disease: Evidence for environmental risk factors. Movement Disorders. 2012;28(1):8-13.
- [Google Scholar]
- Brain-gut-microbiota axis in Parkinson’s disease. World J. Gastroenterol.. 2015;21(37):10609-10620.
- [Google Scholar]
- The prevalence of Parkinson’s disease: a systematic review and meta-analysis. Mov. Disord.. 2014;29(13):1583-1590.
- [Google Scholar]
- Redoxregulated fate of neural stem progenitor cells. Biochim. Biophys. Acta.. 2015;1850:1543-1554.
- [Google Scholar]
- Of pesticides and men: a California story of genes and environment in Parkinson’s disease. Curr. Environ. Health Rep.. 2016;3(1):40-52.
- [Google Scholar]
- Targeting Parkinson’s – Tyrosine hydroxylase and oxidative stress as points of interventions. CNS Neurolog. Disorders – Drug Targets. 2012;11(4):369-380.
- [Google Scholar]
- In vivo characterization of somatodendritic dopamine release in the substantia nigra of 6-hydroxydopamine-lesioned rats. J. Neurochem.. 2004;90:29-39.
- [Google Scholar]
- Bacterial neuroactive compounds produced by psychobiotics. Adv.Exp. Med. Biol.. 2014;817:221-239.
- [Google Scholar]
- Transcranial brain sonography findings in discriminating between parkinsonism and idiopathic Parkinson disease. Arch Neurol.. 2007;64:1635-1640.
- [Google Scholar]
- Effect of acupuncture on the motor and nonmotor symptoms in Parkinson’s disease-a review of clinical studies. CNS Neurosci Ther.. 2016;22(5):333-341.
- [Google Scholar]







